Abstract
BACKGROUND AND PURPOSE:
Hypoplasia of the rostral third of the SSS is a well-known variant and constitutes the most frequent variation of the SSS after preferential drainage to one of the transverse sinuses. Our aim was to describe unilateral hypoplasia of the rostral end of the SSS.
MATERIALS AND METHODS:
CTA performed in 100 consecutive patients studied for conditions other than dural sinus thrombosis was reviewed for the presence of a unilateral or bilateral hypoplastic rostral SSS. Associated dural venous sinus anomalies were recorded as well. The angiographic anatomy of unilateral hypoplastic rostral SSS was illustrated by 2 cases further imaged with DSA.
RESULTS:
Unilateral hypoplastic rostral SSS was found in 7 patients (7%). In all cases, compensatory drainage occurred through a large superior frontal vein that joined the SSS in the region of the coronal suture. Three of the 7 patients with a unilateral hypoplastic rostral SSS had at least another dural venous sinus anomaly. Complete or bilateral hypoplastic rostral SSS was noted in 3 patients (3%).
CONCLUSIONS:
Unilateral hypoplastic rostral SSS is more than twice as frequent as bilateral hypoplastic rostral SSS. It is the most frequently encountered variation of the SSS. Knowledge of this anatomic variation is important to avoid diagnostic pitfalls and to avoid erroneously mistaking it for a thrombosis. Four types of variations of the rostral SSS may be identified: 1) classic anatomy with a fully developed rostral SSS; 2) duplication of the rostral SSS; 3) complete or bilateral hypoplastic rostral SSS; 4) unilateral hypoplastic rostral SSS. The 4 types of rostral SSS variations can be explained by studying the embryologic development of the SSS.
Anatomic variations of the intracranial dural venous sinuses are frequent. They should be recognized to avoid confusion with pathologic conditions, in particular venous sinus thrombosis. Dural sinus anomalies most frequently involve the transverse sinuses,1 though other venous sinuses, such as the SSS, are commonly involved as well. Hypoplasia of the rostral third of the SSS is a frequent variation,2 second only to preferential drainage of the SSS into either of the transverse sinuses. In cases of complete hypoplastic rostral SSS, the absent rostral portion of the SSS is replaced by a pair of large parasagittal superior frontal cortical veins that run dorsally to join the origin of the SSS close to the coronal suture.2,3
The purpose of the present study was to report the existence of a unilateral hypoplastic rostral SSS, which, to the best of our knowledge, has not been previously documented. The potential clinical implications of the variant and its embryologic origin are discussed.
Materials and Methods
The venous phase (CTV) of CTA studies of 100 consecutive patients (including 4 pediatric patients) imaged for conditions other than dural sinus thrombosis were retrospectively evaluated for the presence of a unilateral hypoplastic rostral SSS. Dural sinus thrombosis was excluded on the basis of absence of clinical suspicion and absence of a filling defect within the dural sinus (other than typical arachnoid granulation or septation) or occlusion suggestive of intraluminal thrombosis. CTV cases of complete hypoplastic rostral SSS were also recorded. All CTAs were performed as routine clinical studies. Informed consent for the CTA study was obtained from all patients or their authorized representative. This included administration of intravenous contrast material and inclusion into our institutional review board−approved data base, authorizing the analysis of data obtained during routine diagnostic and interventional clinical activity.
CTA was performed on 320-MDCT (Aquilion One; Toshiba Medical Systems, Tokyo, Japan); 320-MDCT has a longitudinal coverage (z-axis) of 16 cm, which allows whole-head scanning in 1 gantry rotation. CTA was obtained by sequentially scanning the head during the intracranial passage of a bolus of intravenously administered contrast material and by subtracting 3 fused volumes obtained before the arrival of the contrast from the remaining contrast-enhanced volumes. Low-dose parameters (80 kV and 100 mAs) were used to reduce radiation exposure to the patient, resulting in a typical dose of 3.9 mSv per CTA in adults. In the pediatric patients, the number of head volumes scanned was kept to the strict minimum to further reduce radiation exposure to levels below 2.9 mSv. All adult patients received 50–70 mL of iopamidol 370 (Isovue 370; Bracco Diagnostics, Princeton, New Jersey) injected at a rate of 6 mL/s in an antecubital vein. For pediatric patients, the injection rate was set at 2.5–4 mL/s, depending on the size of the child, with a maximum dose of 2 mL/kg.
“Bilateral hypoplastic rostral SSS” was defined as absence of the proximal (rostral) third of the SSS in the presence of a bilaterally located prominent parasagittal superior frontal vein running from the pole of both frontal lobes to the origin of the SSS near or at the coronal suture.
“Unilateral hypoplastic rostral SSS” was defined as:
1) A prominent parasagittal frontal cortical vein coursing on the surface of the brain, assuming a tubular shape (rather than the flattened appearance expected from a dural sinus in a parasagittal location) and joining the middle third of the SSS near or at the coronal suture and
2) In association with a contralateral small midline venous channel clearly embedded in the midsagittal dura mater of the falx cerebri adjacent to the calvaria and directly contiguous with the middle third of the SSS.
Other dural venous sinus variations seen in patients with unilateral or bilateral hypoplastic rostral SSS were recorded as well.
The angiographic appearance of unilateral hypoplastic rostral SSS is further illustrated with 2 observations made during routine DSA studies. An anatomic specimen with bilateral hypoplastic rostral SSS identified during routine anatomic dissections is provided as well to offer a macroscopic correlation of the reported variation.
Results
CTA studies performed in 100 consecutive patients were retained for statistical analysis. One patient with a pathology involving the SSS, a parasagittal meningioma infiltrating the posterior third of the SSS without disrupting its anterior portion, was included in the series. Unilateral hypoplastic rostral SSS was found in 7 patients (7%). Tables 1 and 2 summarize the characteristics of patients with unilateral and bilateral hypoplastic rostral SSS. The number of cases with unilateral hypoplastic rostral SSS was too small to draw significant conclusions in regard to their sex or side predominance.
Table 1:
Characteristics of patients with unilateral hypoplastic rostral SSS on CTV
| Age (yr) | Sex | Side | Clinical Diagnosis | Additional Dural Venous Sinus Anomaly |
|---|---|---|---|---|
| 59 | F | Right | Right paraophthalmic aneurysm | None |
| 44 | F | Right | Ruptured left ACA aneurysm and left frontal AVM | None |
| 28 | F | Left | AcomA aneurysm | Left TS hypoplasia |
| 4 | M | Left | Ruptured right lenticulostriate aneurysm | Left TS hypoplasia |
| 44 | M | Left | Pseudotumor cerebri | None |
| 41 | F | Right | Pseudotumor cerebri | None |
| 26 | M | Left | Giant posterior fossa arachnoid cyst | Torcular herophili displaced by the cyst |
Note:—ACA indicates anterior cerebral artery; AcomA, anterior communicating artery; TS, transverse sinus.
Table 2:
Characteristics of patients with bilateral hypoplastic rostral SSS on CTV
| Age (yr) | Sex | Side | Clinical Diagnosis | Additional Dural Venous Sinus Anomaly |
|---|---|---|---|---|
| 70 | F | Both | Right cavernous carotid aneurysm | None |
| 65 | F | Both | Inferior petrosal sinus sampling work-up | None |
| 55 | F | Both | Pseudotumor cerebri, bilateral TS stenosis | None |
In all cases of unilateral hypoplastic rostral SSS, a large superior frontal venous channel was found ipsilateral to the side of the hypoplasia. The morphologic characteristics of this channel were that of a cortical vein coursing on the surface of the brain, assuming a tubular shape rather than the flattened appearance expected from a dural sinus in a parasagittal location. The hemi-SSS on the other side, however, clearly coursed within the dura, appearing as a smooth anterior continuation of the middle third of the SSS (Fig 1). Additional dural sinus anomalies were noted in 3 of the 7 cases of unilateral hypoplastic rostral SSS. In 2 instances, the proximal third of the left transverse sinus was noted to be hypoplastic. In the third case, the torcular herophili and the straight sinus were displaced cranially by a giant posterior fossa arachnoid cyst (Fig 2).
Fig 1.
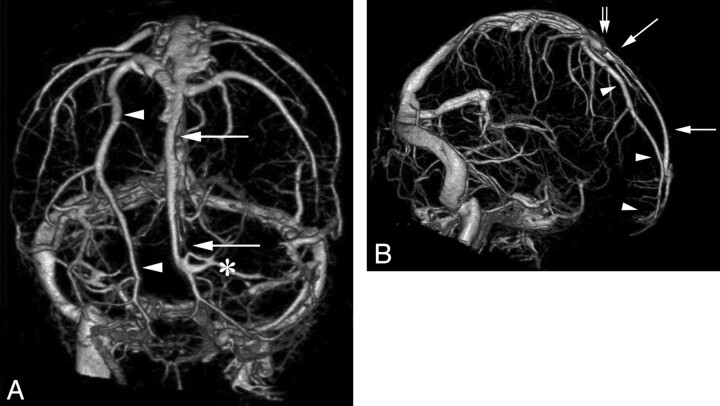
CTA, venous phase, obtained with a 320-MDCT in a 41-year-old woman imaged for pseudotumor cerebri. Anteroposterior (A), and right lateral (B) projections of the subtracted intracranial venous system. Unilateral hypoplastic rostral SSS is documented on the right side. Note the compensatory drainage through an enlarged parasagittal frontal cortical vein (arrowhead), coursing parallel to the midline from the pole of the right frontal lobe to the SSS in the region of the coronal suture (double arrow). A hemi-SSS (arrows) is observed on the left side, directly continuous with the middle third of the SSS. Left frontopolar cortical veins (asterisk) drain into the most rostral portion of the SSS at a right angle.
Fig 2.
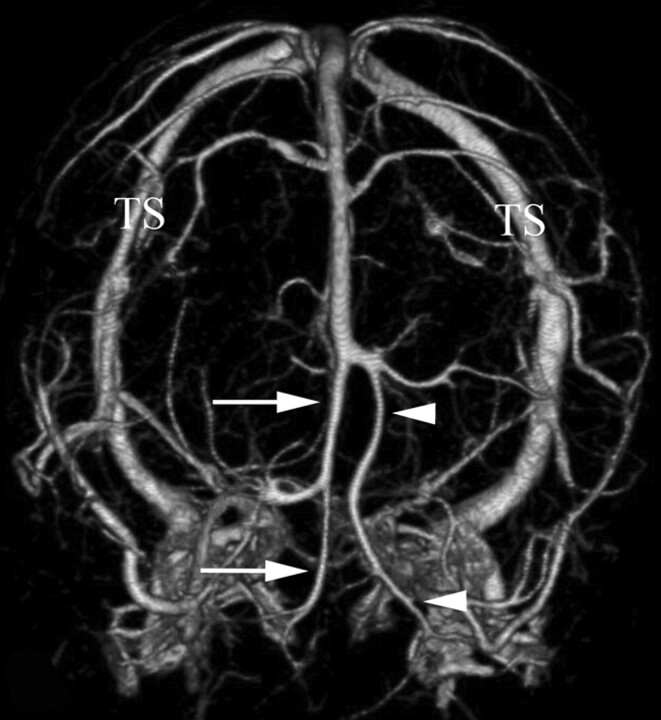
A 26-year-old man with intracranial hypertension secondary to a giant posterior fossa arachnoid cyst. Unilateral hypoplastic rostral SSS is documented on the left side, associated with cranial displacement of the torcular herophili and straight sinus by the arachnoid cyst. These findings are demonstrated on the venous phase from a CTA obtained with 320-MDCT. Anteroposterior view of the subtracted intracranial venous system shows a right hemi-SSS (arrows) coursing in the midsagittal plane. The left hypoplastic rostral SSS is compensated by an enlarged frontal cortical vein (arrowheads). The SSS−transverse sinuses junction is abnormally highly (rostral) situated due to the posterior fossa arachnoid cyst. TS indicates transverse sinus.
Bilateral hypoplastic SSS was found in 3 patients (3%) (Table 2). In 2 cases, a 2- to 4-cm-long stump of the atretic SSS was found anterior to the point of confluence of the superior frontal veins and the SSS. No associated dural venous sinus anomalies were noted.
The clinical information for the 2 patients with unilateral hypoplastic rostral SSS demonstrated by DSA is summarized in Table 3. One of them, a 23-year-old woman with a giant clival chordoma, was found to have 2 additional rare variations of the dural venous sinuses: 1) the ISS drained a large interhemispheric vein from the inner surface of the superior frontal gyrus and the anterior portion of the cingulate gyrus;, and 2) the right basal vein of Rosenthal drained through a dural sinus along the free edge of the tentorium cerebelli into the distal third of the straight sinus (Fig 3).
Table 3:
Characteristics of the 2 patients with unilateral hypoplastic rostral SSS undergoing DSA
| Age (yr) | Sex | Side | Clinical Diagnosis | Additional Dural Venous Sinus Anomaly |
|---|---|---|---|---|
| 62 | F | Right | Left ACA territory infarct after left ICA endarterectomy | None |
| 23 | F | Right | Giant skull base chordoma | Large ISS draining the left mesiofrontal lobe and tentorial drainage of the posterior segment of the right BVR |
Note:—BVR indicates basal vein of Rosenthal; ACA, anterior cerebral artery.
Fig 3.
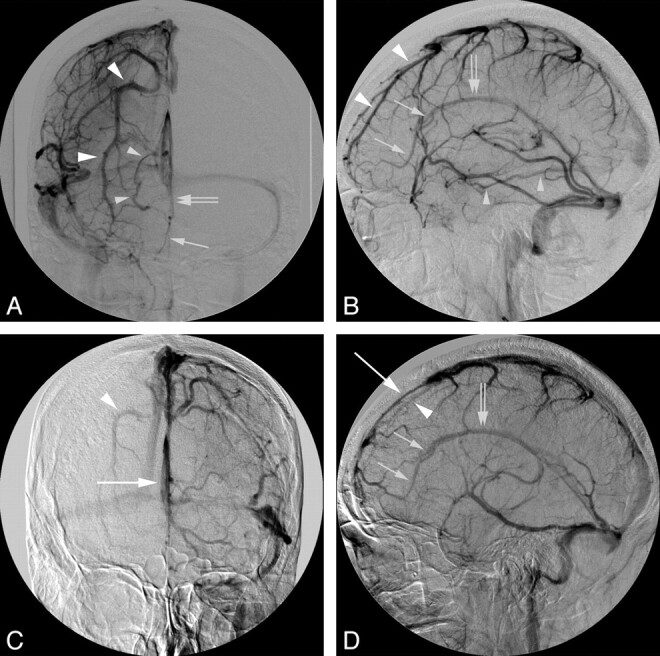
A 23-year-old woman with a giant skull base chordoma. DSA demonstrates right unilateral hypoplastic rostral SSS associated with additional dural venous sinus anomalies. A and B, The venous phase of a right common carotid artery injection in the anteroposterior (A) and lateral (B) projections shows the right unilateral hypoplastic rostral SSS with the characteristic compensatory enlarged parasagittal frontal vein (large arrowheads). In addition, there is compensatory drainage of the mesiofrontal lobe through a frontal interhemispheric vein (small arrows) into the ISS (double arrows). Note the anomalous posterior drainage of the right basal vein of Rosenthal (small arrowheads) into the middle portion of the straight sinus. C and D, The venous phase of a left common carotid artery injection in the anteroposterior (C) and lateral (D) projections demonstrates a left hemi-SSS (large arrow). Due to cross-filling of the right anterior cerebral artery through the anterior communicating artery, the right hypoplastic rostral SSS and other right-sided venous anomalies are also demonstrated during the left common carotid artery venous phase.
The macroscopic appearance of bilateral hypoplastic rostral SSS is illustrated by an observation made during routine anatomic dissection. Two large parasagittal superior frontal veins were identified in the absence of the rostral third of the SSS. Both veins coursed dorsally before curving medially, at right angles, to continue as the middle third of the SSS. The pial/subarachnoid nature of these compensatory frontal cortical veins was well-demonstrated (Fig 4).
Fig 4.
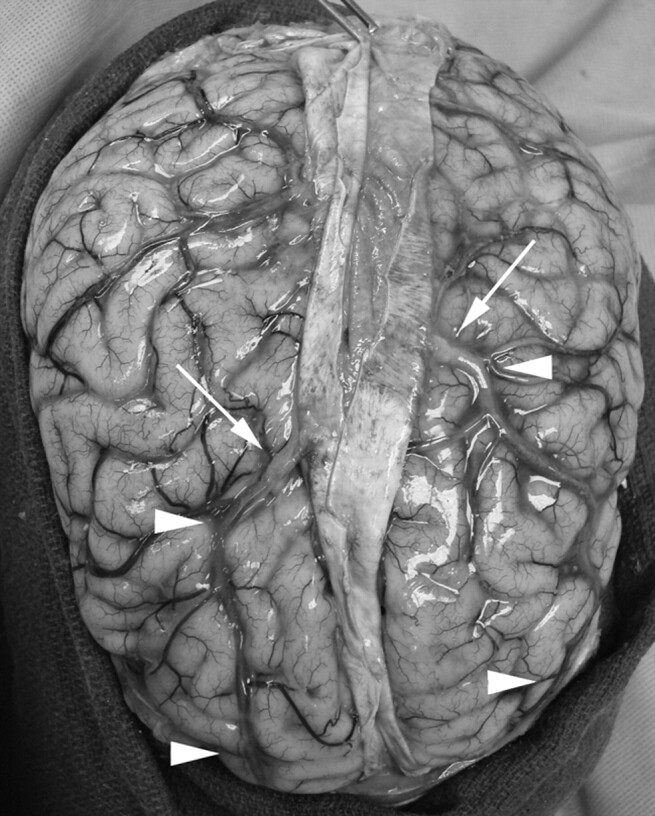
Anatomic preparation showing a case of bilateral hypoplastic rostral SSS. Note that the large compensatory parasagittal frontal veins (arrowheads) are truly pia-arachnoid veins and not dural venous sinuses, in keeping with the CTV and DSA findings reported in our study. There is a transition zone (arrows) in which these veins become thickened before entering the middle third of the SSS in the vicinity of the coronal suture. These findings are to be distinguished from the observations reported by Kaplan and Browder,13 in which the compensatory venous occurred by way of intradural venous drainage channels coursing parallel to the midsagittal plane, strongly suggesting a duplication of the rostral SSS rather than true hypoplastic rostral SSS.
Duplication of the rostral SSS was never observed.
Discussion
Anatomic variations of the dural venous sinuses represent common diagnostic pitfalls, particularly in the setting of suspected dural venous sinus thrombosis.4 For instance, the venous drainage of the posterior fossa is typically asymmetric, in keeping with the independent embryonic development of its constituents, in particular of the transverse and sigmoid sinuses.5–7 The most common segmental variation of the dural venous sinuses is isolated hypoplasia of the proximal portion of a transverse sinus. Anatomic variations of the SSS are not frequent. Segmental or complete duplication of the SSS may be observed,1 sometimes associated with occipital or parietal meningoencephaloceles and an anomalous course of the straight sinus.8,9 Preferential drainage of the rostral and middle portions of the SSS into a persistent falcine sinus in the presence of a hypoplastic dorsal SSS has been reported,10,11 but is very rare. While they are not true anatomic variations of the disposition of the SSS, arachnoid granulations or dural septations may also represent diagnostic pitfalls during routine imaging of the intracranial venous system.12
The most frequently reported variation of the SSS after preferential damage to 1 of the transverse sinuses, is hypoplasia of its rostral third.2,3 In the most extensive form of rostral hypoplasia, the SSS takes its origin in the vicinity of or at the coronal suture.2 In such instances, a rudimentary rostral segment may occasionally be documented by DSA, CTV, or MR venography, though no cortical or meningeal tributaries of this hypoplastic segment can usually be demonstrated by radiologic means. The distinction between hypoplastic rostral SSS and thrombosis of the anterior third of the SSS relies on the demonstration of prominent bilateral superior frontal veins that follow a parasagittal course running from the pole of the frontal lobes to the origin of the SSS at the coronal suture.
Kaplan and Browder13 found evidence of hypoplastic rostral SSS in 7 of 382 (1.8%) anatomic specimens in 1 series and in 12 of 201 (6%) specimens in a second anatomic series. Most interesting, in the first series, the compensatory venous drainage for the hypoplastic rostral SSS was not through the superior frontal veins but by way of an intradural venous channel that drained cortical veins and ran parallel to the midsagittal plane toward the origin of the SSS. The dural nature of these venous channels was confirmed by histology. From an embryologic perspective (see below), this anatomic disposition seems more in keeping with a duplication of the rostral SSS rather than SSS atresia or hypoplasia. The 12 cases from the second series seem to correspond to the more typical disposition of a true hypoplastic rostral SSS, in which the compensatory drainage of the anterior and superior aspects of the frontal lobes occurs through large parasagittal frontal veins ending in the more dorsally located SSS. In the present study of 100 consecutive CTVs, a complete hypoplastic rostral SSS similar to the one reported in Kaplan and Browder's second series13 was encountered in only 3% of the cases. This lower incidence of complete hypoplastic rostral SSS is likely accounted for by the stricter inclusion criteria in our study, in which hypoplastic rostral SSS was only considered to be present when the rostral SSS was missing from the region of the foramen caecum to the coronal suture and large compensatory superior frontal veins were identified.
After an extensive review of the English and French literature, we were unable to find a reference to unilateral hypoplastic rostral SSS with compensatory drainage through 1 superior frontal vein. Kaplan and Browder13 reported 1 case of unilateral hypoplastic rostral SSS with a parasagittal dural venous sinus that collected the venous drainage of the adjacent frontal lobe, which again suggests duplication of the rostral SSS. In the present study, unilateral hypoplastic rostral SSS was found in 7% of the cases, more than twice the incidence of complete hypoplastic rostral SSS. In 1 of the cases demonstrated by DSA, additional compensatory drainage occurred through a large ISS by way of an interhemispheric frontal vein. Cortical drainage through the ISS is usually not conspicuous if at all demonstrable angiographically but may become prominent in cases of SSS outflow obstruction.14 Although no outflow obstruction was demonstrated in our case, the anomalous development of the rostral SSS may have resulted in the rerouting of part of its normal drainage territory toward the ISS. The small number of cases of unilateral hypoplastic rostral SSS in our series limits the significance of statistical analysis, in particular in regard to its association with other abnormalities of dural venous sinuses that share a common embryologic development, such as the proximal transverse, inferior sagittal, and straight sinuses. Hypoplasia of the proximal left transverse sinus was observed in 2 cases, but this is also a common occurrence in the general population, where this variant may be observed in 15% of cases.1,15 The 4 cases (of 7) in which unilateral hypoplastic rostral SSS was the only recognizable dural venous variation confirm that it can exist as an isolated finding.
Embryologic Considerations
According to Padget,5 the primitive marginal sinus is the precursor of the SSS and the medial (proximal) end of the transverse sinuses, which can already be identified in stage 3 of embryonic life (6–12 mm). Streeter16 named this bilateral venous structure stretched between the developing cerebral hemispheres the “sagittal plexus.” The ensuing account of the formation of the SSS is based upon Streeter's work.
In Stages 1 and 2 (5–8 mm), the primitive pial capillary plexus overlying the neural tube of the forebrain drains into the anterior dural plexus. By Stage 3, the sagittal plexus is clearly defined as a subdivision of the anterior dural plexus. This plexus will give rise to individual venous channels that will eventually form a single SSS by 1 of 2 processes: 1) preferential use of 1 of the venous pathways that enlarges to become the mature adult dural sinus; or 2) coalescence of 2 or more parallel channels into a single vessel. The ISS and the straight sinuses are similarly derived from this primitive plexus. This developmental process is still active during stage 7 (40 mm), which marks the beginning of fetal life. It is completed in 50-mm fetuses. The primitive SSS matures in a craniocaudal direction, in a manner consistent with the posterior expansion of the cerebral hemispheres. The caudal extension of the primitive SSS is concomitant with the dwindling of the anterior dural plexus, whose function in the drainage of the forebrain is partially transferred to the SSS.
The pia-arachnoid veins (future cortical veins) of the developing forebrain initially form a primitive capillary network on the surface of the neural tube that collects into the anterior dural plexus found on either side of the head (stage 2, 10–16 cm).5 The expansion of the cerebral hemisphere and the lateral displacement of the anterior dural plexus result in stretching of the pia-arachnoid veins. Many of these veins disappear, while others become enlarged and elongated and establish secondary anastomoses among themselves. As this primary pial network continues to develop, the number of its connections with dural venous channels decreases; this change results in the appearance of regionally dominant cortical drainage pathways. This process of secondary pial venous anastomoses accounts for the variable distribution of cortical veins over the surface of the hemispheres. In addition, the topography and drainage pathway of the cortical veins also depends on the development of the dural venous sinuses in their vicinity. In the absence of their “normal” collecting dural sinus, 1 or several cortical veins may develop variant drainage patterns through the recruitment of an alternate path through the primary pial network.
Based on the existing literature and the findings reported in the present study, 4 types of rostral SSS dispositions can be defined (Fig 5), each of which can be explained by the embryologic processes described above:
A normal rostral SSS receiving cortical veins from both frontal lobes along its course
Duplication of the rostral SSS, explained by the persistence of 2 individual channels (hemi-SSS) derived from the sagittal plexus (of Streeter16) that have failed to coalesce at the midline
Complete hypoplastic rostral SSS with compensatory drainage through bilateral large superior frontal veins. This variation may be explained by failure or delay in the formation of the rostral segment of the sagittal plexus, compensated by the development of longitudinal anastomoses connecting the pia-arachnoid veins of the anterior and superior frontal regions with the venous tributaries of the more dorsally located developing SSS
Unilateral hypoplastic rostral SSS with compensatory drainage through an ipsilateral large superior frontal vein, resulting from the process described for variant 3 on 1 side, combined with a normal developmental pattern for the other side.
Fig 5.
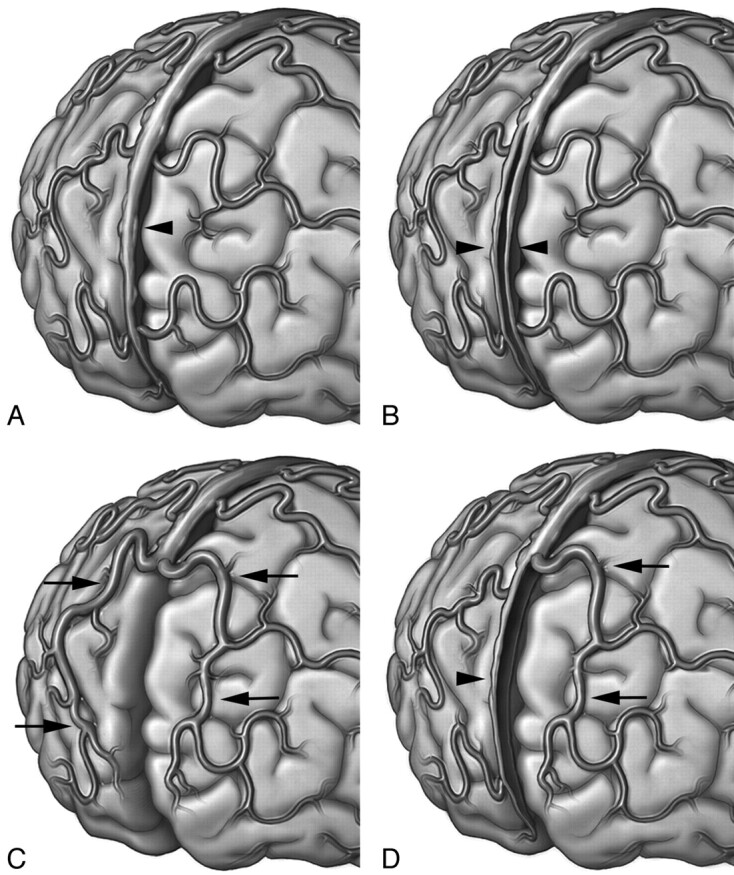
Schematic representation of the variations of the rostral SSS. A, Type 1, classic anatomy, with a fully developed rostral SSS (arrowhead) receiving multiple bilateral frontal veins. B, Type 2, duplication of the rostral SSS, with each hemi-SSS (arrowheads) draining ipsilateral frontal veins. C, Type 3, complete hypoplastic rostral SSS, with compensatory drainage through bilateral parasagittal superior frontal veins (arrows). D, Type 4, unilateral hypoplastic rostral SSS, with compensatory drainage through a parasagittal superior frontal vein on 1 side (arrows) and a hemi-SSS on the other (arrowhead).
Conclusions
Unilateral hypoplastic rostral SSS, a variant in which the rostral SSS is hypoplastic and compensatory drainage occurs through a large parasagittal superior frontal vein, is an unrecognized anatomic variation of the SSS. It is more frequently encountered than bilateral or complete hypoplastic rostral SSS, with a 7% incidence in the present series. Knowledge of this anatomic variation will help to avoid diagnostic pitfalls, particularly when a dural venous sinus thrombosis is suspected.
Acknowledgments
The authors express their gratitude to Ronald S. Wade, Director of the Anatomical Services Division, University of Maryland, for providing generous access to the laboratory and his help in the preparation of anatomic specimens. We also extend our gratitude to Lydia Gregg, Medical Illustrator, Research Associate, The Johns Hopkins School of Medicine, for her help in the preparation of the anatomic specimens and illustrations.
ABBREVIATIONS:
- ISS
inferior sagittal sinus
- MDCT
multidector row CT
- SSS
superior sagittal sinus
Footnotes
Disclosures: Philippe Gailloud, Research Support (including provision of equipment or materials): Siemens, Details: sponsored research; Consultant: Codman.
References
- 1. Huang YP, Okudera T, Ohta T. Anatomy of the dural venous sinuses. In: Kapp JP, Schmidek HH. eds. The Cerebral Venous System and Its Disorders Orlando, Florida: Grune and Stratton; 1984: 109– 67 [Google Scholar]
- 2. Kaplan HA, Browder AA, Browder J. Atresia of the rostral superior sagittal sinus: associated cerebral venous patterns. Neuroradiology 1972; 4: 208– 11 [DOI] [PubMed] [Google Scholar]
- 3. Hacker H. Superficial supratentorial veins and dural sinuses. In: Newton TH, Gordon Potts MDD. eds. Radiology of the Skull and Brain: Angiography. St. Louis: C.V. Mosby Company; 1974: 1851–902 [Google Scholar]
- 4. Leach JL, Fortuna RB, Jones BV, et al. Imaging of cerebral venous thrombosis: current techniques, spectrum of findings, and diagnostic pitfalls. Radiographics 2006; 26 suppl 1: S19– 41, discussion S42–43 [DOI] [PubMed] [Google Scholar]
- 5. Padget DH. The development of the cranial venous system in man, from the viewpoint of comparative anatomy. In: Contributions to Embryology. Washington, DC: Carnegie Institution of Washington; 1957; 247: 81–140 [Google Scholar]
- 6. Butler H. The development of certain human dural venous sinuses. J Anat 1957; 91: 510– 26 [PMC free article] [PubMed] [Google Scholar]
- 7. Butler H. The development of mammalian dural venous sinuses with especial reference to the post-glenoid vein. J Anat 1967; 102: 33– 56 [PMC free article] [PubMed] [Google Scholar]
- 8. Brunelle F, Baraton J, Renier D, et al. Intracranial venous anomalies associated with atretic cephalocoeles. Pediatr Radiol 2000; 30: 743– 47 [DOI] [PubMed] [Google Scholar]
- 9. Otsubo Y, Sato H, Sato N, et al. Cephaloceles and abnormal venous drainage. Childs Nerv Syst 1999; 15: 329– 32 [DOI] [PubMed] [Google Scholar]
- 10. Knott JF. On the cerebral sinuses and their variations. J Anat 1882; 16: 27– 42 [PMC free article] [PubMed] [Google Scholar]
- 11. Manoj KS, Krishnamoorthy T, Thomas B, et al. An incidental persistent falcine sinus with dominant straight sinus and hypoplastic distal superior sagittal sinus. Pediatr Radiol 2006; 36: 65– 67 [DOI] [PubMed] [Google Scholar]
- 12. Liang L, Korogi Y, Sugahara T, et al. Normal structures in the intracranial dural sinuses: delineation with 3D contrast-enhanced magnetization prepared rapid acquisition gradient-echo imaging sequence. AJNR Am J Neuroradiol 2002; 23: 1739– 46 [PMC free article] [PubMed] [Google Scholar]
- 13. Kaplan HA, Browder J. Atresia of the rostral superior sagittal sinus: substitute parasagittal venous channels. J Neurosurg 1973; 38: 602– 07 [DOI] [PubMed] [Google Scholar]
- 14. McCord GM, Goree JA, Jimenez JP. Venous drainage to the inferior sagittal sinus. Radiology 1972; 105: 583– 89 [DOI] [PubMed] [Google Scholar]
- 15. Bono F, Lupo MR, Lavano A, et al. Cerebral MR venography of transverse sinuses in subjects with normal CSF pressure. Neurology 2003; 61: 1267– 70 [DOI] [PubMed] [Google Scholar]
- 16. Streeter G. The development of the venous sinuses of the dura mater in the human embryo. Am J Anatomy 1915; 18: 145– 78 [Google Scholar]