These investigators used ASL perfusion to objectively measure CBF in 11 patients who underwent occlusion of an ICA after passing a test balloon occlusion. All CBF values were normal and there were no differences between the ipsi- and contralateral hemispheres. However, arrival of labeled blood was prolonged on the ipsilateral side. In most patients, collateral flow occurred via both the anterior and posterior communicating arteries.
Abstract
BACKGROUND AND PURPOSE:
Therapeutic carotid occlusion is an established technique for treatment of large and giant aneurysms of the ICA, in patients with synchronous venous filling on angiography during BTO. Concern remains that hemodynamic alterations after permanent occlusion will predispose the patient to new ischemic injury in the ipsilateral hemisphere. The purpose of this study was to assess whether BTO with synchronous venous filling is associated with normal CBF long term after carotid sacrifice.
MATERIALS AND METHODS:
Eleven patients were included (all women; mean age, 50.5 years; mean follow-up, 38.5 months). ASL with single and multiple TIs was used to assess CBF and its temporal characteristics. Selective ASL was used to assess actual territorial contribution of the ICA and BA. Collateral flow via the AcomA or PcomA or both was determined by time-resolved 3D PCMR. Paired t tests were used to compare CBF and timing parameters between hemispheres.
RESULTS:
Absolute CBF values were within the normal range. There was no significant CBF difference between hemispheres ipsilateral and contralateral to carotid sacrifice (49.4 ± 11.2 versus 50.1 ± 10.1 mL/100 g/min). Arterial arrival time and trailing edge time were significantly prolonged on the occlusion side (816 ± 119 ms versus 741 ± 103 ms, P = .001; and 1765 ± 179 ms versus 1646 ± 190 ms, P < .001). Two patients had collateral flow through the AcomA only and were found to have increased timing parameters compared with 9 patients with mixed collateral flow through both the AcomA and PcomA.
CONCLUSIONS:
In this small study, patients with synchronous venous filling during BTO had normal CBF long term after therapeutic ICA occlusion.
For large and giant aneurysms of the ICA, therapeutic carotid artery balloon occlusion is a safe and effective therapy in patients tolerating sacrifice of the carotid artery.1,2 Different methods (including positron-emission tomography, single-photon emission CT, xenon-enhanced CT, and BTO with clinical or angiographic evaluation) have been used to identify patients at risk of developing cerebral ischemia after permanent ICA occlusion.3,4 Field et al5 reported that therapeutic ICA occlusion can result in cerebral infarction despite normal clinical BTO results, and they evaluated outcome in patients who underwent clinical BTO combined with quantitative CBF analysis with xenon-enhanced CT. They concluded that this combined method was a reliable technique for identification of patients at risk of ischemic infarction.5
Tolerance to carotid artery occlusion can also be evaluated reliably by angiographic BTO.1 In a large consecutive series, it has been demonstrated that synchronous venous filling during BTO (a <0.5-second delay of opacification between the cortical veins of the occluded side and the contralateral side) has a high positive predictive value (98%) for tolerance to ICA occlusion.2 After tolerance to test occlusion, acute and delayed symptomatic ischemic events have been described occasionally.6,7 Some concern remains that hypoperfusion after permanent carotid artery occlusion will predispose the patient to the formation of new ischemic injury in the ipsilateral hemisphere, resulting in overt or silent infarction.7–9 In the case of silent infarction, focal neurologic symptoms are absent, while ischemic injury is visible on MR imaging. The prevention of these so-called silent infarcts is important because they appear to be associated with neurocognitive decline.10 Thus far, to our knowledge, no literature is available on actual CBF measurements long term after therapeutic occlusion in patients who passed the angiographic BTO.
ASL is a noninvasive MR perfusion imaging technique that uses arterial blood water protons as an endogenous tracer of flow.11 By acquisition and subtraction of images, with and without previous magnetic labeling of arterial blood water protons, perfusion images are obtained that are used for quantitative estimation of CBF. Previous studies have demonstrated that ASL could be of high value to measure perfusion impairment and identify patients at risk for ischemic events.12,13 By acquiring images at increasing delay times after initial labeling, ASL can be used to assess the temporal characteristics of the inflowing arterial blood such as the arrival time (the time from initial labeling until arrival of the labeled bolus in the brain parenchyma) and the trailing edge time (the time between initial labeling and clearance of the labeled bolus from the brain parenchyma).14,15
Bokkers et al16 were the first to use this technique in patients with symptomatic carotid artery occlusion and measured significant differences in CBF and timing parameters between hemispheres ipsilateral and contralateral to the occlusion side. Recently, a similar technique was used by Macintosh et al,17 who found that prolonged arterial arrival times resided in the affected hemisphere of patients with minor stroke or transient ischemic attack, though functional impairment and lesions on diffusion-weighted imaging were limited.
The purpose of the current study was to assess whether BTO with synchronous venous filling is associated with normal CBF long term after therapeutic occlusion of the ICA for large and giant carotid artery aneurysms. Therefore, we used ASL to measure CBF and timing parameters of arterial blood in patients who underwent therapeutic ICA occlusion for large or giant aneurysms and who tolerated the angiographic BTO. Because the risk of late ischemic complications is largely dependent on the adequacy of the collateral circulation, we also measured collateral flow via the circle of Willis.18
Materials and Methods
Patients
The local ethics committee approved the study protocol. Between January 2003 and November 2009, 24 consecutive patients were treated with ICA balloon occlusion for carotid aneurysms. Previous to permanent occlusion, patients had undergone test occlusion of the ICA. During test occlusion, angiography of the contralateral ICA or vertebral artery was performed. Synchronous venous filling (a <0.5-second delay of opacification between the cortical veins of the occluded carotid artery and the cortical veins of the territory of the examined artery) was considered a predictor for tolerance of permanent occlusion. If collateral flow was mainly via the AcomA, the venous phase of both cerebral hemispheres had to be synchronous on angiograms of the contralateral carotid artery. If collateral flow was mainly via the PcomA, the venous phase of the tested cerebral hemisphere had to be synchronous with filling of the cerebellar and temporo-occipital cerebral cortical veins on vertebral angiography. In case of mixed collateral flow, opacified blood could be diluted, resulting in diminished attenuation of the opacification of the veins in the vascular territory of the occluded carotid artery. If the veins filled synchronously, however, the patient was considered as having passed the test.2
If test occlusion was tolerated, the carotid artery was definitively occluded by a detachable latex balloon (Goldvalve No. 16, Nycomed, Paris, France; and Goldbal 1 and 2, Balt, Montmorency, France) positioned just proximal to the aneurysmal neck, and a second safety balloon was detached in the proximal ICA. In 4 patients, (additional) coils were used for occlusion. No clinical sequelae after occlusion had been reported.
Of the 24 patients who had been treated, 3 were excluded because age older than 70 years was considered an exclusion criterion. In December 2009, 21 patients younger than 70 years of age (20 women, 1 man) were sent an invitation to participate in a long-term MR imaging follow-up study. Two patients were lost to follow-up, 6 patients did not want to participate, and 13 patients were willing to participate with written informed consent obtained. Two of these 13 patients were claustrophobic and were, therefore, excluded from participation. The remaining 11 patients were all women with a mean age of 50.5 years (range, 32.7–65.1 years). All patients except 2 initially presented with cranial nerve palsy (see the Table for patient characteristics, initial symptoms, and date of occlusion). Two patients presented with acute headache due to SAH, confirmed on unenhanced CT. The mean follow-up period was 38.5 months (median, 29.6 months; range, 14.3–78.3 months).
Patient characteristics, clinical, and angiographic results
| No. | Sex | Age (yr) | Clinical Onset | Side | Location | ICA Occlusion | FU | COW (MRA) | Symptoms | Ischemic Lesions (T2WI FLAIR) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 65 | R CN III, V1, VI palsy | R | C4 | Balloons | 14 | Hypoplastic PcomAs | Improved | Both hemispheres, unchanged |
| 2 | F | 32 | R CN III palsy | R | C4 | Balloons | 49 | Complete | Cured | – |
| 3 | F | 48 | Headache nausea, SAH | R | C4 | Balloons | 74 | Complete | Cured | Both hemispheres, unchanged |
| 4 | F | 48 | R decreased visual acuity | R | C4 | Balloons | 23 | Absent contralateral PcomA | Cured | – |
| 5 | F | 64 | R ophthalmoplegia | R | C4 | Coils + balloon | 35 | Absent contralateral PcomA | Cured | – |
| 6 | F | 60 | R CN III palsy | R | C4 | Coils + balloon | 20 | Hypoplastic PcomAs | Improved | Both hemispheres, unchanged |
| 7 | F | 50 | L ophthalmoplegia | L | C4 | Balloons | 30 | Hypoplastic contralateral PcomA | Unchanged | – |
| 8 | F | 55 | Headache, nausea, SAH | R | C5 | Coils | 22 | Complete | Cured | Right hemisphere, increased |
| 9 | F | 59 | R CN VI palsy | R | C4 | Balloons | 58 | Absent contralateral PcomA | Improved | Both hemispheres, unchanged |
| 10 | F | 33 | R CN VI palsy | R | C4 | Balloons | 78 | Complete | Cured | – |
| 11 | F | 41 | R ophthalmoplegia | R | C4 | Coils | 22 | Complete | Improved | Right hemisphere, unchanged |
Note:—FU indicates follow-up in months; Unchanged, unchanged compared with MR imaging at the time of diagnosis/directly postprocedure; R, right; L, left; COW, circle of Willis.
MR Imaging
MR imaging was performed on a 3T Philips MR imaging system (Philips, Best, the Netherlands) by using a sensitivity encoding 8-channel head coil and body coil transmission. The imaging protocol consisted of the following: axial T2/FLAIR-weighted imaging, high-resolution MOTSA 3D-TOF MRA, ASL with a single TI for estimation of CBF, ASL with multiple TIs for calculation of temporal parameters, selective ASL to visualize the actual territorial contribution of the ICA and the BA, and time-resolved 3D PCMR for grading of collateral flow via the circle of Willis.
For the MOTSA 3D-TOF sequences, imaging parameters were as follows: 3D fast-field echo T1-weighted sequence; TR/TE, 25/4 ms; flip angle, 17°; FOV, 200 × 200 mm2; 512 × 324 matrix (reconstructed to 512 × 512); 1.0-mm-thick sections interpolated to 0.5 mm; and 184 sections acquired in 8 slabs; sensitivity encoding, 2.5.
For ASL with a single TI, pCASL was used with additional background suppression pulses and a 2100-ms postlabeling delay.19–21 Imaging parameters were the following: TR/TE, 4000/14 ms; flip angle, 90°; FOV, 240 × 240 mm2; matrix size, 80 × 79; 17 sections; thickness, 7 mm; no gap; gradient-echo single-shot echo-planar imaging; sensitivity encoding, 2.5; postlabeling delay, 2.1 seconds; number of dynamics, 40. Scan duration was 5 minutes and 20 seconds. For ASL with multiple TIs, we used pulsed ASL with TIs ranging from 100 to 2700 ms and a 300-ms interval.15,22 Imaging parameters were the following: TR/TE, 3000/28 ms; flip angle, 30°; FOV, 240 × 240 mm2; matrix size, 80 × 79; 5 sections; thickness, 7 mm; no gap; gradient-echo single-shot echo-planar imaging; sensitivity encoding, 1.2; number of dynamics, 40; labeling gap between the center of the imaging volume and the labeling slab, 25 mm. For selective ASL, we used vessel-encoded pCASL, in which selective labeling was achieved by spatial manipulation of the labeling efficiency in sets of 5 dynamics (75 dynamics were obtained).23 Further imaging parameters for selective ASL were the same as for nonselective pCASL.
Retrospectively gated spoiled gradient-echo PCMR24 measurement was performed in the circle of Willis at a resolution of 0.5 × 0.5 × 0.5 mm3; 25 axial sections; TE/TR, 3.4/6.8 ms; flip angle, 15°. Velocity-encoding was performed in 3 directions simultaneously by using a 4-point method (100 cm/s in each direction). To avoid motion artifacts, we kept the temporal resolution low (4 cardiac phases), and the total scan duration did not exceed 4 minutes.
Data Analysis
All MR imaging and MRA studies were interpreted by 2 experienced neuroradiologists in consensus (C.B.M. and R.v.d.B.) and were compared with the MR imaging and digital subtraction angiograms at the time of carotid artery occlusion to assess the anatomy of the circle of Willis and the incidence of de novo ischemic lesions.
Functional MR Imaging Software Library (http://www.fmrib.ox.ac.uk) and Matlab (MathWorks, Natick, Massachusetts; http://www.mathworks.com) were used for off-line processing of ASL data. Subtraction of labeled and control ASL images yielded whole-brain perfusion-weighted images. Selective ASL scans were processed according to the methods proposed by Wong and Kansagra.25 Voxels with a similar relative labeling efficiency were identified. Subsequent subtraction of the nonlabeled image and the selectively labeled images rendered 2 flow maps, visualizing the actual territorial contribution of the ICA and the BA.
By 3D affine registration, standard perfusion templates of the ACA, MCA, and PCA were registered to the perfusion-weighted images of individual patients. After segmentation of perfusion-weighted images into flow territories, cerebral perfusion was quantified for all flow territories. Quantification of CBF and arterial timing parameters was performed according to the methods previously described by our study group.12,14,19,22 For quantification of CBF, we used a 2-compartment model, taking the difference between the T1 of arterial blood and the T1 of brain tissue into account.26 CBF between hemispheres and flow territories of individual patients was compared by calculating the percentage of the difference between the 2 hemispheres or flow territories.
GT-Flow (Gyrotools, Zurich, Switzerland) was used to assess flow in semiautomatically defined regions of interest placed in and perpendicular to the different circle of Willis arteries (remaining ICA, BA, A1 segments of both ACAs; M1 segment of both MCAs; and P1 segment of both PCAs). Flow was defined in all regions of interest. On the basis of MRA, flow maps, and flow measurements in the circle of Willis arteries, different types of collateral flow via the circle of Willis were identified. For each collateral flow type, the distribution of arterial blood across the cerebral arteries was calculated as a percentage of total flow in the cerebral arteries.
Differences in CBF and timing parameters were tested for significance by using paired t tests (with P values < .05 regarded as significant; Statistical Package for the Social Sciences, Version 16.02, SPSS, Chicago, Illinois).
Results
Clinical, Angiographic, and Structural MR Imaging Results
Eleven patients were included in this study with a mean follow-up of 38.5 months (range, 14–78 months). Four patients had small ischemic lesions in both hemispheres, which were unchanged compared with the T2/FLAIR at the time of diagnosis. Two other patients, both treated with coil occlusion, had small ischemic lesions in the deep white matter of the ipsilateral hemisphere. These lesions had already been diagnosed directly postprocedure. In 1 of these patients, small ischemic lesions in the deep white matter of the right hemisphere had increased on late follow-up. However, this patient did not have any neurologic symptoms. The Table shows patient characteristics and clinical and angiographic results for all patients.
The 2 patients who were claustrophobic and were eventually excluded from participation did not have any neurologic symptoms after carotid balloon occlusion. Of the 6 patients who did not want to participate, 4 patients completely recovered after carotid balloon occlusion and did not have any neurologic symptoms. Two other patients who did not want to participate had neurologic symptoms. These symptoms were, however, not directly related to balloon occlusion of the carotid artery. In 1 patient, who initially presented with temporal epilepsy caused by a giant aneurysm of the right ICA, symptoms remained after permanent occlusion. MR imaging and angiography revealed an increased size of the aneurysm due to retrograde filling via the right ophthalmic artery and PcomA. Before further treatment of the aneurysm, extra-to-intracranial bypass surgery was performed, which was complicated by hemorrhagic infarction and eventually resulted in paresis of the left arm. The other patient had pre-existing right-sided hemiparesis and aphasia, caused by SAH from a basilar tip aneurysm, with secondary vasospasm and infarction of the left hemisphere. This patient was treated for a giant aneurysm of the right medial cerebral artery by balloon occlusion of the right ICA. On follow-up MR images, no ischemic injury was seen in the right hemisphere.
CBF
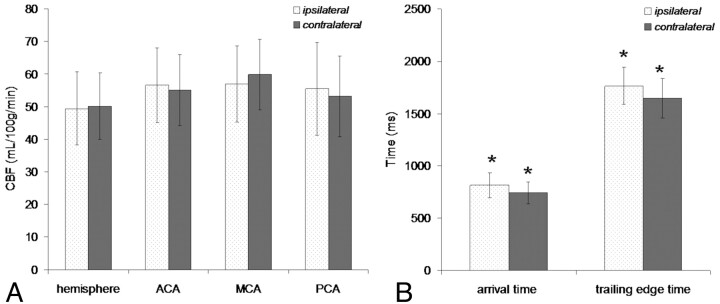
In 5 patients, CBF in the flow territory of the MCA on the occlusion side was 10% lower than that the contralateral MCA flow territory. No association was observed between lowered CBF in the MCA flow territories and the presence of ischemic lesions. In the group analysis, no significant CBF difference was found between the hemispheres (49.4 ± 11.2 versus 50.1 ± 10.1 mL/100 g/min) and between flow territories of the ACA (56.5 ± 11.5 and 55.0 ± 10.9 mL/100 g/min), MCA (56.8 ± 11.7 and 59.8 ± 10.8 mL/100 g/min), and PCA (55.4 ± 14.3 and 53.1 ± 12.4 mL/100 g/min) ipsilateral and contralateral to the occlusion (Fig 1A).
Fig 1.
CBF in hemispheres and flow territories ipsilateral and contralateral to the occlusion side (A) and mean arterial arrival time and trailing edge time ipsilateral and contralateral to the occlusion side (B). Error bars represent corresponding SDs. Significant differences are indicated by an asterisk.
Timing Parameters
The arterial arrival and trailing edge time were significantly prolonged in the hemisphere ipsilateral to carotid artery occlusion (816 ± 119 ms versus 741 ± 103 ms, P = .001; and 1765 ± 179 ms versus 1646 ± 190 ms, P < .001; Fig 1B). Arterial arrival and clearance of the labeled bolus were delayed in the hemisphere ipsilateral to the occlusion (Fig 2).
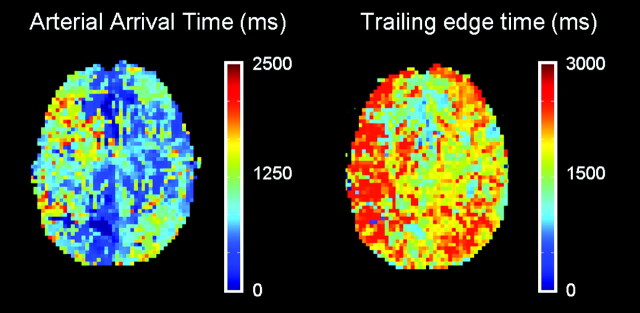
Fig 2.
Axial brain maps showing arterial arrival and trailing edge time in a patient with right ICA occlusion. Delayed arterial arrival and increased trailing edge time can be observed in the right hemisphere.
Collateral Flow
On the basis of MRA, time-resolved PCMR, and selective ASL, 2 different patterns of collateral supply could be distinguished in our population (Fig 3). In 2 patients, an anterior collateral flow pattern through the AcomA only could be observed (Fig 3A). In 9 patients, a mixed collateral flow pattern through the PcomA ipsilateral to the occlusion and AcomA was seen (Fig 3B). The mixed collateral flow pattern was further subdivided in a mixed pattern with (n = 5) and without (n = 4) a patent contralateral PcomA. None of the patients had a posterior collateral flow pattern (collateral flow through the ipsilateral PcomA only).
Fig 3.
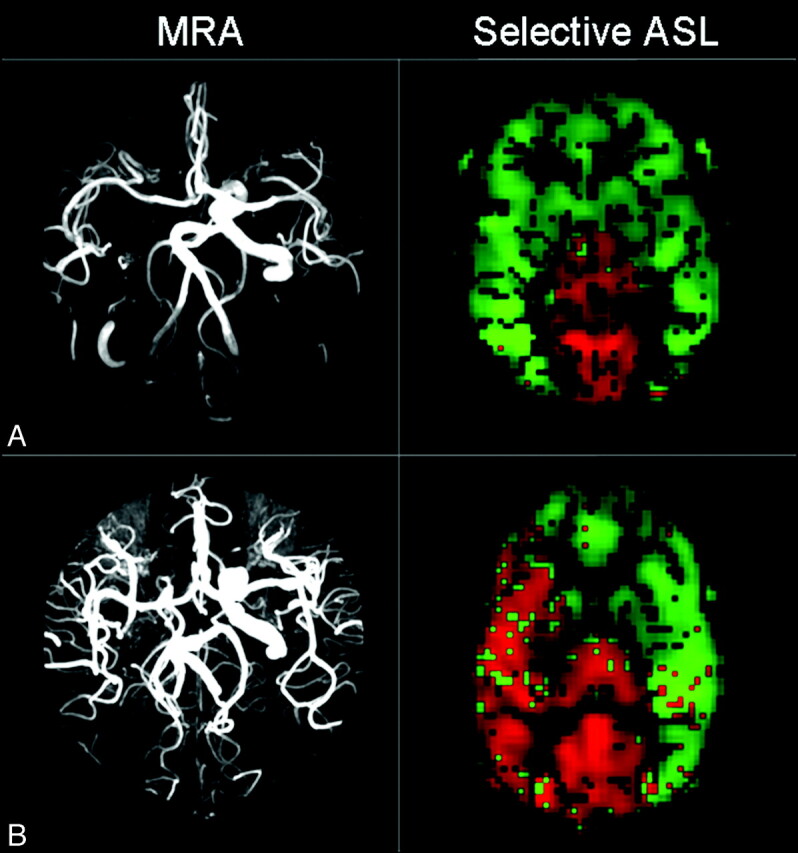
MR imaging data of 2 patients with right ICA occlusion, illustrating 2 different patterns of collateral flow. A, MRA data show hypoplasia of both PcomAs. Flow maps obtained by selective ASL illustrate the compensatory flow from the remaining ICA via the AcomA. The flow territory of the remaining ICA (selective tagging of the ICA) is green, and the flow territory of the BA (selective tagging of the BA) is red. B, MRA data show a complete COW, resulting in mixed collateral flow via the AcomA and PcomA as illustrated by velocity data and flow maps.
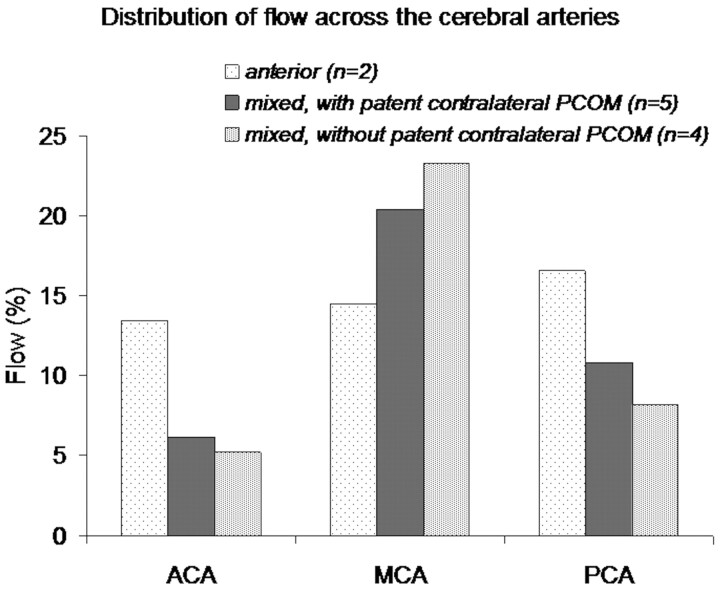
In patients with anterior collateral flow, flow through the cerebral arteries on the occlusion side was equally distributed across the ACA, MCA, and PCA (13%, 14%, and 16%, Fig 4). In patients with mixed collateral flow (AcomA, ipsilateral PcomA), flow through the cerebral arteries on the occlusion side was mainly via the MCA (20% with and 23% without a functioning PcomA contralateral to the occlusion) with considerably less flow via the ACA and PCA (Fig 4).
Fig 4.
Different collateral flow patterns through the circle of Willis and corresponding distribution of flow via the ACA, MCA, and PCA on the occlusion side. Flow has been displayed as a percentage of total flow in the cerebral arteries.
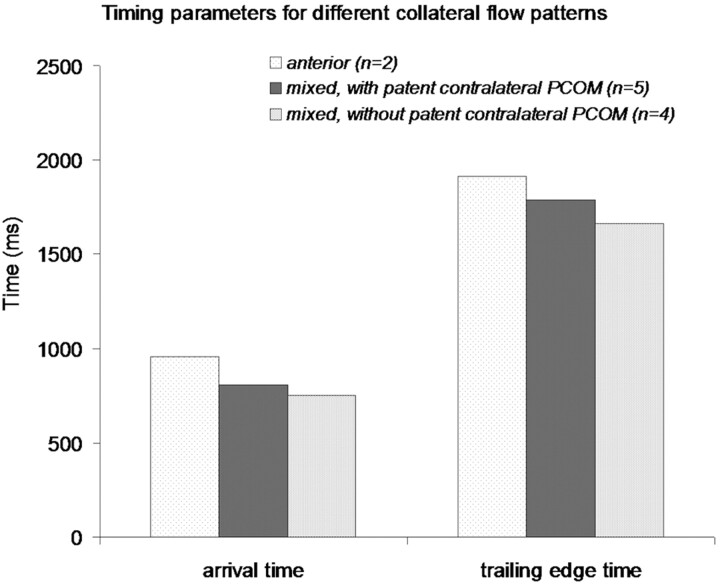
Patients with anterior collateral flow had prolonged timing parameters in the hemisphere on the occlusion side compared with patients with mixed collateral flow of both subtypes (Fig 5). In patients with mixed patterns, the presence of an ipsilateral PcomA and absence of a contralateral PcomA resulted in the shortest timing parameters.
Fig 5.
Timing parameters in the hemisphere on the occlusion side, depicted for each collateral flow type.
Discussion
In this study, we quantitatively evaluated cerebral perfusion long term after therapeutic carotid artery occlusion for large and giant carotid aneurysms in patients with synchronous venous filling during BTO. To our knowledge, this is the first long-term CBF follow-up study after therapeutic carotid artery occlusion. The results of this study show that CBF values are within the normal range without a significant difference in absolute CBF between the hemispheres ipsilateral and contralateral to ICA occlusion.27,28 Also, no significant CBF difference could be observed between the flow territories of the ACA, MCA, and PCA of both hemispheres in the group analysis. The finding that there was no significant CBF difference between the hemispheres ipsilateral and contralateral to carotid occlusion is in concordance with expectations based on test occlusion and clinical outcome of patients after permanent carotid occlusion.2,6,7
Arterial timing parameters were, however, significantly prolonged on the occlusion side. The fact that arterial arrival is delayed (75 ms on average) is not surprising because the labeled blood has to pass an elongated distance over the circle of Willis collateral system before reaching the brain tissue. A prolonged trailing edge time in the ipsilateral hemisphere could be due to a larger temporal spread of the diverted inflowing bolus and also to the presence of compensatory vasodilation resulting in increased cerebral blood volume and longer arterial transit times.
Flow measured in the circle of Willis arteries, combined with selective ASL flow maps of the remaining ICA and the BA, clearly illustrated the collateral flow pattern via the circle of Willis in all patients. Depending on the collateral pathway via the circle of Willis, timing parameters on the occlusion side were more or less affected. Timing parameters were increased in patients with anterior collateral flow compared with patients with mixed collateral flow, due to an elongated pathway via the AcomA. In patients with mixed collateral flow and an absent PcomA contralateral to occlusion, a relatively large amount of flow was via the MCA on the occlusion side. These patients had the shortest arterial timing parameters on the occlusion side. In our population, the absence of the contralateral PcomA thus seems to be in favor of hemodynamics that compensate for ICA occlusion. Future studies in larger patient populations should be undertaken to evaluate the influence of an absent or hypoplastic PcomA contralateral to the ICA occlusion.
Our findings are in clear contrast to the observation of the severely disturbed hemodynamics in patients with symptomatic ICA occlusion. Bokkers et al16,29 and Hendrikse et al30 used pulsed ASL with multiple acquisition times in patients with symptomatic ICA stenosis or occlusion. They found significant CBF reductions and increased timing parameters in the hemisphere ipsilateral to the stenosis or occlusion compared with the contralateral hemisphere and compared with data from healthy controls. Bokkers et al16 reported trailing edge times of 2225 ± 167 ms in the frontal-parietal region of the hemisphere ipsilateral to ICA occlusion compared with 1593 ± 35 ms in healthy controls. In our population, trailing edge times were 1765 ± 179 ms in the hemisphere ipsilateral to the therapeutic occlusion and 1646 ± 190 ms in the contralateral hemisphere.
Most probably, this difference is due to the inclusion of symptomatic patients in the study of Bokkers et al,16 whereas in our study, patients had angiographic BTO with synchronous venous filling preceding therapeutic ICA occlusion. Each study corresponds, therefore, to a different side of the spectrum of (atherosclerotic) disease severity. Most important, this study shows that the presence of an occluded ICA in itself does not result in an impaired hemodynamic status of the brain. The observed hypoperfusion in patients with symptomatic carotid occlusion might be due to poor collateral supply in these subjects or to the presence of atherosclerotic disease in the peripheral cerebral vasculature. Only patients having sufficient collateral supply, as indicated by synchronous opacification of cortical veins during test occlusion, were treated by permanent occlusion.2 In our population, the arterial arrival time in the hemispheres ipsilateral and contralateral to occlusion differed by 75 ms on average, while the difference in trailing edge time was 119 ms. Although arterial timing parameters cannot be directly compared with the venous measures used in test balloon occlusion, they clearly are within the 0.5-second timeframe that is used as a cutoff for synchronous opacification of cortical veins during test occlusion.
In addition to the previous work by Bokkers et al,16,29 Hendrikse et al,30 and Macintosh et al,17 this study demonstrates the feasibility of ASL with multiple TIs to measure perfusion and its temporal characteristics. Whereas previous studies included symptomatic patient populations, this study was the first to assess cerebral perfusion and its temporal characteristics in an asymptomatic patient population in whom increased delay time particularly is a reflection of the elongated route via collateral pathways.
Some limitations should be mentioned. First, we estimated CBF on the basis of an ASL sequence with a single TI because this sequence allows a larger imaging volume, identical to the imaging volume of selective ASL. Although the latter could introduce the risk of underestimation of CBF in the ipsilateral hemisphere due to delayed arrival of collateral flow, this was probably not the case in this study because data were acquired at a relatively long delay time of 2100 ms. Second, due to the low incidence of giant aneurysms (representing only 3%–5% of all intracranial aneurysms9,31), the population included in this study was limited. Also, only female patients were included in this study, attributable to the fact that there is a female predominance in the occurrence of intracranial aneurysms.31 Although the current study population is homogeneous and the number of patients is too small to draw definitive conclusions, the results of this study do strengthen the hypothesis that in patients tolerating BTO, cerebral perfusion endures long term after therapeutic occlusion of the ICA.
Conclusions
In this study, we assessed CBF and timing parameters of inflowing arterial blood long term after therapeutic artery occlusion for large and giant aneurysms in patients who tolerated BTO. Although the arterial arrival and trailing edge times were prolonged in the hemisphere ipsilateral to occlusion, eventually CBF was not significantly affected. Our results, therefore, suggest that synchronous venous filling during angiographic BTO is associated with sustained CBF on the long term.
ABBREVIATIONS
- ACA
anterior cerebral artery
- AcomA
anterior communicating artery
- ASL
arterial spinlabeling
- BA
basilar artery
- BTO
balloon test occlusion
- CN
cranial nerve
- MOTSA
multiple overlapping thin-slab acquisition
- PCA
posterior cerebral artery
- pCASL
pseudo-continuous ASL
- PCMR
phase-contrast MR imaging
- PcomA
posterior communicating artery
- TOF
time-of-flight
Footnotes
Disclosures: Matthias van Osch—UNRELATED: Payment for Lectures, Including Service on Speakers Bureaus: Philips Healthcare.
References
- 1. van Rooij WJ, Sluzewski M, Metz NH, et al. Carotid balloon occlusion for large and giant aneurysms: evaluation of a new test occlusion protocol. Neurosurgery 2000;47:116–21 [DOI] [PubMed] [Google Scholar]
- 2. van Rooij WJ, Sluzewski M, Slob MJ, et al. Predictive value of angiographic testing for tolerance to therapeutic occlusion of the carotid artery. AJNR Am J Neuroradiol 2005;26:175–78 [PMC free article] [PubMed] [Google Scholar]
- 3. Brunberg JA, Frey KA, Horton JA, et al. [15O]H2O positron emission tomography determination of cerebral blood flow during balloon test occlusion of the internal carotid artery. AJNR Am J Neuroradiol 1994;15:725–32 [PMC free article] [PubMed] [Google Scholar]
- 4. Peterman SB, Taylor A, Jr, Hoffman JC, Jr. Improved detection of cerebral hypoperfusion with internal carotid balloon test occlusion and 99mTc-HMPAO cerebral perfusion SPECT imaging. AJNR Am J Neuroradiol 1991;12:1035–41 [PMC free article] [PubMed] [Google Scholar]
- 5. Field M, Jungreis CA, Chengelis N, et al. Symptomatic cavernous sinus aneurysms: management and outcome after carotid occlusion and selective cerebral revascularization. AJNR Am J Neuroradiol 2003;24:1200–07 [PMC free article] [PubMed] [Google Scholar]
- 6. Higashida RT, Halbach VV, Dowd CF, et al. Intracranial aneurysms: interventional neurovascular treatment with detachable balloons—results in 215 cases. Radiology 1991;178:663–70 [DOI] [PubMed] [Google Scholar]
- 7. Larson JJ, Tew JM, Jr, Tomsick TA, et al. Treatment of aneurysms of the internal carotid artery by intravascular balloon occlusion: long-term follow-up of 58 patients. Neurosurgery 1995;36:26–30 [PubMed] [Google Scholar]
- 8. Linskey ME, Sekhar LN, Horton JA, et al. Aneurysms of the intracavernous carotid artery: a multidisciplinary approach to treatment. J Neurosurg 1991;75:525–34 [DOI] [PubMed] [Google Scholar]
- 9. Mawad ME, Cekirge S, Ciceri E, et al. Endovascular treatment of giant and large intracranial aneurysms by using a combination of stent placement and liquid polymer injection. J Neurosurg 2002;96:474–82 [DOI] [PubMed] [Google Scholar]
- 10. Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol 2007;6:611–19 [DOI] [PubMed] [Google Scholar]
- 11. Golay X, Hendrikse J, Lim TC. Perfusion imaging using arterial spin labeling. Top Magn Reson Imaging 2004;15:10–27 [DOI] [PubMed] [Google Scholar]
- 12. Chalela JA, Alsop DC, Gonzalez-Atavales JB, et al. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke 2000;31:680–87 [DOI] [PubMed] [Google Scholar]
- 13. Detre JA, Alsop DC, Vives LR, et al. Noninvasive MRI evaluation of cerebral blood flow in cerebrovascular disease. Neurology 1008;50:633–41 [DOI] [PubMed] [Google Scholar]
- 14. Buxton RB, Frank LR, Wong EC, et al. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med 1998;40:383–96 [DOI] [PubMed] [Google Scholar]
- 15. Petersen ET, Lim T, Golay X. Model-free arterial spin labeling quantification approach for perfusion MRI. Magn Reson Med 2006;55:219–32 [DOI] [PubMed] [Google Scholar]
- 16. Bokkers RP, van Laar PJ, van de Ven KC, et al. Arterial spin-labeling MR imaging measurements of timing parameters in patients with a carotid artery occlusion. AJNR Am J Neuroradiol 2008;29:1698–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Macintosh BJ, Lindsay AC, Kylintireas I, et al. Multiple inflow pulsed arterial spin-labeling reveals delays in the arterial arrival time in minor stroke and transient ischemic attack. AJNR Am J Neuroradiol 2010;31:1892–94 . Epub 2010 Jan 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schomer DF, Marks MP, Steinberg GK, et al. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N Engl J Med 1994;330:1565–70 [DOI] [PubMed] [Google Scholar]
- 19. Dai W, Garcia D, de Bazelaire C, et al. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 2008;60:1488–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu WC, Fernandez-Seara M, Detre JA, et al. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med 2007;58:1020–27 [DOI] [PubMed] [Google Scholar]
- 21. Ye FQ, Frank JA, Weinberger DR, et al. Noise reduction in 3D perfusion imaging by attenuating the static signal in arterial spin tagging (ASSIST). Magn Reson Med 2000;44:92–100 [DOI] [PubMed] [Google Scholar]
- 22. Gevers S, van Osch MJ, Bokkers RP, et al. Intra- and multicenter reproducibility of pulsed, continuous and pseudo-continuous arterial spin labeling methods for measuring cerebral perfusion. J Cereb Blood Flow Metab 2011;31:1706–15. Epub 2011 Feb 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wong EC. Vessel-encoded arterial spin-labeling using pseudocontinuous tagging. Magn Reson Med 2007;58:1086–91 [DOI] [PubMed] [Google Scholar]
- 24. Markl M, Chan FP, Alley MT, et al. Time-resolved three-dimensional phase-contrast MRI. J Magn Reson Imaging 2003;17:499–506 [DOI] [PubMed] [Google Scholar]
- 25. Wong EC, Kansagra AP. Mapping middle cerebral artery branch territories with vessel encoded pseudo-continuous ASL: sine/cosine tag modulation and data clustering in tagging efficiency space. In: Proceedings of the International Society for Magnetic Resonance in Medicine, Toronto, Ontario, Canada. May 3–9, 2008 [Google Scholar]
- 26. Alsop DC, Detre JA. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab 1996;16:1236–49 [DOI] [PubMed] [Google Scholar]
- 27. Chen Y, Wang DJ, Detre JA. Test-retest reliability of arterial spin labeling with common labeling strategies. J Magn Reson Imaging 2011;33:940–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gevers S, Majoie CB, van den Tweel XW, et al. Acquisition time and reproducibility of continuous arterial spin-labeling perfusion imaging at 3T. AJNR Am J Neuroradiol 2009;30:968–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bokkers RP, van der Worp HB, Mali WP, et al. Noninvasive MR imaging of cerebral perfusion in patients with a carotid artery stenosis. Neurology 2009;73:869–75 [DOI] [PubMed] [Google Scholar]
- 30. Hendrikse J, van Osch MJ, Rutgers DR, et al. Internal carotid artery occlusion assessed at pulsed arterial spin-labeling perfusion MR imaging at multiple delay times. Radiology 2004;233:899–904 [DOI] [PubMed] [Google Scholar]
- 31. Pia HW, Zierski J. Giant cerebral aneurysms. Neurosurg Rev 1982;5:117–48 [DOI] [PubMed] [Google Scholar]