SUMMARY:
Flow-territory mapping by MR imaging ASL noninvasively provides a unique insight into the distribution of cerebral perfusion. The introduction of planning-free vessel-encoded pCASL made flow-territory mapping feasible for clinical use, though lack of individual planning could impede reproducibility of this technique. We assessed the reproducibility of planning-free vessel-encoded pCASL in patients and controls. Results indicated that planning-free vessel-encoded pCASL is a reproducible method that could assist in clinical decision-making.
The ability to visualize perfusion territories of individual brain feeding arteries is important for many clinical applications; it may guide endovascular treatment and explain differences in clinical outcome.1 In everyday radiologic practice, standard templates are often used to determine the relationship between pathology and feeding vasculature. Because of the large intersubject variability in perfusion territories of brain-feeding arteries, these templates may be inadequate.2 Intra-arterial DSA is currently the reference standard for visualizing the cerebrovascular tree and collateral pathways via the circle of Willis or leptomeningeal anastomoses. This technique neither enables the identification of flow-territory borders at tissue level nor provides quantitative perfusion information.
The introduction of selective ASL has enabled noninvasive visualization of perfusion territories of individual brain feeding arteries. Selective ASL combined with MRA provides information comparable with DSA on the collateral supply in patients with steno-occlusive disease and has been proved valuable in the classification of cortical and borderzone infarcts.2,3
The first selective ASL methods were clinically difficult to implement because of the need for advanced hardware and time-consuming planning to limit labeling to a single artery.1,4 However, the introduction of vessel-encoded pCASL by Wong5 and Wong and Kansagra6 has enabled planning-free acquisition of all major flow territories within 5 minutes.
The clinical applicability of planning-free vessel-encoded pCASL, further referred to as planning-free selective ASL, is crucially dependent on its robustness and reproducibility, especially because the lack of individualized planning may render this technique more dependent on arterial layout than individually planned methods. Therefore, the aim of our study was to measure the robustness and reproducibility of flow mapping by planning-free selective ASL in healthy controls and in patients with steno-occlusive disease. Robustness and reproducibility were assessed within sessions (intrasession), within centers (intracenter), and between centers (intercenter).
Technique
Intrasession, intracenter, and intercenter robustness and reproducibility of planning-free selective ASL were assessed in healthy volunteers. Robustness and reproducibility in healthy volunteers might not be representative of the performance of the technique in patients, especially in case of large-vessel pathology potentially resulting in longer arterial transit times or lower CBF. Therefore, intrasession robustness and reproducibility were also assessed in patients with steno-occlusive disease.
Three imaging centers in the Netherlands participated in this study. MR imaging investigations were performed on 3T MR imaging scanners (Philips Healthcare, Best, the Netherlands) with the same implementation of planning-free selective ASL. Local ethics committees of all participating imaging centers approved the study protocol. After written informed consent, 6 volunteers (5 men; age range, 25–50 years) without known cerebrovascular disease were scanned twice at each location (1–3 weeks between sessions). Ten patients (7 men; 56–78 years of age) with a recent symptomatic ICA stenosis ≥50% were scanned once at 1 site. Two patients presented with ischemic stroke; 7, with a transient ischemic attack; and 3, with ipsilateral retinal ischemia. Several patients had >1 presenting event. ICA stenosis was 70%–99% in 9 patients and 50%–69% in 1 patient. Contralateral ICA stenosis was 0%–49%.
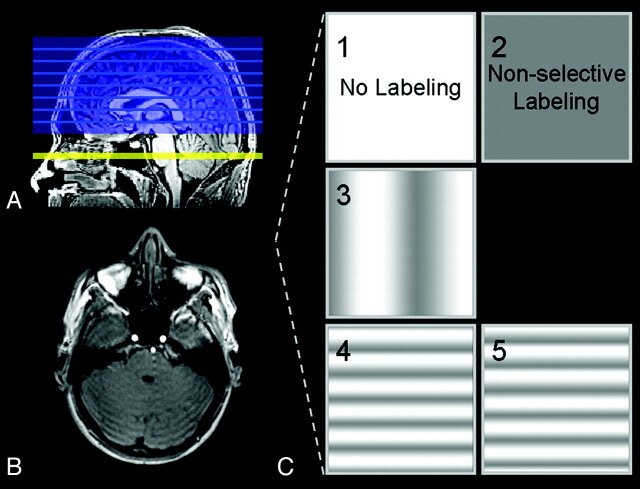
Each session protocol consisted of planning-free selective ASL and a high-resolution 3D T1-weighted anatomic scan for registration and segmentation purposes. All scans were acquired with a sensitivity encoding 8-channel head coil and body coil transmission. Imaging parameters for planning-free selective ASL were the following: TR/TE, 4000/14 ms; FOV, 240 × 240 mm2; matrix size, 80 × 79; 17 sections; thickness, 7 mm; no gap; single-shot echo-planar imaging; labeling duration, 1650 ms; postlabeling delay, 1525 ms; background suppression by a saturation pulse preceding the labeling and 2 inversion pulses, 1680 and 2830 Ω, after the saturation pulse; number of dynamics, 75. Only the imaging volume was planned, whereas the relative position of the labeling section, the phase of the radio-frequency pulses, and the strength and orientation of the flow-territory encoding gradients were fixed to population-based values. The labeling plane was set parallel to and 44 mm proximal to the imaging volume, at a level where the labeling plane is more or less perpendicular to the BA and ICAs in an average subject (Fig 1A,-B). Selective labeling was accomplished by spatial manipulation of the labeling efficiency within the labeling plane in sets of 5 dynamics: no labeling (control), nonselective labeling (globally perfusion weighted), labeling varied in right-left direction (50 mm between full label and control situation), labeling varied in the anteroposterior (AP1) direction (18 mm between full label and control situation); and labeling varied in the anteroposterior direction (AP2, similar to AP1, but shifted 9 mm in the posterior direction compared with the previous dynamic) (Fig 1C).6 For each condition, 15 dynamics were gathered in total. The time for the planning-free selective ASL scanning was 5 minutes.
Fig 1.
A and B, Sagittal and axial 3D T1-weighted images in which the imaging volume and labeling plane of planning-free selective ASL are illustrated. C, Labeling efficiency is spatially manipulated within the labeling plane.
The Functional MR Imaging Software Library (Oxford, United Kingdom) and Matlab (The MathWorks, Natick, Massachusetts) were used for off-line data processing. Because of an expected lower SNR—due to the smaller number of dynamics used and to the possible lower CBF in patients with steno-occlusive disease—spatial smoothing was applied to patient data (Gaussian filter kernel 4.5-mm full width at half maximum).
Flow territories of the right ICA, the left ICA, and the BA were determined by averaging dynamic scans with equal spatial encoding of labeling, resulting in 1 control and 1 globally labeled and 3 selectively labeled perfusion-weighted images. Subtraction of labeled and control images yielded 3 selective perfusion maps and 1 nonselective perfusion image. Following the procedures outlined by Wong and Kansagra,6 we established relative labeling efficiencies of the spatially manipulated scans by voxelwise calculation of the ratio of each selective perfusion image over the global perfusion map.
Robustness was defined as the extent to which the technique provides similar flow territories for repeated scans as determined by visual data inspection. Criteria for visual inspection to rate 2 sets of flow-territory maps as dissimilar were the following: the main flow territories identified as being fed by a different major artery or other gross variations that should be attributed to the suboptimal discriminating power of the flow-territory sequence. Intrasession robustness was determined for 6 controls and 10 patients, all scanned at the same imaging site (16 datasets). Intrasession robustness was based on the first 35 and the last 35 dynamics of each scan, yielding 2 sets of flow territories per scan (32 datasets). Intracenter and intercenter robustness was determined for 6 controls, scanned twice at all participating sites (36 datasets). Intra- and intercenter robustness was based on all 75 dynamics per scan.
The images that resulted in comparable flow territories for repeated measurements were included in the finer scale reproducibility analyses of intrasession, intracenter, and intercenter repetitions. Reproducibility was assessed by calculating the distance between observed flow-territory boundaries of repeated acquisitions. To this end, perfusion images and flow territories were transformed into anatomic space by affine registration on corresponding anatomic scans. Flow territories at 3 different levels in the brain were manually outlined on the basis of the outcome of the clustering algorithm (Fig 2C). Subsequently, the distance between flow-territory boundaries obtained from repeated scans was calculated by searching, for each edge-pixel of the first image and the closest edge-pixel of the second image (Fig 2D). Since the employed definition of distance depends on the order of the 2 images, both orders were averaged. The mean distance and the mean-maximum distance were calculated. The first is sensitive to a consistent mismatch over a large part of the flow territory boundaries, whereas the second reflects the largest encountered mismatch. Intrasession distances and intracenter distances were averaged over all subjects. Intercenter distances were calculated for all permutations between the 3 centers and then averaged over all controls.
Fig 2.
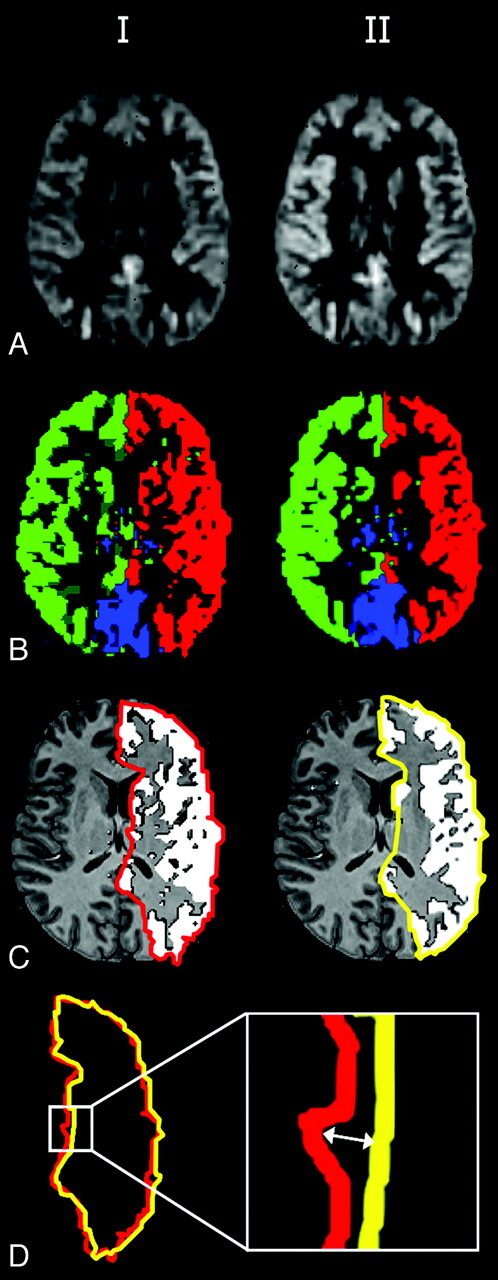
Axial nonselective perfusion-weighted images (A) and flow territories (B) of 2 sessions, manual outlining (C) and the distance between flow-territory boundaries (D).
The nonselective perfusion-weighted image and flow territories of the ICAs and the BA obtained by planning-free selective ASL in a single subject are shown in Fig 2A, B. Intrasession repeated flow mapping yielded comparable flow territories for all controls (100%) and for 8 of 10 patients (80%). Figure 3 shows the flow territories based on the first and second halves of a selective ASL scan in an 80-year-old woman with a right ICA stenosis of 70%–99%. Intrasession repeated flow mapping yielded similar flow territories with enlargement of the contralateral left ICA flow territory, though some differences could be observed at the level of the basal ganglia. In 2 patients, gross motion artifacts led to uninterpretable flow-territory maps.
Fig 3.
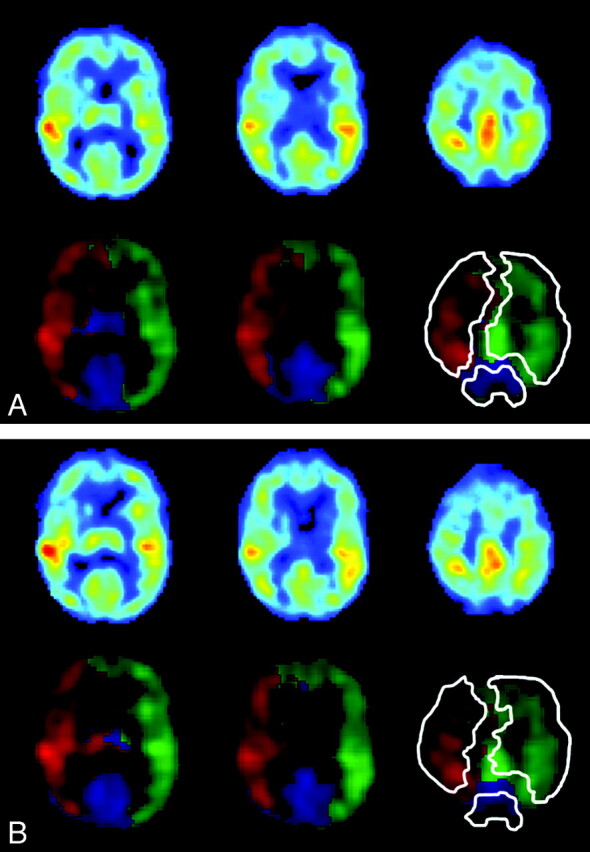
Three axial sections showing perfusion-weighted images, flow territories, and manual outlining of the ICAs and the BA in an 80-year-old female patient with right ICA stenosis of 70%–99%. A and B, Images are based on the first (A) and last (B) 35 dynamics.
All controls and 8 patients were included in the quantitative reproducibility analysis. Intra- and intercenter repeated planning-free selective ASL yielded comparable flow territories in 34 of 36 datasets (94%). In 1 individual, 2 of 6 scans showed an erroneously enlarged posterior perfusion territory, taking over approximately half of the perfusion territory of the left ICA (Fig 4). This volunteer was not included in quantitative reproducibility analysis. Thirty datasets were included in this analysis. The amount of overlap between the perfusion territories that were gathered from all 6 sessions for the remaining 5 control subjects in our study is shown in Fig 4. In the Table, the results of the quantitative reproducibility analysis are presented. Flow-territory boundaries deviated <4 mm on average and approximately 3 voxels maximum.
Fig 4.
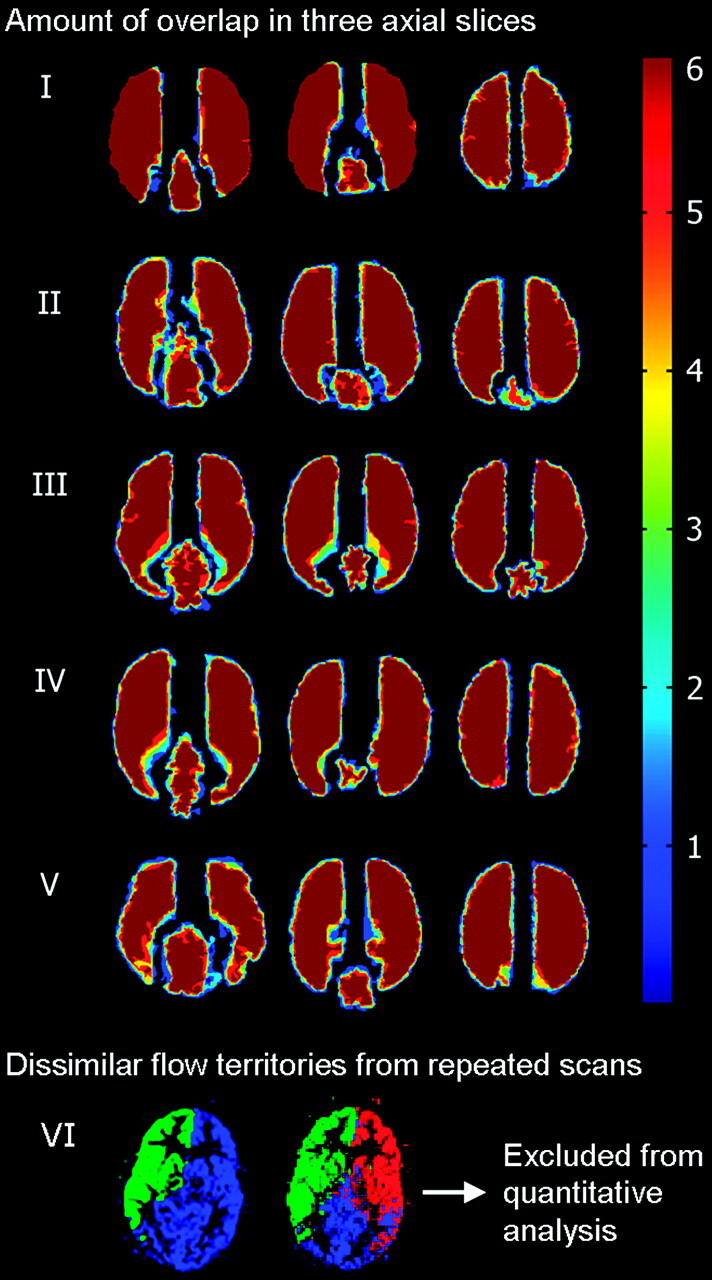
Three axial sections in 5 volunteers who were included in the quantitative analysis show the amount of overlap of flow territories from all sessions and sites (dark blue 1, light blue 2, green 3, yellow 4, red 5, and dark red 6 territories overlapping) and dissimilar flow territories obtained from repeat scanning in 1 volunteer who was excluded from further analysis.
Mean and mean-maximum distances (in millimeters) between edge pixels obtained from intrasession and intra- and intercenter repeated flow-territory mapping
| Mean/Mean-Max | Right ICA | Left ICA | BA |
|---|---|---|---|
| Intrasession | |||
| 8 Patients | |||
| Mean | 2.1 ± 1.0 | 2.4 ± 0.9 | 1.8 ± 0.6 |
| Mean-max | 7.1 ± 2.9 | 8.2 ± 2.2 | 6.9 ± 2.1 |
| 6 Controls | |||
| Mean | 1.6 ± 0.4 | 1.5 ± 0.5 | 1.3 ± 0.4 |
| Mean-max | 6.5 ± 1.8 | 5.9 ± 2.4 | 4.9 ± 0.9 |
| Intracenter sites I, II, III | |||
| 5 Controls | |||
| Mean | 2.2 ± 0.6 | 2.1 ± 0.5 | 2.5 ± 0.7 |
| Mean-max | 7.9 ± 2.3 | 8.4 ± 2.0 | 10.0 ± 4.2 |
| Intercenter | |||
| 5 Controls | |||
| Mean | 2.6 ± 0.3 | 2.5 ± 0.3 | 4.0 ± 2.9 |
| Mean-max | 9.0 ± 1.4 | 9.7 ± 1.5 | 10.6 ± 2.7 |
Discussion
The results of the present study indicate that the robustness and reproducibility of planning-free selective ASL are reasonable in both patients with steno-occlusive disease and controls. Reasonable robustness and reproducibility were even obtained by using only 35 dynamics for flow-territory mapping (scanning time, <3 minutes), as is shown by the results of intrasession analyses, implying that shorter scanning times suffice to obtain robust and reproducible flow territories.
Intrasession repeated flow-territory mapping yielded comparable flow territories in 80% of patients and in 100% of controls. The motion artifacts present in the flow-territory maps of 2 patients could be identified easily. This would result in a report of technical failure without the risk of incorrect interpretation. Intra- and intercenter repeated flow-territory mapping yielded comparable flow territories in 94% of controls. For 1 volunteer, 2 of 6 scans showed a normal right ICA flow territory and an enlarged posterior flow territory, probably composed of both left ICA and BA supply. MRA showed a close course of the BA and the left ICA at the spin-labeling level, causing similar labeling efficiency of blood water protons in both arteries, rendering identification of separate clusters impossible.
Also, in patients with brain disease, the outcome of the clustering algorithm could be obscured due to elongation and curvature of the vasculature at the labeling location, extensive collateral supply, or complete occlusion of brain feeding arteries. In clinical decision-making, flow territories obtained by planning-free selective ASL should be combined with an angiogram at the level of labeling to avoid misinterpretation of the flow territories. In case of complex vascular pathology, one might also choose selective labeling of single arteries.7 However, single-vessel selective ASL sequences are time-consuming when >1 flow territory needs to be imaged.
In the present study, the flow-territory boundaries of repeated selective ASL scans deviated <4 mm on average. Because the acquired in-plane scan resolution was 3 mm, this 4-mm distance is considered small. The quantitative reproducibility analysis was only performed on those datasets that showed comparable flow territories with repeated mapping. This was chosen to differentiate 2 kinds of errors: gross errors in flow territories due to insufficient variations in labeling efficiency of the main brain-feeding arteries and more subtle errors due to decreased SNR in the borderzones. The mean distance for intrasession repeated flow mapping was slightly larger in patients with steno-occlusive disease than in controls (approximately 2.2 versus 1.5 mm). This difference could be attributed to longer arterial transit times and lower CBF in patients (and thus lower SNR) or to the increased blurring kernel size used in patient data.
The current study has several limitations. Robustness and reproducibility of planning-free selective ASL were studied with a single field strength (3T) and scanner brand (Phillips) and a single observer. Lower field strengths would show lower SNR, which could lower the robustness and reproducibility. It is expected, however, that the robustness will be mainly affected by intersubject differences in vascular layout and would, therefore, be comparable at 1.5T. Reproducibility is mainly affected by the low SNR in the white matter. However, whether a lower field strength would degrade SNR in white matter and thus reproducibility remains to be studied.8 Whereas flow territories are 3D surfaces, we calculated the reproducibility in axial sections. Because representative levels were chosen, it is expected that the influence of this limitation is relatively small.
Conclusions
The present study is the first to assess the reproducibility of selective ASL. Planning-free selective ASL yields the same flow territories in repeated scans, though anatomic variations or pathology could result in aberrant flow territories. The results of this study indicate that planning-free selective ASL is a robust and reproducible method that can be used as a diagnostic tool in daily radiologic practice, though an MR angiogram of the label location should be consulted to avoid misinterpretation of flow territories.
ABBREVIATIONS:
- ASL
arterial spin-labeling
- BA
basilar artery
- CBF
cerebral blood flow
- DSA
digital subtraction angiography
- ICA
internal carotid artery
- Mean-max
mean-maximum
- MRA
MR angiography
- pCASL
pseudocontinuous arterial spin-labeling
- SNR
signal intensity–to-noise ratio
Footnotes
Previously presented in part as a poster at: Annual Meeting of the International Society for Magnetic Resonance in Medicine, April 18–24, 2009; Honolulu, Hawaii.
References
- 1. van Laar PJ, van der Grond J, Hendrikse J. Brain perfusion territory imaging: methods and clinical applications of selective arterial spin-labeling MR imaging. Radiology 2008; 246: 354–64 [DOI] [PubMed] [Google Scholar]
- 2. Hendrikse J, Petersen ET, Cheze A, et al. Relation between cerebral perfusion territories and location of cerebral infarcts. Stroke 2009; 40: 1617–22 [DOI] [PubMed] [Google Scholar]
- 3. Chng SM, Petersen ET, Zimine I, et al. Territorial arterial spin labeling in the assessment of collateral circulation: comparison with digital subtraction angiography. Stroke 2008; 39: 3248–54 [DOI] [PubMed] [Google Scholar]
- 4. Hendrikse J, van der Grond J, Lu H, et al. Flow territory mapping of the cerebral arteries with regional perfusion MRI. Stroke 2004; 35: 882–87 [DOI] [PubMed] [Google Scholar]
- 5. Wong EC. Vessel-encoded arterial spin-labeling using pseudocontinuous tagging. Magn Reson Med 2007; 58: 1086–91 [DOI] [PubMed] [Google Scholar]
- 6. Wong EC, Kansagra AP. Mapping middle cerebral artery branch territories with vessel-encoded pseudo-continuous ASL: sine/cosine tag modulation and data clustering in tagging efficiency space. In: Proceedings of the International Society for Magnetic Resonance in Medicine, Toronto, Ontario, Canada. May 3–9, 2008: 182 [Google Scholar]
- 7. Helle M, van Osch MJ, Norris DG, et al. Superselective pseudo-continuous arterial spin labeling. In: Proceedings of the International Society for Magnetic Resonance in Medicine, Toronto, Ontario, Canada. May 3–9, 2008: 183 [Google Scholar]
- 8. van Osch MJ, Teeuwisse WM, van Walderveen MA, et al. Can arterial spin labeling detect white matter perfusion signal? Magn Reson Med 2009; 62: 165–73 [DOI] [PubMed] [Google Scholar]