Abstract
BACKGROUND AND PURPOSE:
Five commercial devices are available for mechanical thrombectomy in acute ischemic stroke. This study evaluated and compared the resultant arterial damage from these devices.
MATERIALS AND METHODS:
Wall damage after 4 wall-contact devices (the Merci retriever, Catch thromboembolectomy system, and Solitaire FR revascularization devices of 4 and 6 mm) and 1 aspiration device (the Penumbra System) was evaluated in the superficial femoral arteries of 20 male swine. Each device was tested with and without intraluminal clot. Twenty control vessels were not subjected to any intervention. Acute histopathologic changes were evaluated.
RESULTS:
In the device samples, endothelial denudation (72.8 ± 29.4% versus 0.9 ± 1.9%, P < .0001), medial layer edema (52 ± 35.9% versus 18.1 ± 27.8%, P = .004), and mural thrombus (5.3 ± 14.2% versus 0%, P = .05) were found to a greater extent compared with the control samples. The aspiration device provoked more intimal layer (100 ± 79.1% versus 58.8 ± 48.9%, P = .27) and medial layer (75 ± 35.4% versus 46.3 ± 34.8%, P = .13) edema than the wall-contact devices.
CONCLUSIONS:
All devices caused vascular injuries extending into the medial layer. The aspiration device was associated with more intimal and medial layer edema, compared with the wall-contact devices except for the Catch thromboembolectomy system.
Recanalization is a powerful predictor of stroke outcome in patients with arterial occlusion treated with either IV rtPA or an endovascular approach.1,2 IV thrombolysis has been limited by its low recanalization rate in the setting of large-artery occlusions,3 whereas mechanical endovascular thrombectomy devices can result in higher rates of recanalization in patients with stroke with similar proximal arterial occlusions.4–6 The recanalization rate with MET is approximately 80%, and approximately 40% of patients have a favorable clinical outcome.7
A number of new thrombectomy devices have recently been introduced, with varying theoretic mechanisms of action. They can be classified into 2 major groups, according to their mechanism of retrieving the clot. Aspiration devices apply force to the proximal base of the thrombus6,8; this group includes various aspiration catheters and systems. Wall-contact devices can be divided into distal devices (brushlike, basketlike, or coil-like devices)9 and stentlike devices (including various self-expandable stent retrievers).10 With both types, the force is applied to the distal base of the clot. So far, to our knowledge, no systematic study has been performed comparing various MET devices under standardized conditions in vivo, in terms of arterial wall response (MEDLINE data base research by using the terms “endovascular,” “mechanical thrombectomy,” “clot removal,” “arterial wall,” and “animal model”).
The purpose of this study was to evaluate and compare arterial wall responses to 5 neurovascular mechanical thrombectomy devices: 4 wall-contact devices (the Merci retriever; Concentric Medical, Mountain View, California; the Catch thromboembolectomy system; Balt, Montmorency, France; and the Solitaire FR revascularization devices, 4 and 6 mm; Covidien, Irvine, California) and 1 aspiration device (Penumbra System; Penumbra, Alameda, California).
Materials and Methods
Animal Care
All procedures were conducted according to international guidelines and were approved by the responsible local authorities. Twenty male swine ranging from 20 to 25 kg (Haute-Vienne Research and Analysis Department, Haute-Vienne, France) were used in this study. The procedures were conducted with the swine under general anesthesia. No further heparin bolus or other thrombolytic drugs were administered. Further details are described in previous studies.9 Vital parameters, such as arterial blood pressure, heart rate, and carbon dioxide levels, were continuously recorded. Expired carbon dioxide levels were maintained at 30–35 mm Hg. One hour after the end of the last retrieval, the animals were euthanized.
Description of the Neurovascular Thrombectomy Devices
We tested 5 devices: 4 wall-contact devices and 1 aspiration device. Their properties are summarized in Table 1. The wall-contact devices were the following:
Two Solitaire FR revascularization devices: diameters of 4 and 6 mm (Solitaire FR 4 mm and Solitaire FR 6 mm). These are self-expanding, closed-cell nitinol stent devices that can be fully deployed and fully resheathed, and their design allows immediate blood-flow restoration and clot retrieval.
The Catch thromboembolectomy system consists of a self-expanding, basketlike device that has a maximum diameter of 4 mm and is fixed to a pusher wire. After the microcatheter, equipped with a microwire (SilverSpeed 14; ev3, Irvine, California), was placed in the common femoral artery, the microcatheter was navigated into the occluded vessel, past the thrombus. The basket was deployed distal to the thrombus. Then both the basket and the microcatheter were pulled back, simultaneously, into the guiding catheter.
The Merci retrieval system is a tapered wire with 5 helical loops of decreasing diameter at its distal end. In this study, the Merci retriever V 2.5 Firm was used, with a maximal loop diameter of 2.5 mm. The microcatheter was advanced beyond the thrombus. Both the microcatheter and the Merci retriever were pulled back to contact the thrombus, and the remaining loops were deployed within the thrombus. During retrieval, aspiration was applied through the guide catheter, by using a 50-mL syringe.
Table 1:
Neurovascular thrombectomy devices
| Description | Solitaire FR 4 mm | Solitaire FR 6 mm | Catch | Merci Retriever V 2.5 Firm | Penumbra 0.32 |
|---|---|---|---|---|---|
| Recommended guide catheter (F) | 6 | 6 | 6 | 9 | 6 |
| Microcatheter (F) | 2.4 | 2.8 | 2.4 | 2.4 | 3.4 |
| Element design | Stent retriever | Stent retriever | Basket-like | 5 Helical loops | Aspiration |
| Outer diameter (mm) | 4 | 6 | 4 | 2.5 | Not described |
| Length (mm) | 20 | 20 | 18 | 6 | 30 |
| Recommended vessel diameter (mm) | 2.0–4.0 | 3.0–5.5 | ≤4 | 2.0–3.5 | 2.0–3.0 |
Note:—F indicates French.
The aspiration device was the Penumbra System 0.32 (Penumbra). This is a platform that includes a reperfusion microcatheter connected to an aspiration pump via aspiration tubing, generating a suction force of 0.9 bar (700 mm Hg). When the microcatheter tip was placed in contact with the thrombus, the aspiration was applied during the clot fragmentation by using a separator.
Clot Preparation
Radiopaque clots were prepared in vitro, as recently described by Kan et al.11 In brief, 20 mL of whole blood was mixed with 2 g of barium sulfate powder to obtain radiopacity, and the mixture in the syringe was incubated at room temperature for 120 minutes. The solid component was separated from the serum component, and a piece of clot was resected. Each prepared thrombus, which measured 25 mm in length, was inserted into a Cole-Parmer silicone tube (Vernon Hills, Illinois) with saline and was prepared for injection.
Study Protocol
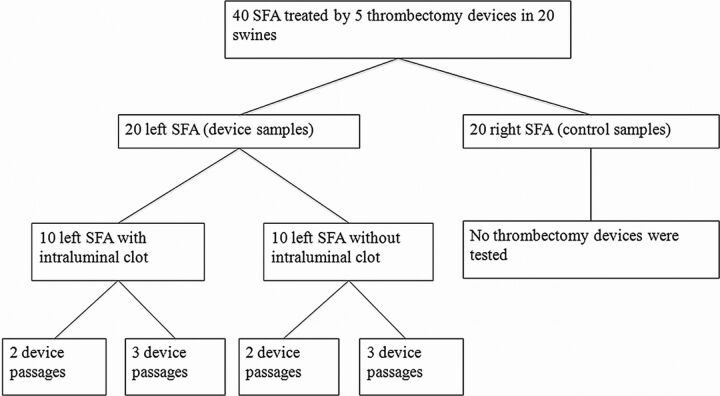
The protocol is detailed in Fig 1. Twenty swine had their superficial femoral arteries dissected bilaterally. The 20 right SFA samples were not subjected to any kind of intervention (control samples). The 20 left SFA samples were randomly submitted to thrombectomy with 1 of the 5 devices (device samples) (Fig 2A). Ten of the samples had an intraluminal clot, and 10 were normally patent. Each device was retrieved 2 times (2 passages) or 3 times (3 passages), resulting in 4 target arteries per device.
Fig 1.
Study protocol.
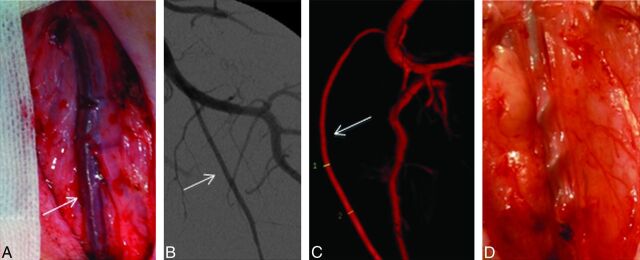
Fig 2.
Mechanical endovascular thrombectomy in the left SFA by using the Merci retrieval system. A, Photograph shows the superficial position of the left SFA (arrow), allowing easy resection. B, Anteroposterior angiogram of the left SFA (arrow). C, 3D reconstruction of the SFA (arrow). Note the size (inner diameter, 2.7–2.9 mm) of this artery, which reproduces the characteristics of proximal intracranial arteries, such as the MCA or basilar artery. D, Photograph shows the extension of the Merci retrieval system inserted into the vessel lumen.
Concerning the aspiration system, the clot retrieval was conducted according to 2 scenarios: 100 and 200 passages of the fragmentation separator under aspiration of 0.9 bar (similar to the clinical use).
Angiography and Clot Placement
A short 6F catheter sheath (Terumo, Tokyo, Japan) was inserted into the right common femoral artery and continuously flushed with physiologic saline. A 6F guiding catheter (Neuropath guiding catheter; DePuy, Raynham, Massachusetts) was introduced and continuously flushed with physiologic saline. Angiography of the left SFA was performed on a biplane system (Integris; Philips, Best, the Netherlands) (Fig 2B). Rotational angiography, followed by 3D reconstruction of the native projections (Fig 2C), was performed just before the thrombectomy procedure was begun. The diameters of the vessels were measured in 3D reconstruction images.
For selective thromboembolization, the preformed thrombus was injected into a 5F guide catheter (Chaperon; MicroVention, Aliso Viejo, California) positioned in the left SFA. After connecting the silicone tube containing a prepared clot into the guiding catheter, we injected 2 mL of saline into the tube to propel the clot into the catheter. The clot migrated to the SFA to achieve the occlusion. After an angiogram had been obtained to document occlusion, the guiding catheter was retrieved. The occlusion was maintained for 1 hour (± 30 minutes) before mechanical retrieval was attempted (Fig 2D).
All procedures were performed with the swine under general anesthesia through a 6F guiding catheter placed in the left SFA. The retrieval attempts were conducted according to the requirements of the manufacturers. During retrieval, only the microwire, microcatheter, and device entered the target vessel. For repeated attempts, the devices were cleaned, inspected, and, if still intact, reinserted.
Study End Point
The protocol was strictly designed to assess the degree of vascular damage. The recanalization rate, time to achieve recanalization, vasospasm, arterial perforation, and distal embolism were not evaluated.
Histopathologic Evaluation
Both of the SFAs were dissected away from the surrounding tissue, and each was cut transversely into 6 individual pieces, numbered proximally to distally, with regard to arterial blood flow. The most proximal and distal 2 cm of the SFAs were excluded to avoid ligature sites occurring during surgery. The vessel sections from each artery were processed and embedded in paraffin, according to standard laboratory operating procedures. They were subsequently sectioned at 5–7 μm and stained with hematoxylin-eosin and orcein (elastic fibers). The sections were examined with an optical microscope by using magnification ranging from 40 to 400 times by 2 independent board-certified pathologists who were blinded to the device used (A.G. and C.Y., who had 5 and 21 years of experience, respectively).
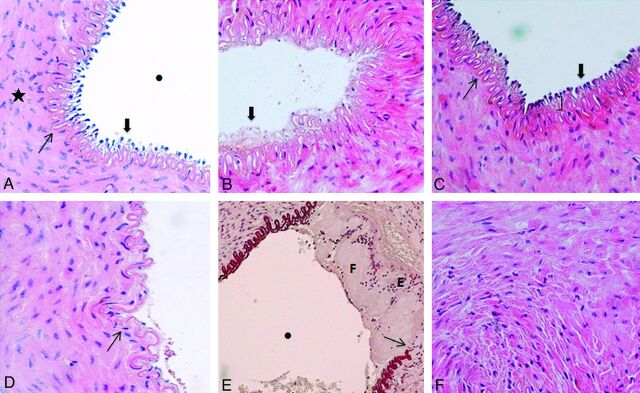
A grading system to evaluate the acute mural response to the MET devices was developed (Fig 3). We assessed the following: 1) amount of endothelial denudation (percentage of surface area), 2) presence of mural thrombus (vascular occlusion percentage), 3) intimal layer edema (inner intima circumference percentage), 4) medial layer edema (inner media circumference percentage), and 5) internal elastic lamina fracture (percentage).
Fig 3.
Histopathologic grading systems. A, Microscopic view of a right SFA (control sample) (hematoxylin-eosin [HE] staining, original magnification × 400) demonstrates well-maintained tissue integrity throughout the internal layers of the vessel. Endothelial cells (thick arrow) protruded toward the vascular lumen (dot), and the IEL (thin arrow) is intact. The mediaI layer (star) has homogeneous layers of smooth muscle cells. B, Microscopic view of a left SFA (device sample) (HE staining, original magnification × 400) shows total denudation of the endothelium (thick arrow) (100% of the surface area). C, Microscopic view of a left SFA (device sample) (HE staining, original magnification × 400) shows focal intimal thickening (parentheses) due to edema. Endothelial cells (thick arrow) and the IEL (thin arrow) are intact. D, Microscopic view of a left SFA (device sample) (HE staining, original magnification × 400) shows a focal fracture (thin arrow) of the IEL and denudation of the endothelium. E, Microscopic view of a left SFA (device sample) (orcein staining, original magnification × 200) demonstrates a fracture of the IEL (thin arrow) and mural thrombus with a layered pattern including both fibrin-rich (F) and erythrocyte-rich (E) layers. The mural thrombus does not compromise the lumen of the vessel (dot). F, Microscopic view of a left SFA (device sample) (HE staining, original magnification × 400) demonstrates edema within the media layer visualized as interstitial infiltration among smooth-muscle cells.
Statistical Analysis
The data from the histologic grading were independently analyzed by using SAS, Version 9.1.3 (SAS Institute, Cary, North Carolina). The statistical differences in arterial damage grades among the 5 devices were analyzed by the Mann-Whitney test. A P value < .05 was considered significant.
Results
Forty SFAs with diameters of 2.0–3.5 mm from 20 swine were studied. The model of arterial occlusion was effective in all target vessels. The five devices were applied to 20 vessels (4 vessels each)—2 with clots and 2 without—and they were successfully retrieved in every case.
Histopathologic Results
All of the MET devices caused lesions, which extended from the intimal layer to the medial layer. In the device samples versus the control samples, these were characterized by significant endothelial denudation and medial layer edema (Table 2). Intimal layer edema and fracture of the IEL were also found in all of the device samples but with insignificant differences for all device samples combined compared with the control samples. No other lesions were found deeper in the external elastic lamina or adventitia, nor were there perforations or dissections in any samples.
Table 2:
Comparison of arterial damage in the device and control samplesa
| Solitaire 4 Samples (n = 4) | Solitaire 6 Samples (n = 4) | Catch Samples (n = 4) | Merci Retriever Samples (n = 4) | Penumbra Samples (n = 4) | All Device Samples (n = 20) | Control Samples (n = 20) | P Valueb | |
|---|---|---|---|---|---|---|---|---|
| Mural thrombus (%) | 14.1 ± 28.1 | 0 | 9.4 ± 15.6 | 3.1 ± 6.2 | 0 | 5.3 ± 14.2 | 0 | .05 |
| Endothelial denudation (%) | 85.8 ± 22.9 | 76.2 ± 12.8 | 76.1 ± 21.2 | 85.9 ± 12.1 | 40.1 ± 47.6 | 72.8 ± 29.4 | 0.9 ± 1.9 | .0001 |
| Intimal layer edema (%) | 43.8 ± 51.5 | 81.3 ± 65.7 | 43.8 ± 37.5 | 66.5 ± 47.1 | 100 ± 89.1 | 67 ± 56.2 | 44.6 ± 43.6 | .25 |
| IEL fractured (%) | 12.5 ± 14.4 | 25 ± 35.4 | 18.8 ± 23.9 | 50 ± 100 | 12.5 ± 14.4 | 23.7 ± 46.2 | 20.6 ± 33.3 | .78 |
| Medial layer edema (%) | 25 ± 0 | 37.5 ± 43.3 | 81.3 ± 23.9 | 41.5 ± 35.2 | 75 ± 35.4 | 52 ± 35.9 | 18.1 ± 27.8 | .004 |
| EEL fractured (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Not applicable |
| Adventitia edema (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Not applicable |
Data are means +/− standard deviations.
Mann-Whitney test comparing all device samples combined with control samples.
There were no statistically significant differences in the arterial damage among the 5 device groups. However, the aspiration device (Fig 4) created less endothelial denudation and mural thrombus than the wall-contact device samples (Fig 5), but more intimal and medial layer edema, though none of these differences were significant (Table 3).
Fig 4.
Histopathologic findings after mechanical thrombectomy by using the Penumbra system. A, Microscopic view of a right SFA (control sample) shows all layers preserved (hematoxylin-eosin [HE] staining, original magnification × 400). B and C, Microscopic view of a left SFA demonstrates the intact internal elastic lamina (thin arrow) and a single layer of endothelial cells (thick arrow). The parentheses show edema of the subendothelial layer in B and the mediaI layer in C. There is no thrombus (HE staining, original magnification × 400). D, Anteroposterior angiogram of the left SFA immediately after thrombectomy shows moderate diffuse vasospasm (thin arrows).
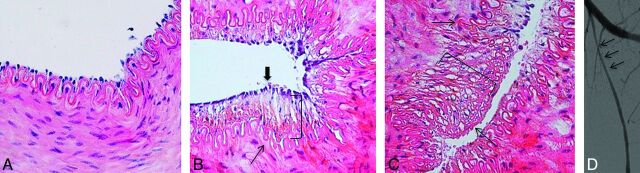
Fig 5.
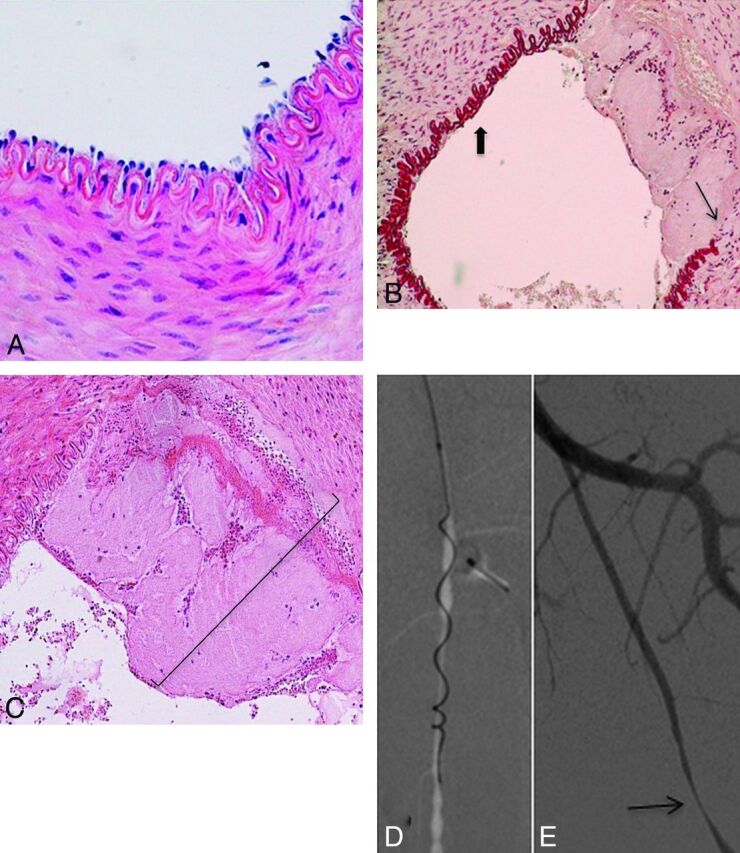
Histopathologic findings after mechanical thrombectomy by using the Catch thromboembolectomy system. A, Microscopic view of a right SFA (control sample) shows all layers preserved (hematoxylin-eosin [HE] staining, original magnification × 400). B, Microscopic view of a left SFA demonstrates the denudation of endothelial cells (thick arrow) and the broken IEL (thin arrow) (orcein staining, original magnification × 200). C, Microscopic view of a left SFA shows a mural clot (parentheses) and the IEL fractured. (HE staining, original magnification × 200). D, Anteroposterior angiogram of the left SFA shows the Merci clot device during the retrieval. E, Anteroposterior angiogram of the left SFA immediately after thrombectomy demonstrates focal stenosis (thin arrow).
Table 3:
Comparison of arterial damage by the wall-contact devices and the aspiration devicea
| Wall-Contact Devices (n = 16) | Aspiration System (n = 4) | P Valueb | |
|---|---|---|---|
| Mural thrombus (%) | 6.6 ± 15.7 | 0 ± 0 | .28 |
| Endothelial denudation (%) | 81.0 ± 16.8 | 40.1 ± 47.5 | .15 |
| Intimal layer edema (%) | 58.8 ± 48.9 | 100 ± 79.1 | .27 |
| IEL fractured (%) | 26.6 ± 51.2 | 12.5 ± 14.4 | .92 |
| Medial layer edema (%) | 46.3 ± 34.8 | 75 ± 35.4 | .13 |
Data are means +/− standard deviations.
Mann-Whitney test comparing wall-contact device samples combined with aspiration system (Penumbra) samples.
Mural Thrombus
Overall, the MET devices caused more mural thrombus in the arterial lumen of the device samples compared with control samples (Table 2). Three MET devices (the Solitaire FR 4 mm, Merci retrieval system, and Catch thromboembolectomy system) caused mural thrombus after their use, but the Penumbra system and Solitaire FR 6 mm did not.
Intraluminal Clot
There was a predominance of intimal layer edema in arteries without intraluminal clots, compared with the clotted ones, but there were no other significant histopathologic differences (Table 4).
Table 4:
Comparison of arterial damage by the device samples according to the presence or absence of a clot and the number of retrievalsa
| With Clot (10 Samples) | Without Clot (10 Samples) | P Valueb | Two Retrievals (10 Samples) | Three Retrievals (10 Samples) | P Valuec | |
|---|---|---|---|---|---|---|
| Mural thrombus (%) | 0.5 ± 1.6 | 10.1 ± 19.3 | .21 | 3.2 ± 10.3 | 7.3 ± 17.6 | .31 |
| Endothelial denudation (%) | 81.8 ± 18.9 | 63.8 ± 35.8 | .25 | 68.9 ± 28.8 | 76.6 ± 30.0 | .41 |
| Intimal layer edema (%) | 35 ± 33.4 | 99.1 ± 57 | .009 | 84.1 ± 62.8 | 50 ± 45.6 | .24 |
| IEL fractured (%) | 10 ± 17.5 | 37.5 ± 61.5 | .18 | 40 ± 61.5 | 7.5 ± 12.1 | .27 |
| Medial layer edema (%) | 45 ± 43.8 | 59.1 ± 26.5 | .44 | 56.6 ± 37 | 47.5 ± 36.2 | .58 |
Data are means +/− standard deviations.
Mann-Whitney test for samples with versus without clots.
Mann-Whitney test for 2 versus 3 retrievals.
Number of Attempts
For each device, 2 and 3 retrieval attempts were performed in samples both with and without a clot (resulting in 4 procedures per device). The mean arterial damage for each retrieval attempt with and without clot is summarized in Table 4. The number of attempts with each device in both groups of arteries (with and without clot) did not influence the histopathologic results; there was no statistical difference shown, but variability was large and sample sizes were small.
Discussion
The approved medical therapy in acute ischemic stroke is IV rtPA within 4.5 hours.12 Recently, mechanical thrombectomy has been demonstrated to successfully restore large-vessel patency and therefore provide an alternative and synergistic method for flow restoration.4–6,10,13,14 Thrombectomy devices offer many potential advantages over pharmacologic thrombolysis, including more rapid achievement of recanalization, enhanced efficacy in treating large-vessel occlusions, and a potentially lower risk for hemorrhagic events.15 However, mechanical manipulation of a thrombus in intracranial arteries might cause complications such as vasospasm, dissection, or perforation and may worsen clinical outcome at discharge.16,17
Nonetheless, arterial wall changes after endovascular treatment with mechanical devices in acute stroke are poorly reported and compared. There are no standards to evaluate such devices. The first in vivo model dedicated to MET in ischemic stroke was reported by Gralla et al.18 It permits an angiographic evaluation and comparison of neurovascular mechanical thrombectomy devices.9,19–21 However, no study has evaluated the histopathologic responses induced by devices, to our knowledge. Recently, a model has been specifically developed in swine, by using the superficial cervical artery to evaluate arterial structural changes.22 Compared with this model, surgical access to swine SFA is not technically difficult, due to its superficial position. The normal histologic characteristics of the porcine femoral artery are well known23 and raise the possibility of evaluating pathologic lesions. In a postmortem study of 5 patients who died acutely after MET procedures, vascular pathologic abnormalities affecting the proximal arteries were variable, but 1 patient (20%) showed a subintimal dissection with resultant occlusion of the middle cerebral artery.24 In the current study, 5 mechanical devices (4 wall-contact devices and 1 aspiration device) were evaluated and the grading scale analyzing the acute extent of the injury caused by the device (from the inner layer to the adventitia layer) and the presence of swelling or an intraluminal thrombus were reported.
It was observed that the devices caused significant endothelial and medial damage to the vessel walls. Intimal and subintimal damage seemed higher, but no intimal dissection was found histologically. Little is known about the response of the arterial wall after mechanical thrombectomy, partly due to limited angiographic/histologic data from the treated arteries. Recently, an angiographic follow-up study in 261 patients with 265 embolic vessel occlusions revealed 26.0% vasospasm and 0.4% dissection in the acute phase.25 On follow-up, 0.9% target-vessel occlusion and 3.4% of de novo stenosis were reported. These data may suggest that arterial wall injuries are probably reversible in the long term.
The aspiration device was responsible for more intimal and medial layer edema. However, these differences were not statistically significant, though this result could be due to the small animal sample. These results are probably related to the fact that the designs and mechanisms of these 2 types of devices are completely different. The aspiration device applies aspiration force to the proximal base of the thrombus.6,8,9 As opposed to this suction device, wall-contact devices capture the clot by exerting continuous radial force against the vessel wall, which may injure the endothelium in the process.9,10 These devices can lead to diverse effects on the vascular wall, as described in this article. These findings are in accordance with the animal study by Gralla et al,9 in which histologic findings were not reported. The authors compared the in vivo effectiveness and thrombus/device interaction of a proximal approach (such as the Penumbra system) and a distal approach (such as the Catch thromboembolectomy system). The authors found that the former appeared to reduce irritation in the vessel wall, thus lowering the risk and severity of vasospasm, because it does not require repositioning and passing procedures.9
An important finding was the presence of less arterial wall damage in vessels with clots. The clot samples had significantly less intimal layer edema than those without clots (35 ± 33.4% versus 99.11 ± 57%, P = .009). Regarding these results, we deduced that the length of the device should be close to the length of the clot, to reduce the lesions on the wall. Also, wall damage was not significantly increased when the devices were passed 3 times rather than twice.
Another point was the presence of a mural thrombus in the vessel walls submitted to 3 of the wall-contact devices, specifically the Merci retrieval system, the Catch thromboembolectomy system, and the Solitaire FR 4 mm. However, the lesion was limited to the intimal layer attached to the endothelium, and it was not associated with significant reduction of the arterial lumen. The Solitaire FR 6 mm device caused less endothelial damage and no mural thrombus compared with the Solitaire FR 4 mm device. The Solitaire FR 6 mm exerts a greater radial force at all diameters compared with the Solitaire FR 4 mm. The increased intimal tearing could be attributed to the greater length of the Solitaire FR 4 mm (31.8 versus 26.2 mm),21 having more surface area in contact with the vessel wall. Thus radial force could be considered a less important contributor to arterial wall injury.
Because this study was restricted to the acute phase, performing further studies at later stages—subacute and chronic—would be of great value to identify the progression of these lesions. Previous research has shown that the extent and depth of the vascular lesion may be contributing factors in promoting early atherosclerotic and accelerated hyperplastic intimal and medial changes.26 Large areas of endothelial denudation without substantial medial trauma caused only mild intimal thickening, whereas focal endothelial denudation with substantial medial trauma produces marked delayed intimal thickening.27 These results are substantiated by those of other studies that have shown that smooth muscle cells are normally quiescent with respect to proliferation but that deep injuries result in the expression of a phenotype of vascular smooth-muscle cells that exhibits a high proliferative response to other mitogens.28
Given these results, the aspiration device resulted in a less traumatic acute injury in the vascular wall. However, this study did not analyze the vascular recanalization rate; therefore, deducing that the aspiration device would have better functional results is not appropriate. These findings warrant further study of these devices in a model with a longer follow-up.
We acknowledge several limitations of the current study and proposed animal model, as with any in vivo experimental model. First, the swine SFAs are less tortuous without atherosclerosis compared with intracranial arteries in humans. Second, the present study compared arterial wall responses in 5 mechanical thrombectomy devices uncorrelated with their angiographic efficacy. We can imagine that 1 passage may suffice with stent retrievers, while many more passages may be necessary with aspiration devices. It is probably total injuries for effective recanalization that count. Another limitation is that endothelial denudation is difficult to quantify with routine pathologic stains. Further methodologic limitations of the study are the small number of experiments per device and the lack of follow-up angiographic and pathologic data to assess whether damage leads to stenosis. In addition, because of the differences in the arterial wall (lack of adventitia in intracranial arteries), the histologic results of this model may not be applicable to cerebral arteries in clinical practice. Our new model does not represent a counterpart to the entire phenomenon seen in patients with stroke. However, we believe that it may expand the analysis of the safety profile and offer the possibility of an experimental model for the preclinical development and evaluation of mechanical devices.
Conclusions
We have demonstrated in this swine model that both the aspiration device and the wall-contact devices caused vascular injuries extending into the medial layer. However, it would appear that the aspiration device was associated with more intimal and medial-layer edema, compared with the wall-contact devices except for the Catch thromboembolectomy system.
Acknowledgments
We thank Heath Bowman, MSc, for assistance in the concept and design of the study and the provision of thrombectomy devices, and Karine Durand, PhD, for her statistical assistance.
ABBREVIATIONS:
- EEL
external elastic lamina
- IEL
internal elastic lamina
- MET
mechanical endovascular thrombectomy
- SFA
superficial femoral artery
Footnotes
Disclosures: Sebastien Ponsonnard—UNRELATED: Payment for Manuscript Preparation: web-anesthesie.fr (Wolters-Kluwer), Comments: about a lung sonography article. Charbel Mounayer—UNRELATED: Consultancy: MicroVention,* Expert Testimony: MicroVention. *Money paid to the institution.
This work was supported by MicroVention Inc.
REFERENCES
- 1. Mazighi M, Serfaty JM, Labreuche J, et al. Comparison of intravenous alteplase with a combined intravenous-endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): a prospective cohort study. Lancet Neurol 2009;8:802–09 [DOI] [PubMed] [Google Scholar]
- 2. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 2007;38:967–73 [DOI] [PubMed] [Google Scholar]
- 3. del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol 1992;32:78–86 [DOI] [PubMed] [Google Scholar]
- 4. Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005;36:1432–38 [DOI] [PubMed] [Google Scholar]
- 5. Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke 2008;39:1205–12 [DOI] [PubMed] [Google Scholar]
- 6. Penumbra Pivotal Stroke Trial Investigators. The Penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke 2009;40:2761–68 [DOI] [PubMed] [Google Scholar]
- 7. Rouchaud A, Mazighi M, Labreuche J, et al. Outcomes of mechanical endovascular therapy for acute ischemic stroke: a clinical registry study and systematic review. Stroke 2011;42:1289–94 [DOI] [PubMed] [Google Scholar]
- 8. Bose A, Henkes H, Alfke K, et al. The Penumbra system: a mechanical device for the treatment of acute stroke due to thromboembolism. AJNR Am J Neuroradiol 2008;29:1409–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gralla J, Schroth G, Remonda L, et al. Mechanical thrombectomy for acute ischemic stroke: thrombus-device interaction, efficiency, and complications in vivo. Stroke 2006;37:3019–24 [DOI] [PubMed] [Google Scholar]
- 10. Roth C, Papanagiotou P, Behnke S, et al. Stent-assisted mechanical recanalization for treatment of acute intracerebral artery occlusions. Stroke 2010;41:2559–67 [DOI] [PubMed] [Google Scholar]
- 11. Kan I, Yuki I, Murayama Y, et al. A novel method of thrombus preparation for use in a swine model for evaluation of thrombectomy devices. AJNR Am J Neuroradiol 2010;31:1741–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bluhmki E, Chamorro A, Dávalos A, et al. Stroke treatment with alteplase given 3.0–4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol 2009;8:1095–102 [DOI] [PubMed] [Google Scholar]
- 13. Castaño C, Dorado L, Guerrero C, et al. Mechanical thrombectomy with the Solitaire AB device in large artery occlusions of the anterior circulation: a pilot study. Stroke 2010;41:1836–40 [DOI] [PubMed] [Google Scholar]
- 14. Costalat V, Machi P, Lobotesis K, et al. Rescue, combined, and stand-alone thrombectomy in the management of large vessel occlusion stroke using the Solitaire device: a prospective 50-patient single-center study: timing, safety, and efficacy. Stroke 2011;42:1929–35 [DOI] [PubMed] [Google Scholar]
- 15. Leary MC, Saver JL, Gobin YP, et al. Beyond tissue plasminogen activator: mechanical intervention in acute stroke. Ann Emerg Med 2003;41:838–46 [DOI] [PubMed] [Google Scholar]
- 16. Gupta R. Arterial vasospasm during mechanical thrombectomy for acute stroke. J Neuroimaging 2009;19:61–64 [DOI] [PubMed] [Google Scholar]
- 17. Shi ZS, Liebeskind DS, Loh Y, et al. Predictors of subarachnoid hemorrhage in acute ischemic stroke with endovascular therapy. Stroke 2010;41:2775–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gralla J, Schroth G, Remonda L, et al. A dedicated animal model for mechanical thrombectomy in acute stroke. AJNR Am J Neuroradiol 2006;27:1357–61 [PMC free article] [PubMed] [Google Scholar]
- 19. Brekenfeld C, Schroth G, El-Koussy M, et al. Mechanical thromboembolectomy for acute ischemic stroke: comparison of the Catch thrombectomy device and the Merci retriever in vivo. Stroke 2008;39:1213–19 [DOI] [PubMed] [Google Scholar]
- 20. Mordasini P, Hiller M, Brekenfeld C, et al. In vivo evaluation of the Phenox CRC mechanical thrombectomy device in a swine model of acute vessel occlusion. AJNR Am J Neuroradiol 2010;31:972–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mordasini P, Frabetti N, Gralla J, et al. In vivo evaluation of the first dedicated combined flow-restoration and mechanical thrombectomy device in a swine model of acute vessel occlusion. AJNR Am J Neuroradiol 2011;32:294–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yuki A, Kan I, Golshan A, et al. A swine model to analyze arterial structural changes induced by mechanical thrombectomy. AJNR Am J Neuroradiol 2013;34:E87–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Solanes N, Rigol M, Ramirez J, et al. Histological basis of the porcine femoral artery for vascular research. Anat Histol Embryol 2005;34:105–11 [DOI] [PubMed] [Google Scholar]
- 24. Yin NS, Benavides S, Starkman S, et al. Autopsy findings after intracranial thrombectomy for acute ischemic stroke: a clinicopathologic study of 5 patients. Stroke 2010;41:938–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chidi CC, DePalma RG. Atherogenic potential of the embolectomy catheter. Surgery 1978;83:549–57 [PubMed] [Google Scholar]
- 26. Kurre W, Pérez MA, Horvath D, et al. Does mechanical thrombectomy in acute embolic stroke have long-term side effects on intracranial vessels? An angiographic follow-up study. Cardiovasc Intervent Radiol 2012. October 20. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27. Walker LN, Ramsay MM, Bowyer DE. Endothelial healing following defined injury to rabbit aorta: depth of injury and mode of repair. Atherosclerosis 1983;47:123–30 [DOI] [PubMed] [Google Scholar]
- 28. Grünwald J, Haudenschild CC. Intimal injury in vivo activates vascular smooth muscle cell migration and explant outgrowth in vitro. Arteriosclerosis 1984;4:183–88 [DOI] [PubMed] [Google Scholar]