Abstract
BACKGROUND AND PURPOSE:
Current classifications of cerebral cavernous malformations focus solely on morphologic aspects. Our aim was to provide a morphologic classification that reflects hemorrhage rates.
MATERIALS AND METHODS:
We retrospectively categorized 355 cavernous malformations of 70 children and adolescents according to their morphologic appearance on MR imaging and calculated prospective hemorrhage rates on the basis of survival functions for 255 lesions in 25 patients with a radiologic observation period of >180 days.
RESULTS:
Overall, there were 199 MR imaging examinations with 1558 distinct cavernous malformation observations during a cumulative observation period of 1094.2 lesion-years. The mean hemorrhage rate of all 355 cavernous malformations was 4.5% per lesion-year. According to Kaplan-Meier survival models, Zabramski type I and II cavernous malformations had a significantly higher hemorrhage rate than type III and IV lesions. The presence of acute or subacute blood-degradation products was the strongest indicator for an increased hemorrhage risk (P = .036, Cox regression): The mean annual hemorrhage rate and mean hemorrhage-free interval for cavernous malformations with and without signs of acute or subacute blood degradation products were 23.4% and 22.6 months and 3.4% and 27.9 months, respectively. Dot-sized cavernous malformations, visible in T2* and not or barely visible in T1WI and T2WI sequences, had a mean annual hemorrhage rate of 1.3% and a mean hemorrhage-free interval of 37.8 months.
CONCLUSIONS:
It is possible to predict hemorrhage rates based on the Zabramski classification. Our findings imply a tripartite classification distinguishing lesions with and without acute or subacute blood degradation products and dot-sized cavernous malformations.
Cerebral cavernous malformations (CCMs) are common vascular malformations with a prevalence of 0.2%–0.5%.1 CCMs may have a considerable clinical impact due to their high annual hemorrhage rates of up to 60%.2
The appearance of CCMs on MR imaging is manifold, and knowledge of specific imaging features and how these relate to hemorrhage rates can influence surgical treatment considerations and the frequency of radiologic follow-up. Zabramski et al3 (Table 1 and Fig 1) and Mottolese et al4 (On-line Table 1) presented MR imaging classifications characterizing the varied appearances of CCMs. However, neither classification elucidates the relationship between morphologic CCM type and clinical risk. Therefore, these classifications are rarely used in clinical practice. Recently, Jeon et al5 investigated hemorrhage rates of Zabramski types I–III in an adult population and found that nonhemorrhagic type III CCMs were associated with a significantly lower hemorrhage rate than hemorrhagic type I and II CCMs. Unfortunately, the authors excluded children and adolescents and did not analyze dot-sized type IV CCMs, thus leaving out an important type of CCM frequently encountered in hereditary forms of the disease.6
Table 1:
Original MRI classification of CCMs according to Zabramski et al3
| Lesion Type | MRI Signal Characteristics | Pathologic Characteristics |
|---|---|---|
| Type I | T1: hyperintense core | Subacute hemorrhage, surrounded by a rim of hemosiderin-stained macrophages and gliotic brain |
| T2: hyper- or hypointense core with surrounding hypointense rim | ||
| Type II | T1: reticulated mixed-signal core | Loculated areas of hemorrhage and thrombosis of varying ages, surrounded by gliotic, hemosiderin-stained brain; in large lesions, areas of calcification may be seen |
| T2: reticulated mixed-signal core with surrounding hypointense rim | ||
| Type III | T1: iso- or hypointense core | Chronic resolved hemorrhage, with hemosiderin staining within and around the lesion |
| T2: hypointense with a hypointense rim that magnifies the size of the lesion | ||
| GE: hypointense with greater magnification than T2 | ||
| Type IV | T1: poorly seen or not visualized at all | Two lesions in the category were pathologically documented as telangiectasias |
| T2: poorly seen or not visualized at all | ||
| GE: punctate hypointense lesions |
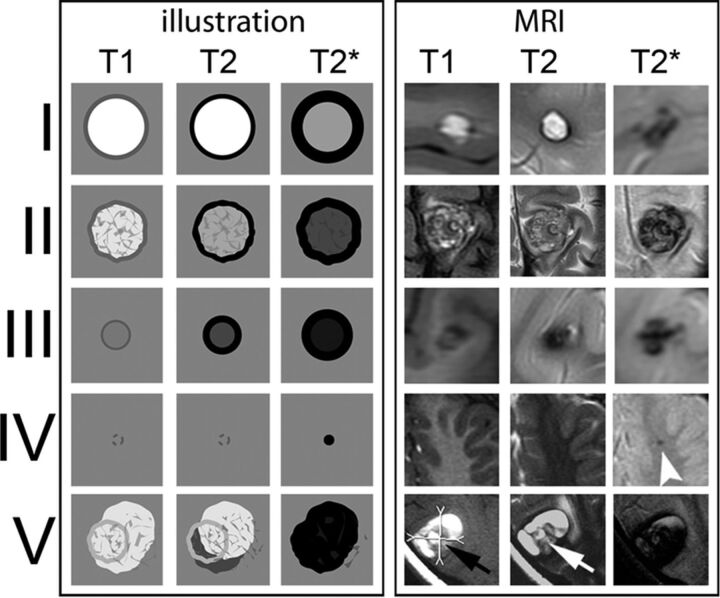
Fig 1.
CCM types according to the Zabramski classification. Graphic illustration (left 3 rows) and corresponding MR images (right 3 rows) of CCMs according to the MR imaging classification of Zabramski et al.3 Type IV CCM: arrowhead indicates a small T2* lesion. Type V: arrows indicate parts of the actual CCMs that are visible in the center of the hemorrhage; however, the CCM is not fully distinguishable from hemorrhage.
Other authors identified prior hemorrhage as an important risk factor for subsequent hemorrhage by comparing hemorrhage rates of CCMs with and without prior hemorrhage.1,2,5,7–9 According to these studies, hemorrhage rates ranged from 0%–6% and 4.5%–60%, depending on whether prior hemorrhage was or was not present, respectively.1,2,5,7–9 However, these articles have a lack of comparability because of differing hemorrhage definitions.10 Hence, our aim was to evaluate whether the different CCM types according to the Zabramski classification, including dot-sized type IV lesions, are associated with different hemorrhage rates and to provide a simple, yet comprehensive morphologic classification that reflects hemorrhage rates best.
Materials and Methods
Patients
After approval from the local institutional ethics board, we retrospectively reviewed data from the medical files from the department of Pediatric Neurosurgery of Hôpital Necker Enfants Malades, Paris, France, between January 1, 1993, and December 31, 2009. We identified 74 patients younger than 18 years of age with clear radiologic and/or pathologic criteria of CCMs, of whom 4 were excluded from the study due to a history of radiation. The remaining 70 patients had 356 CCMs. One small histologically proved CCM was found to be adjacent to a larger CCM during an operation and was removed. It was excluded from our analysis because it was not visible in previous imaging. In the end, we included 355 CCMs in 70 patients in this study. In addition to neuroimaging features, we assessed clinical and demographic information, such as age, sex, medical history, treatment, family history, and histologic findings by chart review. Two neuroradiologists, blinded to all clinical data, analyzed the imaging data. Unblinded consensus readings were performed to obtain a reference standard for statistical analyses.
Definitions
All lesions were radiologically defined according to the Zabramski classification.3 An additional category (type V) accounting for CCMs presenting with gross extralesional hemorrhage was added to take these lesions into account (Fig 1).
Only MR imaging studies performed at 1.5T were included, to minimize variation through magnetic field strength on susceptibility artifacts. All examinations were evaluated on T1WI and T2WI sequences with gradient-echo (GRE) imaging considered when present.
CCM diameters on T1WI and T2WI were averaged in all cases except in dot-sized CCMs (Zabramski type IV). The diameter of these lesions, which are only visible on T2*, was arbitrarily defined as 1 mm, because measuring lesion diameters on the basis of susceptibility artifacts alone will result in different results depending on the amount of hemosiderin depositions, section thickness, and section orientation. Overall hemorrhage size was assessed if the discrimination of a distinct lesion within the hemorrhage was not possible. Lesion growth was defined as a visually distinct increase of lesion diameter of at least 1 mm.
Hemorrhage Definition
To differentiate hemorrhage and simple intralesional thrombosis of blood, we postulated a radiologic hemorrhagic event if there was extralesional hemorrhage (acute or subacute blood-degradation products) or if there was intralesional hemorrhage (acute or subacute blood-degradation products) accompanied by lesion growth, mass effect, or edema. In accordance with criteria proposed by Al-Shahi Salman et al,10 neither lesion growth without signal changes nor the presence of hemosiderin without signs of recent hemorrhage was considered a hemorrhagic event. In addition, the appearance of subacute blood-degradation products without lesion growth or edema was not considered a hemorrhagic event. Because assessment of lesion growth is, by definition, not possible for supposedly hemorrhagic lesions at first imaging, intralesional hemorrhage at first imaging accompanied by corresponding clinical symptoms was also regarded as hemorrhage.3 A hemorrhagic event was considered symptomatic if there was a relation between clinical symptoms and hemorrhage age, anatomic location, or electrophysiologic examination findings. Lesions were regarded as de novo only when their new appearance could be shown in 2 comparable (ie, section thickness and orientation) consecutive series.
Hemorrhage Rate Calculations
Hemorrhage rates were calculated by 2 methods:
Lifetime hemorrhage rates were calculated as hemorrhagic events per time. The observation period of de novo lesions was defined as the period between radiologic diagnosis and last imaging. The observation period of lesions that were present at first imaging (presumably congenital lesions) was defined as the period between birth and last imaging. The lifetime hemorrhage rate was calculated as the average of hemorrhage rates of de novo and presumably congenital lesions. All 355 lesions were included in these calculations.
Prospective hemorrhage rates were calculated on the basis of Kaplan-Meier survival models and were analyzed with the Breslow test, log-rank test, and Cox regression. Because CCMs are dynamic lesions that may change in appearance with time, we analyzed prospective hemorrhage rates depending on the lesion appearance at any given observation point.6,11 Only lesions with a radiologic follow-up of >180 days were included in these calculations.
Statistical Analysis
Standard statistical tests (Student t test, Fisher exact test, Pearson χ2 test, Breslow test, log-rank test, Cox regression) were performed when applicable. P values under the α level of .05 were defined as significant. All statistical analyses were performed with SPSS software (Version 20; IBM, Armonk, New York).
Results
Demographics and Genetics
There were 355 CCMs in 48 male and 22 female patients. Twenty of 70 patients had multiple CCMs. The mean age of all 70 patients at first radiologic diagnosis of a CCM was 8.9 ± 4.5 years, ranging from 6.7 months to 17.3 years (median 9.5 years).
If one considered only lesions with a radiologic observation period of at least 180 days, there were 255 lesions in 10 female and 15 male patients. Of these 255 lesions, 248 were observed in 18 patients with multiple lesions. Six of these 18 children were tested for CCM1, CCM2, and CCM3 mutations. Three patients had a CCM1 mutation, and 3 children had a CCM3 mutation.
Lesion Characteristics
In initial imaging, there were 33 type I, 15 type II, 74 type III, 190 type IV, and 30 type V CCMs. In addition, there was 1 hemorrhagic, predominantly cystic CCM. Initial lesion type could not be classified in 12 cases because of a lack of adequate MR imaging examinations at the date of the finding. Overall, there were 91 frontal, 72 parietal, 64 temporal, 59 occipital, and 5 subependymal CCMs. Twenty-five CCMs were located in deep brain structures, 19 in the brain stem, and 20 in the cerebellum. Sixteen of 355 CCMs were associated with developmental venous anomalies. The initial average diameter of all CCMs excluding dot-sized CCMs was 12.0 ± 12.3 mm, ranging from 1 to 50 mm (median 5 mm). The diameter of lesions with extralesional hemorrhage (mean, 22.5 ± 11.8 mm; median, 20 mm; range, 5–50 mm) was greater than that of lesions with intralesional hemorrhage (mean, 9.4 ± 8.8 mm; median, 5 mm; range, 1–35 mm) and that of nonhemorrhagic type III lesions (mean, 3.4 ± 3 mm; median, 2 mm; range, 1–15 mm) (P < .001, Student t test).
Clinical Findings
Overall, 63 of 70 patients were symptomatic during a mean clinical observation period of 4.0 years. Seizures, signs of raised intracranial pressure, focal neurologic deficits, and isolated headache were observed in 24, 22, 20, and 2 patients, respectively. A cross-tabulation illustrating the co-occurrence of initial symptoms can be found in On-line Table 2. Infratentorial lesions were more likely to present with symptoms related to mass effect (ie, intracranial pressure and focal neurologic deficits), while supratentorial lesions were more likely to present with seizures (P < .001, Pearson χ2 test). Seven patients with 36 CCMs were symptom-free at diagnosis. All 7 patients remained symptom-free during the entire clinical observation period (mean, 5.1 ± 4.5 years; median, 3.9 years; range, 7 months to 13.0 years).
Radiologic Events
If one considered all 355 CCMs, there were 131 radiologic hemorrhagic events. Seventy-four of 131 hemorrhagic events were clinically symptomatic, while the remaining 57 hemorrhagic events were clinically silent. In addition to the 74 clinically symptomatic cases, there were 10 further reported clinical events in which an association between a clinical event and hemorrhage was not determinable due to a lack of adequate MR imaging examinations. Clinical symptoms were likely to be caused by hemorrhage (P = .004, Fisher exact test). Hemorrhage was more likely to be clinically symptomatic if the CCM was located in the brain stem (P = .004, Fisher exact test).
Intralesional signal changes without fulfillment of our hemorrhage criteria were present in 97 cases. These intralesional signal changes were likely to be asymptomatic (P < .001, Pearson χ2 test).
Three cases of recurring seizures were associated with nonhemorrhagic type III lesions. All dot-sized CCMs (Zabramski type IV) in our series were asymptomatic.
Conventional Hemorrhage Rate Calculations
Overall, 199 MR imaging studies were performed (mean: 4.4 studies per child; range, 1–12 MR imaging studies per child). There were 1558 distinct observations of 355 CCMs with a cumulative radiologic observation period of 1094.2 lesion-years. The lifetime hemorrhage risk with regard to all 355 lesions was 4.5% per lesion-year on average, based on 20 hemorrhagic events in 95 de novo lesions during a mean observation period of 3.8 years (5.5% per lesion-year) and 111 hemorrhagic events in 260 presumed congenital CCMs during a mean observation period of 12.3 years (3.5% per lesion-year).
Prospective Hemorrhage Rate Calculations
The mean radiologic observation period of 255 CCMs with a radiologic observation period of at least 180 days was 4.2 ± 2.9 lesion-years, ranging from 217 days to 13.0 years (median 3.2 years). There were 1398 radiologic observations of 255 lesions. The mean time between imaging was 8.8 months, ranging from 2 days to 29.9 ± 7.7 months (median 5.6 months). Table 2, On-line Table 3, and Figs 2 and 3 provide an overview of hemorrhage rates based on survival functions.
Table 2:
Hemorrhage-free survivala
| CCM Type | Mean Hemorrhage-Free Survival |
|||
|---|---|---|---|---|
| Estimator | Standard Error | 95% CI |
||
| Lower | Upper | |||
| I | 18.82 | 1.52 | 15.84 | 21.80 |
| II | 24.92 | 2.91 | 19.21 | 30.63 |
| III | 27.88 | 0.38 | 27.13 | 28.63 |
| IV | 37.78 | 0.40 | 37.00 | 38.57 |
| V | 21.34 | 2.67 | 16.10 | 26.59 |
| I, II, V | 22.63 | 1.50 | 19.68 | 25.57 |
| All | 36.06 | 0.59 | 34.91 | 37.21 |
Demonstrating mean hemorrhage-free survival in months depending on the extended CCM type of Zabramski et al.3
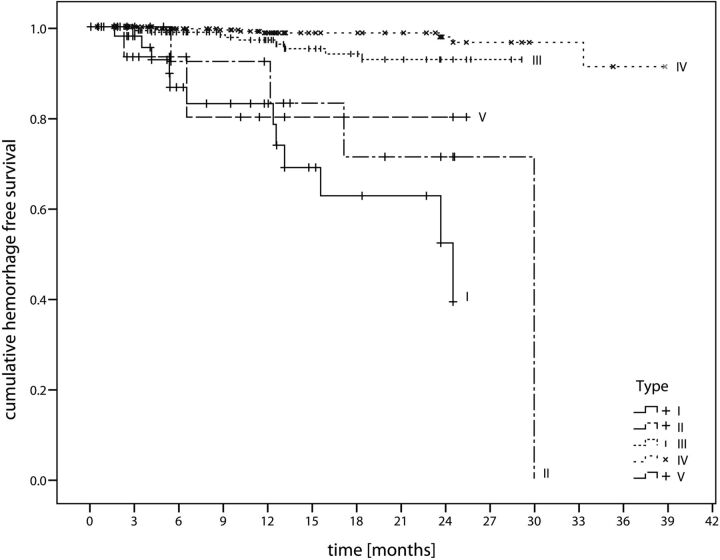
Fig 2.
Hemorrhage-free survival. Kaplan-Meier diagram illustrates hemorrhage-free survival depending on the Zabramski CCM type.
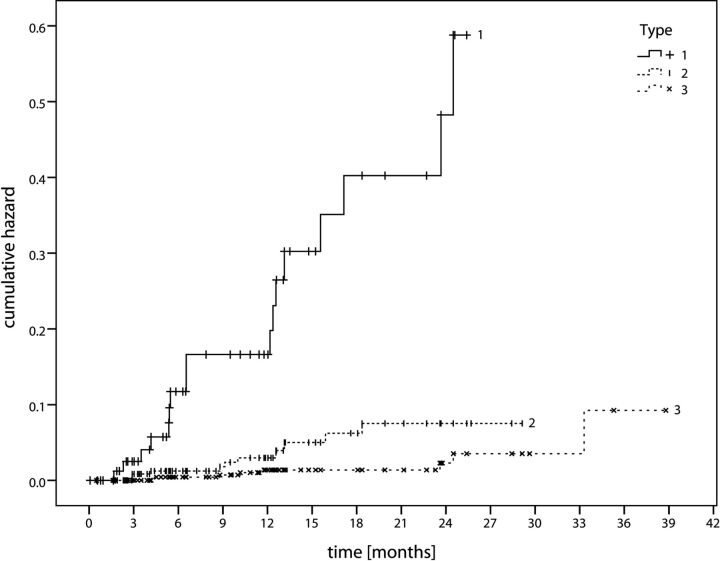
Fig 3.
Cumulative hazard. Diagram illustrates the cumulative hazard for hemorrhage according to our proposed CCM classification, 1) CCM with signs of acute or subacute hemorrhage, 2) CCM without signs of acute or subacute hemorrhage, and 3) dot-sized CCMs.
Sixty-one of 131 radiologic hemorrhagic events occurred in the excluded 100 lesions. Fifty-three of these 61 hemorrhagic events corresponded to CCMs that were hemorrhagic at first imaging. Only 8 of 61 excluded hemorrhagic events corresponded to consecutive hemorrhagic events of lesions that eventually were resected within 180 days.
Prospective Hemorrhage Rates Based on Zabramski Type
The annual hemorrhage rate was 29.8% for Zabramski type I, 20.1% for type II, 3.4% for type III CCMs, and 1.3% for type IV CCMs. The hemorrhage rate was 23.1% for our proposed new type V CCM and 23.4% for combined type I, II, and V CCMs.
Hemorrhage rates of type III and IV CCMs differed significantly (Breslow test: P = .015; log-rank test: P = .014). There was no significant difference between hemorrhage rates of type I, II, and V CCMs (Breslow test: P ≥ .133; log-rank test: P ≥ .247; On-line Table 3). Mean event-free intervals of type I, II, and V lesions were significantly shorter compared with type III and IV CCMs (Breslow test: P ≤ .006; log-rank test: P ≤ .044; On-line Table 3).
Prospective Hemorrhage Rates Based on Other Factors
Univariate analyses revealed that solitary CCMs had a higher hemorrhage risk in the long term than CCMs in the context of multiple CCMs (Breslow test: P = .082; log-rank test: P = .002). In addition, CCMs located in the brain stem had an increased hemorrhage rate (Breslow test: P = .007; log-rank test: P = .044). CCMs associated with a developmental venous anomaly had a higher hemorrhage risk in the shortterm (Breslow test: P = .032; log-rank test: P = .213).
CCM size (cutoffs: 1.5, 2, 2.5, 3 cm) had no significant impact on hemorrhage rates, when dot-sized CCMs were excluded from analysis (Breslow test: P ≥ .352; log-rank test: P ≥ .299). CCMs located close to gray matter had no increased hemorrhage rate (Breslow test: P = .723; log-rank test: P = .759). Neither sex nor mutation type (CCM1 versus CCM3) had a significant influence on hemorrhage rates (Breslow test: P = .203; log-rank test: P = .071; and Breslow test: P = .173; log-rank test: P = .232, respectively).
Eventually, multivariate analyses (Cox regression) revealed that the presence of acute or subacute blood-degradation products had a significant impact on hemorrhage rates (P = .036), whereas mass effect (P = .161), the presence of extralesional hemorrhage (P = .307), CCMs in the context of multiplicity (P = .139), the presence of a developmental venous anomaly (P = .511), and localization in the brain stem (P = .761) had no significant influence on hemorrhage rates.
Discussion
Prospective Hemorrhage Rates Based on the Zabramski Classification
When a CCM is diagnosed, the most important issue is to predict the risk for future hemorrhage and clinical symptoms. Thus, the aim of any classification should be to predict hemorrhage (and consequently clinical) risks. Prior hemorrhage has already been described as an important risk factor for subsequent hemorrhage.1,2,7–9 However, the term “hemorrhage” is used to describe both cerebral bleeding and a clinical symptom in the published literature, making accurate comparisons of hemorrhage rates difficult, with some authors calculating hemorrhage rates per patient and indicating clinical event rates instead of radiologic event rates (Table 3).10 While the assessment of clinical events appears practical, there is a distinct risk of false generalization: The same CCM will lead to different clinical event rates depending on whether it is located in the precentral or the middle frontal gyrus. Accordingly, hemorrhage of brain stem CCMs was more likely to be symptomatic than other CCMs in our series. Therefore, we advocate the assessment of lesion-based radiologic hemorrhage rates because the occurrence of clinical events (with the exception of seizures) depends primarily on lesion location and the extent of hemorrhage.12,13 Clinical event rates can then be deduced when the localization and hemorrhage rate of a given CCM are known.11
Table 3:
Prospective hemorrhage rates in the literaturea
| Study | No. of Patients | No. of Lesions | Hemorrhage Assessment | Hemorrhage Rate without Prior Hemorrhage | Hemorrhage Rate with Prior Hemorrhage |
|---|---|---|---|---|---|
| Al-Shahi Salman et al1 | 134 | NI | MRI/clinical | 2.4% | 29.5% |
| Kondziolka et al8 | 122 | NI | MRI/clinical | 0.6% | 4.5% |
| Moriarity et al15 | 68 | 228 | MRI/clinical | 3.1%b | NI |
| Porter et al16 | 173 | NI | MRI/clinical | 1.6%b | NI |
| Robinson et al17 | 57 | 66 | MRI/clinical | 0.7%b,c | NI |
Note:—NI indicates not indicated.
Hemorrhage rates of supratentorial and infratentorial CCMs found in prospective registry studies. Hemorrhage rates are in patient-years.
Overall hemorrhage rate.
Hemorrhage rates in lesion-years.
We analyzed hemorrhage rates on the basis of the most common CCM classification by Zabramski et al3 and found that it is possible to predict radiologic hemorrhage rates on the basis of this classification. Even though Zabramski type I, II, and (proposed) V CCMs have distinctive morphologic features, these lesions share statistically similar high annual hemorrhage rates of 20.1%–29.8%. Annual hemorrhage rates of type III and dot-sized CCM are significantly lower (3.4% and 1.3%, respectively). Our results imply that the presence of acute or subacute blood-degradation products, present in Zabramski type I, II, and V CCMs, is the strongest predictor of hemorrhage. These findings are highly in accordance with results provided by Jeon et al,5 who performed a comparable analysis on 410 mostly solitary-type I, II, and III CCMs in a population older than 18 years of age: The authors reported an increased annual hemorrhage risk of type I and II lesions (27.6% and 15.4%, respectively) compared with type III lesions (5.4%) (P < .001).5 Additionally, the authors found that female sex, age, infratentorial localization, multiplicity, size, and the presence of venous angioma were no risk factors for hemorrhage, which is also in accordance with our results.5 The outstanding role of signs of prior hemorrhage is also supported by previous published results, in which an increased risk for hemorrhagic CCM has been reported despite different definitions of hemorrhage (Table 3).1,2,7–9 A possible explanation might be the destruction of microstructural integrity after a first hemorrhagic event.14
Our findings and data from the literature imply that a simple tripartite classification might be more useful in clinical practice:
CCMs with acute or subacute blood-degradation products (Zabramski I, II, V). High hemorrhage risk of 23.4% (literature: 4.5%–60%).1,2,5,8 Mean hemorrhage-free interval: 22.63 months. Association with acute or subacute clinical symptoms.
CCMs without acute or subacute blood-degradation products (Zabramski III). Intermediate annual hemorrhage risk of 3.4% (literature: 0%–6%).1,2,5,8,15–17 Mean hemorrhage-free interval: 27.88 months. They may be symptomatic particularly in the context of seizures: Ten percent of seizures in our series were associated with these CCMs.
Dot-sized lesions, visible in T2* and not or barely visible in T1WI and T2WI (Zabramski IV). The lowest hemorrhage rate of 1.3%. Mean hemorrhage-free interval: 37.78 months. Appear to be asymptomatic unless they are hemorrhagic.6
Other Possible Risk Factors
Whereas univariate analyses implied that brain stem localization, the presence of a developmental venous anomaly, and a solitary CCM are associated with an increased hemorrhage risk, these factors did not prove significant in our multivariate analyses. In fact, data from various authors imply that an apparent increased hemorrhage risk of brain stem lesions is likely to be caused by a selection bias: Brain stem hemorrhage is more likely to be symptomatic than supratentorial hemorrhage and is therefore more likely to lead to MR imaging.9,12,18,19 Furthermore, while the relationship between developmental venous anomalies and CCMs is not fully understood yet, neither Flemming et al20 nor Jeon et al,5 who performed analyses comparable with ours found that the presence of developmental venous anomalies was associated with an increased hemorrhage risk. Finally, it has been reported that patients with multiple CCMs and patients with CCM3 mutations have a more aggressive clinical course. Flemming et al,20 for example, indicated an odds ratio of 2.65 for hemorrhage in patients with multiple lesions. However, a more aggressive clinical course does not necessarily imply an increased hemorrhage risk. Current evidence suggests that a more aggressive clinical course is possibly caused by an increased cumulative hemorrhage rate rather than an increased hemorrhage rate of each CCM.5,21,22 Paradoxically, our data demonstrated an increased long-term hemorrhage rate of solitary lesions. However, this result may have been biased because most of the solitary lesions were more likely to be resected early on and thus were absent from long-term radiologic follow-up analyses. Although there were limited patient data with mutations in our analyzed population, our results did not suggest that hemorrhage rates of each CCM type depended on a specific mutation type. Results in the literature suggest that those carrying the CCM3 mutation are likely to present with an increased number of CCMs at an early age.6,22 Again, a more aggressive clinical course in patients with CCM3 mutations may be caused by an increased cumulative risk due to a high number of CCMs.6,21,22
Limitations
Our retrospective approach involves a degree of selection bias. An ideal study dealing with hemorrhage rates should be prospective and standardized. However, examining a considerable number of patients with CCMs on a regular basis—ideally monthly to reliably diagnose new hemorrhage—can be a tedious task, which is reflected by the fact that all published prospective studies dealing with the natural history of CCMs are registry studies.1,8,15–17
Furthermore, it has been discussed controversially whether CCMs in children bear an increased hemorrhage risk.23 Mottolese et al,4 for instance, reported that CCMs in children are more likely to be symptomatic. In the end, the sometimes reported lower proportion of asymptomatic incidental CCMs in children is likely to be caused by a selection bias because routine examinations of the brain are more common in adults than in children. Additionally, hemorrhage rates in the literature are commonly calculated under the assumption that CCMs are congenital, despite the proof of de novo lesions.7–9,15–17 Consequently, hemorrhage rates calculated under this assumption are always inversely proportional to the mean age of a patient population, leading to a higher hemorrhage rate in younger populations. In fact, Jeon et al,5 who performed a prospective hemorrhage rate calculation similar to ours, reported comparable hemorrhage rates for a population older than 18 years of age.
A further limitation of our study is a diagnostic uncertainty concerning dot-sized lesions.6 The size and signal of dot-sized lesions depend strongly on technical MR imaging parameters such as magnetic field strength and section thickness. It is conceivable that dot-sized CCMs simply correspond to small nonhemorrhagic CCMs.6 Nonetheless, our data suggest that these different CCM types have statistically significant distinctive clinical and radiologic features that justify differentiation of these lesions.
In summary, we believe that analyzing CCMs in children and adolescents with multiple CCMs is a practical approach in the absence of prospective studies with serial MR imaging examinations; examining CCMs in the context of multiple lesions allows a longitudinal analysis of different CCMs of varying types, sizes, and locations at the same time and underlying the same possible influence factors. Furthermore, differential diagnosis of dot-sized T2* lesions in adults comprises microangiopathic, amyloid angiopathic, or drug-induced (eg, anticoagulant therapy) microbleeds, which are rare in children and adolescents.
Conclusions
This study has shown that it is possible to predict hemorrhage rates of CCMs on the basis of the most common morphologic CCM classification proposed by Zabramski et al3 when a further category accounting for CCMs with gross extralesional hemorrhage is added. Our findings and data from the literature imply that a simpler tripartite classification predicts hemorrhage risks best. Nevertheless, further prospective research needs to be done to establish whether our results prove correct and practical in daily clinical work.
Supplementary Material
ABBREVIATIONS:
- CCM
cerebral cavernous malformation
- GRE
gradient-echo
Footnotes
Disclosures: Indran Davagnanam—RELATED: Grant: University College London Biomedical Research Centre,* Comments: University College London Biomedical Research Centre funding for 1 consultant session in 2014–2016. Martin Wiesmann—UNRELATED: Consultancy: Stryker Neurovascular; Grants/Grants Pending: Covidien*; Payment for Lectures (including service on Speakers Bureaus): Siemens, Stryker Neurovascular, Bracco. *Money paid to the institution.
References
- 1. Al-Shahi Salman R, Hall JM, Horne MA, et al. ; Scottish Audit of Intracranial Vascular Malformations (SAIVMs) collaborators. Untreated clinical course of cerebral cavernous malformations: a prospective, population-based cohort study. Lancet Neurol 2012;11:217–24 10.1016/S1474-4422(12)70004-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang CC, Liu A, Zhang JT, et al. Surgical management of brain-stem cavernous malformations: report of 137 cases. Surg Neurol 2003;59:444–54; discussion 454 10.1016/s0090-3019(03)00187-3 [DOI] [PubMed] [Google Scholar]
- 3. Zabramski JM, Wascher TM, Spetzler RF, et al. The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg 1994;80:422–32 10.3171/jns.1994.80.3.0422 [DOI] [PubMed] [Google Scholar]
- 4. Mottolese C, Hermier M, Stan H, et al. Central nervous system cavernomas in the pediatric age group. Neurosurg Rev 2001;24:55–71 10.1007/PL00014581 [DOI] [PubMed] [Google Scholar]
- 5. Jeon JS, Kim JE, Chung YS, et al. A risk factor analysis of prospective symptomatic haemorrhage in adult patients with cerebral cavernous malformation. J Neurol Neurosurg Psychiatry 2014;85:1366–70 10.1136/jnnp-2013-306844 [DOI] [PubMed] [Google Scholar]
- 6. Nikoubashman O, Wiesmann M, Tournier-Lasserve E, et al. Natural history of cerebral dot-like cavernomas. Clin Radiol 2013;68:e453-59 10.1016/j.crad.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 7. Aiba T, Tanaka R, Koike T, et al. Natural history of intracranial cavernous malformations. J Neurosurg 1995;83:56–59 10.3171/jns.1995.83.1.0056 [DOI] [PubMed] [Google Scholar]
- 8. Kondziolka D, Monaco EA 3rd, Lunsford LD. Cavernous malformations and hemorrhage risk. Prog Neurol Surg 2013;27:141–46 10.1159/000341774 [DOI] [PubMed] [Google Scholar]
- 9. Li D, Yang Y, Hao SY, et al. Hemorrhage risk, surgical management, and functional outcome of brainstem cavernous malformations. J Neurosurg 2013;119:996–1008 10.3171/2013.7.JNS13462 [DOI] [PubMed] [Google Scholar]
- 10. Al-Shahi Salman R, Berg MJ, Morrison L; Angioma Alliance Scientific Advisory Board. Hemorrhage from cavernous malformations of the brain: definition and reporting standards. Stroke 2008;39:3222–30 10.1161/STROKEAHA.108.515544 [DOI] [PubMed] [Google Scholar]
- 11. Clatterbuck RE, Moriarity JL, Elmaci I, et al. Dynamic nature of cavernous malformations: a prospective magnetic resonance imaging study with volumetric analysis. J Neurosurg 2000;93:981–86 10.3171/jns.2000.93.6.0981 [DOI] [PubMed] [Google Scholar]
- 12. Gross BA, Batjer HH, Awad IA, et al. Brainstem cavernous malformations: 1390 surgical cases from the literature. World Neurosurg 2013;80:89–93 10.1016/j.wneu.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 13. Rosenow F, Alonso-Vanegas MA, Baumgartner C, et al. ; Surgical Task Force, Commission on Therapeutic Strategies of the ILAE. Cavernoma-related epilepsy: review and recommendations for management—report of the Surgical Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2013;54:2025–35 10.1111/epi.12402 [DOI] [PubMed] [Google Scholar]
- 14. Tu J, Stoodley MA, Morgan MK, et al. Ultrastructural characteristics of hemorrhagic, nonhemorrhagic, and recurrent cavernous malformations. J Neurosurg 2005;103:903–09 10.3171/jns.2005.103.5.0903 [DOI] [PubMed] [Google Scholar]
- 15. Moriarity JL, Wetzel M, Clatterbuck RE, et al. The natural history of cavernous malformations: a prospective study of 68 patients. Neurosurgery 1999;44:1166–71; discussion 1172–73 10.1227/00006123-199906000-00003 [DOI] [PubMed] [Google Scholar]
- 16. Porter PJ, Willinsky RA, Harper W, et al. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg 1997;87:190–97 10.3171/jns.1997.87.2.0190 [DOI] [PubMed] [Google Scholar]
- 17. Robinson JR, Awad IA, Little JR. Natural history of the cavernous angioma. J Neurosurg 1991;75:709–14 10.3171/jns.1991.75.5.0709 [DOI] [PubMed] [Google Scholar]
- 18. Maraire JN, Awad IA. Intracranial cavernous malformations: lesion behavior and management strategies. Neurosurgery 1995;37:591–605 10.1227/00006123-199510000-00001 [DOI] [PubMed] [Google Scholar]
- 19. Hauck EF, Barnett SL, White JA, et al. Symptomatic brainstem cavernomas. Neurosurgery 2009;64:61–70; discussion 70–71 10.1227/01.NEU.0000335158.11692.53 [DOI] [PubMed] [Google Scholar]
- 20. Flemming KD, Link MJ, Christianson TJ, et al. Prospective hemorrhage risk of intracerebral cavernous malformations. Neurology 2012;78:632–36 10.1212/WNL.0b013e318248de9b [DOI] [PubMed] [Google Scholar]
- 21. Schneble HM, Soumare A, Hervé D, et al. Antithrombotic therapy and bleeding risk in a prospective cohort study of patients with cerebral cavernous malformations. Stroke 2012;43:3196–99 10.1161/STROKEAHA.112.668533 [DOI] [PubMed] [Google Scholar]
- 22. Spiegler S, Najm J, Liu J, et al. High mutation detection rates in cerebral cavernous malformation upon stringent inclusion criteria: one-third of probands are minors. Mol Genet Genomic Med 2014;2:176–85 10.1002/mgg3.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Al-Holou WN, O'Lynnger TM, Pandey AS, et al. Natural history and imaging prevalence of cavernous malformations in children and young adults. J Neurosurg Pediatr 2012;9:198–205 10.3171/2011.11.PEDS11390 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.