SUMMARY:
Minimally invasive stereotactic tumor ablation is a viable option for the treatment of benign and malignant intracranial lesions. Although surgical excision constitutes first-line therapy for various brain pathologies, it can cause irreversible neurologic deficits. Additionally, many patients who may benefit from surgery do not qualify as surgical candidates due to multiple comorbidities. Recent advancements in laser interstitial thermal therapy, namely the ability to monitor ablation in real-time under MR imaging, have improved the safety and efficacy of the procedure. MRI-guided laser interstitial thermal therapy is currently used as a minimally invasive treatment for brain metastases, radiation necrosis, glioma, and epilepsy. This article will discuss the principles, suggested indications, complications, and imaging characteristics of MRI-guided laser interstitial thermal therapy as they pertain to the treatment of brain pathology.
Laser interstitial thermal therapy (LITT) is a stereotactically guided percutaneous minimally invasive procedure, which delivers light energy to target tissue via a fiberoptic catheter, resulting in selective thermal ablation of malignant and benign lesions. LITT was described in 1983 by Bown1 and was first applied in the treatment of brain lesions in 1990 by Sugiyama et al.2 Acceptance of the procedure has been slow due to initial technologic shortcomings, which resulted in low efficacy and an unacceptably high risk of thermal damage to the surrounding normal brain parenchyma. Recent improvements have led to the development of percutaneous MRI-guided laser interstitial thermal therapy (MRgLITT), which enables monitoring of tissue ablation in real-time. Multiple academic centers are now using MRgLITT as a minimally invasive treatment for brain metastases, radiation necrosis, gliomas, and epilepsy. Successful management of patients undergoing MRgLITT requires interdisciplinary collaboration among neurosurgeons, neuroradiologists, neurologists, anesthesiologists, radiation oncologists, and neuro-oncologists. This article will discuss the principles, suggested indications, complications, and imaging characteristics of MRgLITT as they pertain to the treatment of brain pathology.
Background
Minimally invasive stereotactic tumor ablation is a viable option for the treatment of benign and malignant intracranial lesions. Surgical excision constitutes first-line therapy for many malignant brain tumors followed by chemotherapy and/or radiation therapy. Aggressive complete resection or surgical debulking of newly diagnosed glioblastoma (GBM) improves survival,3 compared with chemotherapy and radiation therapy alone.4,5 A problem arises when patients do not qualify for surgical resection due to the presence of deep-seated lesions, low functional scores, comorbidities, or an inability to tolerate general anesthesia. In such cases, survival is limited; this outcome underscores the need for minimally invasive alternatives. In the case of inoperable brain metastases, stereotactic radiosurgery (SRS) presents a viable minimally invasive option. SRS achieves an 80%–90% local control rate over metastatic lesions6,7 but plays a limited role in the treatment of glioma or medically intractable seizures. Metastatic recurrence after SRS and whole-body radiation therapy is common, with estimates of up to 46.8% at 1-year follow-up.8 Repeat use of radiation therapy after recurrence is limited due to concerns about cumulative adverse radiation effects, such as radiation necrosis.
Delayed radiation necrosis (RN) is a known complication of SRS, with reported rates ranging from 5% to 50%.9 The true incidence of RN in the setting of prior SRS is unknown because of diagnostic difficulties in distinguishing RN and recurrence. Imaging-wise, radiation necrosis and tumor recurrence can both appear as enlarging previously treated lesions with a variable increase in internal enhancement or as a new thickening and/or irregularity of a peripherally enhancing rim with surrounding edema.10 Although commonly used modalities such as MR perfusion imaging, MR imaging spectroscopy, SPECT, and PET may provide useful clues about the identity of a previously treated enlarging lesion, their utility is limited. Current MR imaging and MR spectroscopy concepts under investigation include lesion quotient,11 T1/T2 mismatch,12 percentage signal recovery,13 relative cerebral blood volume,14 and multivoxel proton MR spectroscopy,15 but specificities and sensitivities are variable. Unfortunately, the specificity of biopsy can also be low due to sampling error.16 Definitive diagnosis is further limited because up to 33% of enlarging lesions after treatment with SRS represent a combination of RN and recurrence rather than a pure form of either entity.17 Given the common coexistence of RN and recurrence, Rao et al18 postulated that an ablation technique such as LITT, which treats both RN and recurrent metastatic lesions, may circumvent diagnostic difficulties by providing the same standardized treatment for both types of lesions.
Another entity that could benefit from minimally invasive treatment is epilepsy. One-third of seizures are refractory to pharmacologic therapy. The success rate of additional pharmacologic therapy after 2 failed regimens is ≤3%.19 In the setting of medically intractable seizures, open resection of well-defined epileptic foci has been shown to achieve a control rate of up to 75%–80%.20 However, many of the lesions are deep-seated, requiring excessive dissection during resection, thus increasing the risk of iatrogenic complications and residual permanent neurologic deficits. Minimally invasive procedures such as SRS and radiofrequency ablation are currently of limited utility in the treatment of epilepsy due to the inability to visualize ablation in real-time.
The ideal minimally invasive procedure should reduce intra- and postoperative morbidity and mortality, shorten hospital stays, decrease health care costs, and offer effective treatment options to patients who are otherwise not eligible for open surgery or other debulking treatments. To date, multiple modalities have been used for stereotactic lesion ablation, including cryoablation, radiofrequency, sonography, microwave, ionizing radiation, and laser. Recent technologic advances in laser ablation have significantly improved safety and efficacy by providing the ability to monitor ablation in real-time. Other advantages include shorter ablation times and sharper ablation zone boundaries. Both SRS and LITT can treat difficult-to-access lesions, can be performed with the patient under minimal sedation, and do not require discontinuation of ongoing systemic therapy. Although MRgLITT is an invasive intracranial procedure, which requires a burr-hole and possible general anesthesia, it provides a potential benefit over the noninvasive SRS: MRgLITT delivers nonionizing radiation to target lesions, thus avoiding long-term adverse radiation effects and theoretically allowing retreatment of the same lesion. However, the long-term effects of thermal necrosis have not yet been clearly elucidated.
Physics of MRgLITT
During LITT, photons emitted from a fiberoptic laser are absorbed by tumor chromophores, organic molecules that absorb, transmit, and reflect light. Light absorption leads to molecular excitation and subsequent release of thermal energy within the target tissue. Thermally induced irreversible cell damage occurs between temperatures of 46°C and 60°C.21 Temperatures above 60°C result in instantaneous coagulation necrosis.22 The absorption coefficient determines the extent of photon absorption within a target tissue. In general, the absorption coefficient of pathologic lesions and coagulation necrosis is higher than that in normal brain parenchyma, leading to preferential ablation of the target tissue.23 There is a sharp temperature fall-off at the border of the ablation zone,24 creating a sharp margin between viable and nonviable tissue, which can be monitored with real-time MRI thermal imaging (MRTI).
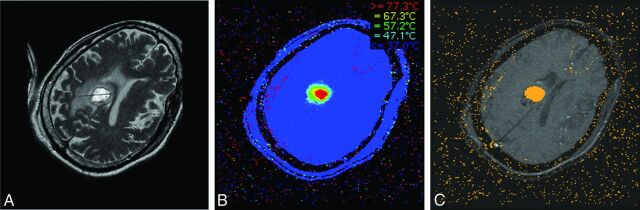
Proton resonance frequency is the most widely used temperature-sensitive MR imaging parameter for real-time MRTI.25 Proton resonance frequency–based MRTI is the basis of MRgLITT. The physical mechanism of proton resonance frequency–based MRTI depends on the presence of hydrogen bonds within a tissue.26–28 As the temperature increases, the number of hydrogen bonds decreases. This decrease leads to a more uniform distribution of electron clouds within a molecule. The more evenly distributed electron clouds are better able to shield the 1H nuclei from the full force of the external magnetic field supplied by the MR imaging machine. Thus, as the temperature increases, the local magnetic field experienced by a 1H nucleus decreases due to increased shielding by the surrounding electrons. The decrease in the local magnetic field decreases the Larmour precession frequency of the 1H nucleus, which, in turn, alters the phase of gradient recalled-echo phase images.25 Measurements are obtained by subtracting “thermal” fast-spoiled gradient recalled phase images (obtained after administration of thermal energy) from a “reference” fast-spoiled gradient recalled phase image (obtained at body temperature before any energy pulse is delivered).26,27 The phase difference, or phase shift, between the 2 images is proportional to the overall temperature change. As such, proton resonance frequency–based MRTI does not measure the absolute temperature of a sample, but simply measures the temperature difference between the sample and a designated reference temperature image.25,28 Temperature information and time of ablation can be incorporated into a mathematic model of thermal tissue destruction (Arrhenius model) to provide a real-time quantitative estimate of tissue necrosis,29 displayed in real-time as an orange “damage zone” (Fig 1).
Fig 1.
Once the location of the laser probe is confirmed, ablation can proceed. A, T2 image demonstrates a properly positioned laser probe within a metastatic melanoma lesion. B, Heat map: during ablation, temperatures surrounding the laser tip are continuously updated and depicted with various colors. C, Damage zone images: orange color depicts the area of tissue that has been successfully ablated (damage zone) on the basis of the Arrhenius model of thermal tissue ablation.
LITT Setup and Procedure
Two major LITT platforms are in use today. NeuroBlate (Monteris Medical Corporation, Minneapolis, Minnesota), which received 510(k) FDA clearance in May 2009, uses a 12-W 1064-nm neodymium-doped yttrium aluminium garnet laser with a CO2 cooled side-firing probe.30 Visualase (Medtronic, Minneapolis, Minnesota), which received FDA clearance in 2007, is the platform used in our institution. The major components of the Visualase system include a 15-W 980-nm diode laser, a disposable saline-cooled diffusing laser applicator probe with a 1-cm-long 1.65-mm diameter outer cooling catheter, and a computer workstation, which communicates with MR imaging. MRTI generates “thermal” images, which are then used to generate “damage” images (Fig 1). Treatment concludes when the “damage zone” in a damage image covers the entire target area. The software allows the programming of temperature limit points near the tip of the probe and in the periphery of the lesion. If temperatures exceed the programmed thresholds, the laser shuts down automatically. Recommended temperature limit points are 90°C near the tip of the probe as a safeguard against overheating, carbonization, and vaporization and 50°C at the periphery to prevent damage to adjacent normal brain tissue.31
The procedure can be performed with the patient under light sedation or general anesthesia, depending on patient positioning and preference. The preoperative MR imaging examination identifies the target lesion and entry site. Using intraoperative neuronavigation, we make a small burr-hole by using a twist drill. A bone anchor is then placed into the skull in the exact target trajectory identified by neuronavigation. The cooling catheter is advanced through the anchor to the desired target and fixed to the bone anchor. The laser probe is then inserted into the cooling catheter and locked into place. T2 imaging is performed to confirm the exact placement of the probe (Fig 1A). Fast-spoiled gradient recalled phase images are obtained at the patient's body temperature to serve as a baseline for all intraprocedural thermal measurements. Once the cooling system begins to circulate, a test pulse of 3–4 W for 30–60 seconds is administered to determine the exact location of the distal 1-cm segment of the laser probe. This is important because thermal energy is emitted from the distal-most 1- cm segment of the laser fiber, and knowing its exact location within the target lesion is crucial to ensuring the accuracy of ablation. Ablation is performed by applying treatment doses of 10–15 W for 30–180 seconds until the damage zone covers the entire area of the target lesion. After completion of the procedure, we remove the probe, catheter, and anchor and close the small skin puncture site with a running Monocryl stitch (Ethicon, Cincinnati, Ohio). The reported average hospital stay varies, but most studies report 24–48 hours for cases without complications.29,31–33
Imaging Protocol
On the day before the procedure, a contrast-enhanced T1WI fiducial study is performed for neuronavigation purposes and to provide a map of the surrounding vasculature. The neuroradiologist assesses change in lesion size, appearance, and enhancement. Gradient recalled echo or susceptibility sequences are used to assess acute intracranial hemorrhage, which may be confirmed on follow-up CT. Acute intracranial hemorrhage is a contraindication to MRgLITT because of the risk of increased postprocedural hemorrhage, edema, and the resultant increase in intracranial pressure. Additionally, the presence of blood products can lead to suboptimal lesion ablation due to the propensity of blood to absorb heat emitted from the laser probe.
On the day of the procedure, the patient is taken to the operating room, where the probe is surgically advanced into the lesion as described above. The patient is then transferred to the MR imaging suite, where probe tip placement is confirmed. Intraoperative imaging and ablation are performed using a head coil capable of accommodating the MR imaging calvarial bone anchor, cooling catheter, and laser probe. Intraprocedural MRTI allows the neurosurgeon to visualize ablation in real-time. This is particularly useful for lesions located adjacent to fluid-filled structures with internal flow, such as vessels or ventricles. Structures with internal flow are potential sources of heat loss via a flow-induced heat sink effect, which can lead to asymmetric ablation of adjacent target lesions. MRTI can detect suboptimal heating within a segment of the lesion, thus prompting the neurosurgeon to apply additional energy until ablation throughout the entire lesion is achieved.
Immediate postprocedural contrast-enhanced T1WI is performed to evaluate the effectiveness of ablation. An additional contrast-enhanced study is performed 24 hours after the procedure to better characterize the ablated lesion. Additionally, because MRgLITT-treated lesions evolve in a somewhat predictable manner (discussed below), this study serves as a baseline for all follow-up imaging. The role of the neuroradiologist is to be able to recognize the normal temporal radiographic evolution of a treated lesion and to differentiate it from disease recurrence. Specific imaging pearls are discussed in the following sections.
MRTI Artifacts
The accuracy of MRTI can be hindered by the following: 1) susceptibility artifacts from calcifications or pre-existing surgical hardware, 2) the presence of fat, 3) misregistration artifacts due to motion, and 4) magnetic field inhomogeneities. In general, if a lesion cannot be adequately visualized, then ablation should not be attempted. Large amounts of susceptibility artifacts adjacent to a lesion are a relative contraindication to MRgLITT, though no publications exist in support of this claim, to our knowledge. The presence of intralesional calcifications has not been addressed in the literature, but we have verbal confirmation from Visualase representatives of successful treatment of partially calcified lesions at other institutions (in conversation with David Simon, PhD, January 22, 2015). The presence of fat is a potential source of susceptibility in proton resonance frequency–based MRTI because fat does not contain hydrogen bonds.27 The lack of hydrogen bonds in fat makes proton resonance frequency less susceptible to temperature change, thus leading to inaccurate temperature measurements and susceptibility effects.26,27 This characteristic implies that ablation of fat-containing lesions cannot be accurately monitored with proton resonance frequency–based MRTI. To our knowledge, there are no published reports on the use of MRgLITT in the treatment of fat-containing lesions. Misregistration artifacts from motion can be prevented with mechanical immobilization of the cranium, proper sedation, and stabilization of the laser tip with a bone anchor. Magnetic field inhomogeneities can be eliminated by subtracting baseline reference images from thermal images.25
Imaging and Radiologic-Pathologic Correlation of Lesions Treated with LITT
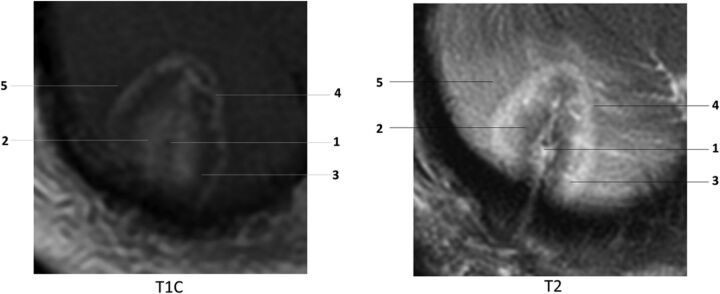
Ablated lesions demonstrate a thin peripheral rim of enhancement, variable T1 and T2 central signal due to presence of blood and protein products and surrounding edema on T1WI contrast-enhanced images obtained 24 hours after treatment. At the microscopic level, these changes correspond to 5 histologically separate concentric zones (Fig 2).34 At the core is the probe track, which may be filled with CSF or blood. The probe track is surrounded by the central zone of coagulation necrosis, which contains damaged cell membranes and stains positive for markers of apoptosis. On 24-hour follow-up, the central zone may appear hyperintense on T1 and hypointense on T2 because of the presence of subacute blood products and protein coagulation. Alternatively, it may appear hypointense on T1 and hyperintense on T2, depending on the age of the blood products and relative concentration of protein.
Fig 2.
Concentric zones: T1 contrast-enhanced (T1C) and T2WI 24 hours after laser ablation of a recurrent right cerebellar metastatic lesion in a 71-year-old female patient with history of breast carcinoma: 1) probe track, 2) central zone, 3) peripheral zone, 4) peripherally enhancing rim, 5) marginal zone. Note that the concentric zones appear as inverse images on T1C and T2 images.
The peripheral zone makes up the next concentric layer, which contains thrombosed vessels and distended cell bodies. This area undergoes delayed liquefaction necrosis and tends to enlarge during the first 1–40 days, followed by a continuous reduction in size thereafter.35 On MR imaging, this layer appears hypointense on T1 and hyperintense on T2 due to edema. The peripheral zone contains a thin peripherally enhancing rim on T1-weighted contrast-enhanced images, secondary to blood-brain barrier damage. The peripheral rim gradually changes in circumference and enhancement in accordance with changes in the entire peripheral zone. It generally decreases with time or may remain stable (Fig 3). Residual enhancement persists on long-term-follow-up, likely due to reactive inflammatory/granulation tissue. According to Rao et al,18 most lesions return to pretreatment size within 16 weeks. The outermost layer of the LITT lesion is the marginal zone, an area of reversible postsurgical perifocal edema, which appears hypointense on T1 and hyperintense on T2. This layer contains viable edematous tissue and demonstrates axonal swelling without thrombosis. It increases in size, reaching maximum dimensions at 1–3 days and gradually decreases in size during the course of 15 days to 2 months (Fig 4). In some cases, the marginal zone may demonstrate high T1 signal without corresponding susceptibility artifacts, likely due to the presence of myelin breakdown products. Patients are instructed to return for follow-up 1 month after ablation. Depending on imaging findings, clinical presentation, and type of disease, subsequent follow-up may be performed on a monthly basis or may be extended to longer intervals. There are no official follow-up recommendations.
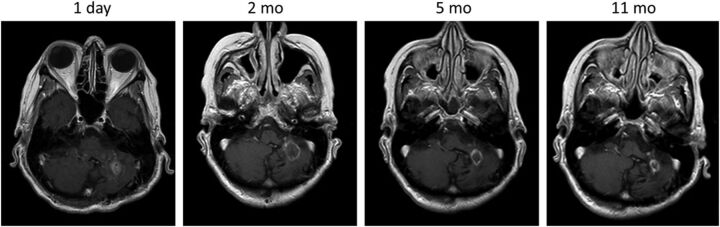
Fig 3.
T1 contrast-enhanced images demonstrating the normal evolution of a LITT-treated metastatic left cerebellar lesion, which recurred after SRS in a 70-year-old female patient with history of ovarian adenocarcinoma. Note the expected increase in the size of the treated lesion at 2-month follow-up and a steady decrease in size on subsequent follow-up.
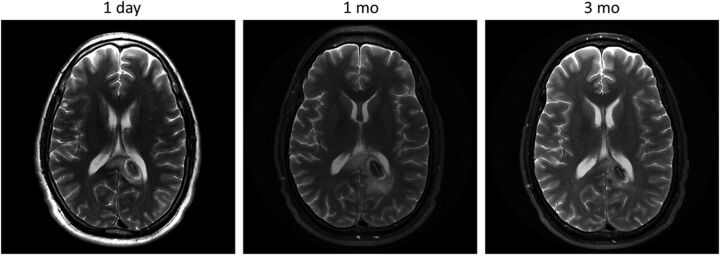
Fig 4.
T2WI demonstrating the normal evolution of an LITT-treated left splenial low-grade astrocytoma in a 30-year-old male patient with a history of type 1 neurofibromatosis. Note the expected increase in the size of the lesion and marginal zone at 1-month follow-up and subsequent decrease at 3 months.
Recurrence
Serial follow-up performed >40 days after the procedure should demonstrate a continuous decrease in the size of the ablated lesion and stable or decreased enhancement (Fig 3). Overall, the entire ablated lesion decreases to 50% of its original size within 93 days of treatment35 and continues to decrease in size for 6–15 months, becoming more homogeneous in appearance. Peripheral enhancement that persists or gradually decreases in size is a sign of the normal evolution of the ablated lesion. Any interval increase in lesion size, heterogeneity, peripheral nodular enhancement, restricted diffusion, CBF/CBV, and surrounding edema in a lesion treated >40–60 days prior should raise suspicion for recurrence (Fig 5).36,37 Recurrence usually occurs within the peripheral rim of enhancement and presents as new or enlarging peripheral enhancing nodularity (Figs 5) or simply as thickening of the rim of enhancement (Fig 6). Comparison with prior images is vital in monitoring tumor recurrence because normally evolving lesions can demonstrate asymmetric enhancement similar to that of recurring lesions. Therefore, irregularly enhancing lesions require close-interval follow-up. Any increase in size, enhancement, or surrounding edema should be further assessed with adjunctive techniques such as MR spectroscopy, perfusion imaging, or PET.
Fig 5.
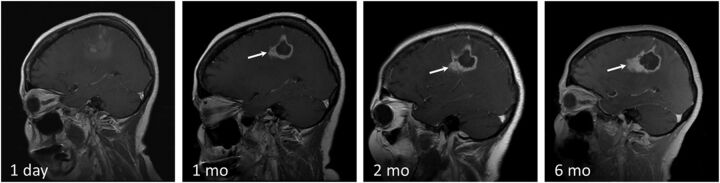
Disease recurrence after treatment with LITT. T1 contrast-enhanced images in a 58-year-old man with GBM status post surgical excision, chemoradiation, and SRS for a recurrent left parietal lobe lesion. The second recurrence was treated with LITT. Note irregular peripheral nodular enhancement at 1-month follow-up (white arrow), which progressively increases in size at 2 and 6 months. Findings are consistent with disease recurrence.
Fig 6.
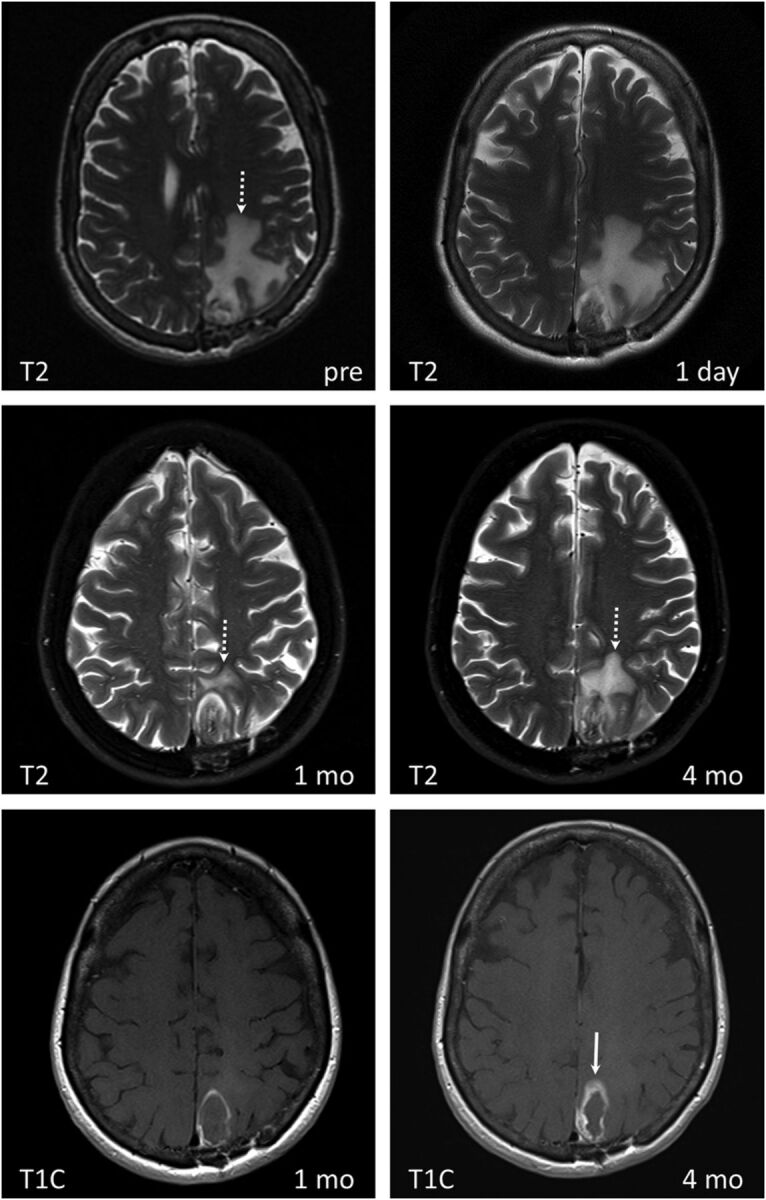
LITT of radiation necrosis with subsequent disease recurrence in a 68-year-old female patient with lung squamous cell carcinoma status post surgical excision and SRS of a metastatic brain lesion in the left parietal lobe, which subsequently resulted in radiation necrosis. Medically intractable radiation necrosis was treated with LITT. Pre-LITT imaging demonstrated an enhancing lesion in the left parietal lobe on T1 contrast-enhanced (not shown) with significant vasogenic edema on T2 images (dashed white arrow on T2 image labeled “pre”). Dynamic imaging pre-LITT (not shown) did not demonstrate a significant increase in CBF or CBV. The patient was not treated with bevacizumab, and RN was favored over recurrence. Note a significant decrease in vasogenic edema 1 month after treatment (dashed white arrow), coinciding with symptomatic improvement. T2 images obtained at 4-month follow-up demonstrate a significant increase in peritumoral vasogenic edema. There is significant thickening of the peripheral zone of enhancement (white arrow) on T1 contrast-enhanced images at 4 months compared with 1 month. Findings are concerning for tumor recurrence within a treated RN lesion, which was corroborated on PET CT (not shown).
Clinical Applications
Studies during the past 20 years report the use of LITT to treat a variety of brain lesions. The most studied lesions include glioma2,30,31,33,38–48 and metastases.2,18,29,32,40,41,44,49–51 Epilepsy51–55 and radiation necrosis50,56 represent a much smaller subset of treated lesions reported in the literature. Additionally, MRgLITT has been used to treat refractory cerebral edema57 and tumors such as ependymoma, meningioma, primitive neuroectodermal tumor, hemangioblastoma, and chordoma.31,58 The survival benefits of LITT after treatment of various brain lesions vary from favorable to statistically insignificant. Evidence is limited because to date, all studies consist of noncontrolled, nonrandomized retrospective reports, case series, or case reports, thus predisposing to selection bias. Many of the studies mix multiple disease entities to increase the number of enrolled subjects; this mixture makes the evaluation of survival benefits for a given disease entity difficult. Another major limitation is the use of variable inclusion criteria by selecting patients with either recurrent, newly diagnosed, previously treated, or untreated tumors or a mixture of any of the above. However, the above studies offer a plethora of evidence on the safety profile of the procedure. The variable complications of MRgLITT will be addressed below after a brief discussion of the 4 most common indications for the procedure: gliomas, metastatic lesions, radiation necrosis, and medically intractable epilepsy.
Gliomas
Gliomas are subdivided into multiple subtypes, of which GBM is the most common primary brain neoplasm in adults. Median survival of newly diagnosed patients after treatment with maximal safe resection, radiation therapy, and adjuvant chemotherapy is 12–15 months.59 Survival decreases with inoperable deep-seated lesions. GBM poses multiple treatment challenges due to its diffusely infiltrative nature and strong resistance to therapy. As a result, all GBMs recur, and median survival after recurrence is 3–5 months.60 Surgical resection increases survival but is not feasible in cases of difficult-to-access tumors, thus the need for minimally invasive surgical procedures. The use of SRS in newly diagnosed GBM demonstrated no survival benefit,61 while data on MRgLITT are inconclusive. Local therapies, such as MRgLITT, SRS, or open surgical resection, do not address the infiltrative component of GBM and can only be used for palliative/salvage therapy. Examples of MRgLITT-treated gliomas are provided in Figs 4 and 5.
Metastatic Brain Tumors
Metastatic brain tumors are 10 times more prevalent than primary brain tumors, accounting for approximately 200,000 cases of the total of 225,000 cases of brain tumors per year.32 The incidence of brain metastases is increasing due to effective oncologic treatments of primary malignancies, resulting in longer survival. The first-line treatment for new brain metastatic lesions is radiation therapy (with SRS, whole-brain radiation therapy, or both) and surgical resection.29 Local recurrence within 1 year of treatment is approximately 10% after resection and radiation therapy, compared with 46% after surgical resection alone.62 Similar to GBM, no consensus exists on the treatment of recurrent metastatic brain lesions, and repeat use of radiation therapy is limited due to concerns over adverse cumulative radiation effects. To date, studies have shown that MRgLITT is a safe, minimally invasive alternative. Further evidence is needed to determine whether there are definitive survival benefits over currently accepted treatments. Examples of treated metastatic lesions are provided in Figs 1–3 and 6.
Radiation Necrosis
Radiation necrosis is a common entity in neuro-oncology. Estimated incidence varies between 5% and 10% for all radiation therapy modalities, but the risk may be as high as 50% in the setting of prior SRS, particularly at treatment doses between 16 and 22 Gy.9 RN, also known as delayed neurotoxicity, represents a specific type of radiation injury and occurs at least 3 months after radiation therapy. The process is irreversible, and approximately 85% of cases occur within 2 years of treatment.10 Histologically, RN consists of a central zone of necrosis surrounded by a peripheral zone of altered astrocytes, which release large quantities of proinflammatory factors such as hypoxia-inducing factor 1α and vascular endothelial growth factor, inducing severe inflammation and edema.63 First-line treatment is steroids to decrease inflammation. A new effective non-FDA-approved treatment is bevacizumab, a humanized mouse monoclonal vascular endothelial growth factor antibody acting as vascular endothelial growth factor inhibitor, though its use is limited due to high cost, increased risk of deep venous thrombosis and pulmonary emboli, and the need to stop other concurrent systemic therapy.9 Surgical resection is reserved for medically refractory RN, but its role is limited in the setting of deep or difficult-to-access lesions and in patients with multiple comorbidities who are unable to tolerate general anesthesia. LITT induces resolution of RN,50,56 but long-term data are limited due to low numbers and lack of sufficient long-term follow-up. The postulated mechanism of action of LITT in the setting of RN is ablation of the peripheral zone of altered astrocytes, thus terminating the proinflammatory signaling cascade induced by vascular endothelial growth factor.9 Figure 6 demonstrates an example of MRgLITT-treated RN in which metastatic disease subsequently recurred.
Epilepsy
Medically intractable focal epilepsy is generally treated with surgical resection of epileptic foci. Given the deep location of many seizure foci, surgical resection can be difficult and can leave patients with persistent neurologic and cognitive deficits secondary to collateral tissue damage. Available minimally invasive treatment options such as SRS64,65 and RF ablation65 offer the potential benefit of decreasing iatrogenic complications, but the inability to monitor ablation in real-time diminishes their margin of safety. A pilot study by Curry et al52 first demonstrated the feasibility of MRgLITT in the successful treatment of medically intractable epileptic foci. To date, studies report laser ablation of tuberous sclerosis, hypothalamic hamartoma, mesial temporal sclerosis, cortical dysplasia, and periventricular nodular hyperplasia with follow-up ranging from 2 to 13 months.51–54 Seven of a total of 9 patients remained seizure-free at 6- to 13-month follow-up, depending on the study. The remaining 2 patients experienced seizure recurrence at 2 and 3 months after LITT and underwent subsequent definitive open surgical treatment.52,54 This result suggests that MRgLITT does not preclude future invasive therapy. Given these findings, Esquenazi et al54 postulated that laser ablation could be used as a first-line treatment for deep-seated medically intractable epileptic foci and that surgical resection be reserved for patients who fail therapy with LITT. More recently, a report by Willie et al55 showed that seizure-free rates for mesiotemporal epilepsy by using MRgLITT closely approximate those of open temporal lobectomies while potentially improving postprocedural neurocognitive outcomes. Figure 7 provides an imaging example of ablation of the left hippocampo-amygdalar area to treat mesial temporal sclerosis.
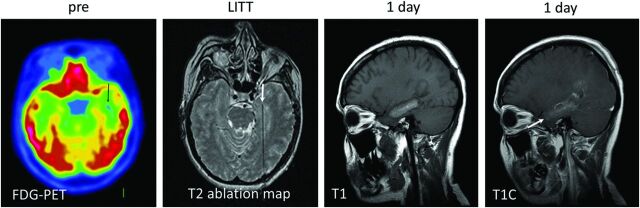
Fig 7.
Mesial temporal sclerosis confirmed on preprocedural FDG-PET (black arrow), which showed decreased FDG uptake in the left hippocampal/parahippocampal region. The lesion was not noted on prior contrast-enhanced MR imaging and did not demonstrate enhancement (not shown). Intraprocedural T2 ablation map demonstrates the laser probe tip within the left medial temporal lobe (white arrow). T1 and T1 contrast-enhanced images 24 hours after LITT demonstrate an oval, rather than round, postablation lesion with signal characteristics similar to those of contrast-enhancing lesions (see Fig 2). The elongated shape is due to sequential probe retraction during ablation to cover the entire left hippocampo-amygdalar area.
Complications
Although the survival benefits and clinical outcomes of MRgLITT are difficult to estimate on the basis of the currently available studies, enough data are available to evaluate the safety profile of the procedure. We performed an analysis of all LITT studies conducted on human subjects to date with complications as an end point. We tallied the different complications and calculated their rates. The most common reported complication of LITT is transient neurologic deficit, accounting for 13% of all complications. Reported symptoms include dysphagia, weakness, hemianopsia, or minor seizures. The symptoms either resolved spontaneously or responded to steroid administration within days to weeks.
The next most common complications include new progressive or permanent neurologic symptoms (3%), intracranial hemorrhage (2.5%), infection (2.5%), and deep venous thrombosis (2.5%). Life-threatening complications include intracranial hemorrhage, ventriculitis, meningitis, and 1 case of refractory intracranial hypertension after simultaneous use of multiple probes to treat a large irregular lesion.31,33,48,51 Two deaths have been reported, both in patients with GBM, one from intractable intracranial hemorrhage and the other from meningitis.48,51 Sloan et al33 suggested that pretreatment MRA or CTA and fiber-tract imaging with DTI may be useful modalities in presurgical planning to identify and potentially avoid critical vascular and white matter structures. Jethwa et al58 recommended that LITT treatment of lesions of >3 cm should be staged. Administration of high-dose preprocedural steroids should be considered.31
Conclusions
LITT appears to be a safe palliative alternative for the treatment of malignant high-grade gliomas and recurrent metastatic lesions. While several life-threatening complications have been reported in the setting of GBM, preliminary data suggest that their overall incidence is acceptably low. Furthermore, the incidence of such complications may be decreased with appropriate preoperative imaging and as neurosurgeons overcome the steep learning curve associated with the procedure. Because research on the use of MRgLITT is in its infancy, indications and contraindications are relative and are still being worked out. The most common postprocedural complications of MRgLITT are non-life-threatening and transient. LITT may also provide a safe curative option in cases of radiation necrosis and in certain types of medically intractable epilepsy.
ABBREVIATIONS:
- GBM
glioblastoma multiforme
- LITT
laser interstitial thermal therapy
- MRgLITT
MRI-guided laser interstitial thermal therapy
- MRTI
MRI thermal imaging
- RN
delayed radiation necrosis
- SRS
stereotactic radiosurgery
REFERENCES
- 1. Bown S. Phototherapy in tumors. World J Surg 1983;7:700–09 [DOI] [PubMed] [Google Scholar]
- 2. Sugiyama K, Sakai T, Fujishima I, et al. Stereotactic interstitial laser-hyperthermia using Nd-YAG laser. Stereotact Funct Neurosurg 1990;54:501–05 [DOI] [PubMed] [Google Scholar]
- 3. Sanai N, Polley MY, McDermott MW, et al. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 2011;115:3–8 [DOI] [PubMed] [Google Scholar]
- 4. Simpson JR, Horton J, Scott C, et al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys 1993;26:239–44 [DOI] [PubMed] [Google Scholar]
- 5. Vuorinen V, Hinkka S, Färkkilä M, et al. Debulking or biopsy of malignant glioma in elderly people—a randomised study. Acta Neurochir (Wien) 2003;145:5–10 [DOI] [PubMed] [Google Scholar]
- 6. Loeffler JS, Barker FG, Chapman PH. Role of radiosurgery in the management of central nervous system metastases. Cancer Chemother Pharmacol 1999;43(suppl):S11–14 [DOI] [PubMed] [Google Scholar]
- 7. Young RF. Radiosurgery for the treatment of brain metastases. Semin Surg Oncol 1998;14:70–78 [DOI] [PubMed] [Google Scholar]
- 8. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 2006;295:2483–91 [DOI] [PubMed] [Google Scholar]
- 9. Rahmathulla G, Marko NF, Weil RJ. Cerebral radiation necrosis: a review of the pathobiology, diagnosis and management considerations. J Clin Neurosci 2013;20:485–502 [DOI] [PubMed] [Google Scholar]
- 10. Shah R, Vattoth S, Jacob R, et al. Radiation necrosis in the brain: imaging features and differentiation from tumor recurrence. Radiographics 2012;32:1343–59 [DOI] [PubMed] [Google Scholar]
- 11. Dequesada IM, Quisling RG, Yachnis A, et al. Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery 2008;63:898–903; discussion 904 [DOI] [PubMed] [Google Scholar]
- 12. Kano H, Kondziolka D, Lobato-Polo J, et al. T1/T2 matching to differentiate tumor growth from radiation effects after stereotactic radiosurgery. Neurosurgery 2010;66:486–91; discussion 491–92 [DOI] [PubMed] [Google Scholar]
- 13. Barajas RF, Chang JS, Sneed PK, et al. Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol 2009;30:367–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitsuya K, Nakasu Y, Horiguchi S, et al. Perfusion weighted magnetic resonance imaging to distinguish the recurrence of metastatic brain tumors from radiation necrosis after stereotactic radiosurgery. J Neurooncol 2010;99:81–88 [DOI] [PubMed] [Google Scholar]
- 15. Chernov M, Hayashi M, Izawa M, et al. Differentiation of the radiation-induced necrosis and tumor recurrence after gamma knife radiosurgery for brain metastases: importance of multi-voxel proton MRS. Minim Invasive Neurosurg 2005;48:228–34 [DOI] [PubMed] [Google Scholar]
- 16. Mullins ME, Barest GD, Schaefer PW, et al. Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR Am J Neuroradiol 2005;26:1967–72 [PMC free article] [PubMed] [Google Scholar]
- 17. Forsyth PA, Kelly PJ, Cascino TL, et al. Radiation necrosis or glioma recurrence: is computer-assisted stereotactic biopsy useful? J Neurosurg 1995;82:436–44 [DOI] [PubMed] [Google Scholar]
- 18. Rao MS, Hargreaves EL, Khan AJ, et al. Magnetic resonance-guided laser ablation improves local control for postradiosurgery recurrence and/or radiation necrosis. Neurosurgery 2014;74:658–67 [DOI] [PubMed] [Google Scholar]
- 19. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000;342:314–19 [DOI] [PubMed] [Google Scholar]
- 20. Téllez-Zenteno JF, Hernández Ronquillo L, Moien-Afshari F, et al. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res 2010;89:310–18 [DOI] [PubMed] [Google Scholar]
- 21. Larson TR, Bostwick DG, Corica A. Temperature-correlated histopathologic changes following microwave thermoablation of obstructive tissue in patients with benign prostatic hyperplasia. Urology 1996;47:463–69 [DOI] [PubMed] [Google Scholar]
- 22. Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol 2000;174:323–31 [DOI] [PubMed] [Google Scholar]
- 23. Yaroslavsky AN, Schulze PC, Yaroslavsky IV, et al. Optical properties of selected native and coagulated human brain tissues in vitro in the visible and near infrared spectral range. Phys Med Biol 2002;47:2059–73 [DOI] [PubMed] [Google Scholar]
- 24. McNichols RJ, Gowda A, Kangasniemi M, et al. MR thermometry-based feedback control of laser interstitial thermal therapy at 980 nm. Lasers Surg Med 2004;34:48–55 [DOI] [PubMed] [Google Scholar]
- 25. Rieke V, Butts Pauly K. MR thermometry. J Magn Reson Imaging 2008;27:376–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Poorter J, De Wagter C, De Deene Y, et al. Noninvasive MRI thermometry with the proton resonance frequency (PRF) method: in vivo results in human muscle. Magn Reson Med 1995;33:74–81 [DOI] [PubMed] [Google Scholar]
- 27. De Poorter J. Noninvasive MRI thermometry with the proton resonance frequency method: study of susceptibility effects. Magn Reson Med 1995;34:359–67 [DOI] [PubMed] [Google Scholar]
- 28. Quesson B, de Zwart JA, Moonen CT. Magnetic resonance temperature imaging for guidance of thermotherapy. J Magn Reson Imaging 2000;12:525–33 [DOI] [PubMed] [Google Scholar]
- 29. Carpentier A, McNichols RJ, Stafford RJ, et al. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery 2008;63(1 suppl 1):ONS21–28; discussion ONS28–29 [DOI] [PubMed] [Google Scholar]
- 30. Mohammadi AM, Schroeder JL. Laser interstitial thermal therapy in treatment of brain tumors–the NeuroBlate system. Expert Rev Med Devices 2014;11:109–19 [DOI] [PubMed] [Google Scholar]
- 31. Jethwa PR, Barrese JC, Gowda A, et al. Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: initial experience. Neurosurgery 2012;71(1 suppl operative):133–44; 144–45 [DOI] [PubMed] [Google Scholar]
- 32. Carpentier A, McNichols RJ, Stafford RJ, et al. Laser thermal therapy: real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg Med 2011;43:943–50 [DOI] [PubMed] [Google Scholar]
- 33. Sloan AE, Ahluwalia MS, Valerio-Pascua J, et al. Results of the NeuroBlate System first-in-humans phase I clinical trial for recurrent glioblastoma: clinical article. J Neurosurg 2013;118:1202–19 [DOI] [PubMed] [Google Scholar]
- 34. Schober R, Bettag M, Sabel M, et al. Fine structure of zonal changes in experimental Nd:YAG laser-induced interstitial hyperthermia. Lasers Surg Med 1993;13:234–41 [DOI] [PubMed] [Google Scholar]
- 35. Schwabe B, Kahn T, Harth T, et al. Laser-induced thermal lesions in the human brain: short- and long-term appearance on MRI. J Comput Assist Tomogr 1997;21:818–25 [DOI] [PubMed] [Google Scholar]
- 36. Tracz RA, Wyman DR, Little PB, et al. Magnetic resonance imaging of interstitial laser photocoagulation in brain. Lasers Surg Med 1992;12:165–73 [DOI] [PubMed] [Google Scholar]
- 37. Tracz RA, Wyman DR, Little PB, et al. Comparison of magnetic resonance images and the histopathological findings of lesions induced by interstitial laser photocoagulation in the brain. Lasers Surg Med 1993;13:45–54 [DOI] [PubMed] [Google Scholar]
- 38. Bettag M, Ulrich F, Schober R, et al. Stereotactic laser therapy in cerebral gliomas. Acta Neurochirurgica 1992;52:81–83 [DOI] [PubMed] [Google Scholar]
- 39. Ascher P, Justich E, Schröttner O. A new surgical but less invasive treatment of central brain tumours preliminary report. Acta Neurochirurgica 1992;52:78–80 [DOI] [PubMed] [Google Scholar]
- 40. Roux FX, Merienne L, Fallet-Bianco C, et al. Stereotaxic laser interstitial thermotherapy: a new alternative in the therapeutic management of some brain tumors [in French]. Neurochirurgie 1992;38:238–44 [PubMed] [Google Scholar]
- 41. Kahn T, Bettag M, Ulrich F, et al. MRI-guided laser-induced interstitial thermotherapy of cerebral neoplasms. J Comput Assist Tomogr 1994;18:519–32 [DOI] [PubMed] [Google Scholar]
- 42. Leonardi MA, Lumenta CB, Gumprecht HK, et al. Stereotactic guided laser-induced interstitial thermotherapy (SLITT) in gliomas with intraoperative morphologic monitoring in an open MR-unit. Minim Invasive Neurosurg 2001;44:37–42 [DOI] [PubMed] [Google Scholar]
- 43. Leonardi MA, Lumenta CB. Stereotactic guided laser-induced interstitial thermotherapy (SLITT) in gliomas with intraoperative morphologic monitoring in an open MR: clinical experience. Minim Invasive Neurosurg 2002;45:201–07 [DOI] [PubMed] [Google Scholar]
- 44. Schulze P, Vitzthum H, Goldammer A, et al. Laser-induced thermotherapy of neoplastic lesions in the brain–underlying tissue alterations, MRI-monitoring and clinical applicability. Acta Neurochir (Wein) 2004;146:803–12 [DOI] [PubMed] [Google Scholar]
- 45. Schwarzmaier HJ, Eickmeyer F, von Tempelhoff W, et al. MR-guided laser irradiation of recurrent glioblastomas. J Magn Reson Imaging 2005;22:799–803 [DOI] [PubMed] [Google Scholar]
- 46. Schwarzmaier HJ, Eickmeyer F, von Tempelhoff W, et al. MR-guided laser-induced interstitial thermotherapy of recurrent glioblastoma multiforme: preliminary results in 16 patients. Eur J Radiol 2006;59:208–15 [DOI] [PubMed] [Google Scholar]
- 47. Carpentier A, Chauvet D, Reina V, et al. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers Surg Med 2012;44:361–68 [DOI] [PubMed] [Google Scholar]
- 48. Mohammadi AM, Hawasli AH, Rodriguez A, et al. The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: a multicenter study. Cancer Med 2014;3:971–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hawasli AH, Ray WZ, Murphy RK, et al. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for subinsular metastatic adenocarcinoma: technical case report. Neurosurgery 2012;70(2 suppl operative):332–37; discussion 338 [DOI] [PubMed] [Google Scholar]
- 50. Rahmathulla G, Recinos PF, Valerio JE, et al. Laser interstitial thermal therapy for focal cerebral radiation necrosis: a case report and literature review. Stereotact Funct Neurosurg 2012;90:192–200 [DOI] [PubMed] [Google Scholar]
- 51. Hawasli AH, Bagade S, Shimony JS, et al. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for intracranial lesions: single-institution series. Neurosurgery 2013;73:1007–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Curry DJ, Gowda A, McNichols RJ, et al. MR-guided stereotactic laser ablation of epileptogenic foci in children. Epilepsy Behav 2012;24:408–14 [DOI] [PubMed] [Google Scholar]
- 53. Tovar-Spinoza Z, Carter D, Ferrone D, et al. The use of MRI-guided laser-induced thermal ablation for epilepsy. Childs Nerv Syst 2013;29:2089–94 [DOI] [PubMed] [Google Scholar]
- 54. Esquenazi Y, Kalamangalam GP, Slater JD, et al. Stereotactic laser ablation of epileptogenic periventricular nodular heterotopia. Epilepsy Res 2014;108:547–54 [DOI] [PubMed] [Google Scholar]
- 55. Willie JT, Laxpati NG, Drane DL, et al. Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery 2014;74:569–84; discussion 584–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Torres-Reveron J, Tomasiewicz HC, Shetty A, et al. Stereotactic laser induced thermotherapy (LITT): a novel treatment for brain lesions regrowing after radiosurgery. J Neurooncol 2013;113:495–503 [DOI] [PubMed] [Google Scholar]
- 57. Fabiano AJ, Alberico RA. Laser-interstitial thermal therapy for refractory cerebral edema from post-radiosurgery metastasis. World Neurosurg 2014;81:652.e1–4 [DOI] [PubMed] [Google Scholar]
- 58. Jethwa PR, Lee JH, Assina R, et al. Treatment of a supratentorial primitive neuroectodermal tumor using magnetic resonance-guided laser-induced thermal therapy. J Neurosurg Pediatr 2011;8:468–75 [DOI] [PubMed] [Google Scholar]
- 59. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96 [DOI] [PubMed] [Google Scholar]
- 60. Barker FG 2nd, Chang SM, Gutin PH, et al. Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery 1998;42:709–20; discussion 720–23 [DOI] [PubMed] [Google Scholar]
- 61. Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys 2004;60:853–60 [DOI] [PubMed] [Google Scholar]
- 62. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998;280:1485–89 [DOI] [PubMed] [Google Scholar]
- 63. Nonoguchi N, Miyatake S, Fukumoto M, et al. The distribution of vascular endothelial growth factor-producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles. J Neurooncol 2011;105:423–31 [DOI] [PubMed] [Google Scholar]
- 64. Quigg M, Barbaro NM. Stereotactic radiosurgery for treatment of epilepsy. Arch Neurol 2008;65:177–83 [DOI] [PubMed] [Google Scholar]
- 65. Liscak R, Malikova H, Kalina M, et al. Stereotactic radiofrequency amygdalohippocampectomy in the treatment of mesial temporal lobe epilepsy. Acta Neurochir (Wien) 2010;152:1291–98 [DOI] [PubMed] [Google Scholar]