We all know the infratentorial arteries are never involved in Moyamoya disease whereas the posterior cerebral ones are commonly affected. These authors sought to determine whether age-related differences in clinical manifestations were associated with age-related angiographic differences in 78 patients. In patients younger than 4 years of age, the prevalence of infarctions on MR images was highest, and along with severity of steno-occlusive lesions of the posterior cerebral artery, the prevalence was significantly higher than in older children (aged 4–7 years). The severity of steno-occlusive lesions in the internal carotid artery and the degree of transdural collaterals did not differ significantly. The prevalence of infarctions did not differ significantly in older children whereas ICA and PCA lesions and transdural collaterals correlated positively with diagnostic age. In conclusion, the high prevalence of infarctions diagnosed before 4 years of age and is associated with advanced steno-occlusive lesions of the PCA. In patients 4 years and older at diagnosis, transdural collaterals develop in parallel with advancement of ICA and PCA lesions, which may contribute to the nearly constant prevalence of completed strokes with permanent neurologic deficit.
Abstract
BACKGROUND AND PURPOSE:
At diagnosis, the primary clinical manifestations of pediatric Moyamoya disease are TIA or CSs. CSs are reported to be more prevalent in younger than in older children. We sought to determine whether age-related differences in clinical manifestations are associated with age-related angiographic differences.
MATERIALS AND METHODS:
We divided 78 patients diagnosed with Moyamoya disease before 16 years of age into four 4-year age groups and examined the relationships between age at diagnosis and clinical manifestations and angiographic and MR imaging findings.
RESULTS:
Among the 4 diagnostic age groups, in those younger than 4 years of age, the prevalence of CSs and of infarctions on MR images was highest, and along with severity of steno-occlusive lesions of the PCA, the prevalence was significantly higher than that in the next diagnostic age group (4–7 years), though the severity of steno-occlusive lesions in the ICA and the degree of transdural collaterals did not differ significantly. The prevalence of CSs and infarctions did not differ significantly in the 3 oldest diagnostic age groups, whereas ICA and PCA lesions and transdural collaterals correlated positively with diagnostic age.
CONCLUSIONS:
The high prevalence of CSs and infarctions in patients diagnosed before 4 years of age is associated with advanced steno-occlusive lesions of the PCA. In patients 4 years of age and older at diagnosis, transdural collaterals develop in parallel with advancement of ICA and PCA lesions, which may contribute to the nearly constant prevalence of CSs.
Moyamoya disease is an idiopathic disorder in which the terminal segments of the ICAs undergo progressive steno-occlusive changes, and abnormally dilated net-like vessels (Moyamoya) develop at the base of the brain.1 The PCAs are often involved following advancement of ICA lesions,2–5 and transdural collaterals frequently develop from the ophthalmic artery or the ECA to compensate for the reduced intracerebral anterior and posterior circulation.1,6
In pediatric (0–15 years of age) patients with Moyamoya disease, the primary clinical manifestations at diagnosis are TIAs and CSs.6–14 Studies of the clinical manifestations have found that CSs occur more frequently in patients younger than those who develop TIAs.7,9 A recent study that reported a higher prevalence of CSs and more progressive clinical courses in patients younger than 3 years of age compared with patients 3–6 years of age observed no substantial difference between patients 3–6 years of age and those 7–16 years of age.13 The authors therefore recommended early or aggressive surgical treatment in children younger than 3 years of age.
We investigated whether the clinical features seen in very young patients are causally linked to age-related vascular differences on conventional angiography or MR angiography. Such a causal relationship would affect therapeutic strategies in younger children with Moyamoya disease.
In this study, we divided 78 patients diagnosed with Moyamoya disease before 16 years of age into four 4-year age groups and examined the relationships between age at diagnosis and clinical manifestations and angiographic and MR imaging findings, focusing mainly on the characteristics of patients diagnosed at the youngest age (before 4 years of age).
Materials and Methods
Patients
We evaluated 78 consecutive Japanese patients younger than 16 years of age (32 boys, 46 girls; median age, 7 years [25th percentile, 4.75 years; 75th percentile, 10.25 years]), who were definitively diagnosed with Moyamoya disease at our institution or affiliated hospitals between 1997 and 2008 and were registered at the time of diagnosis with the Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan (hereafter referred to as Ministry Research Committee).10,11
Clinical Manifestations at Diagnosis
In all 78 patients, we reviewed the clinical manifestations at diagnosis registered with the Ministry Research Committee,10,11 and on the basis of symptoms and MR imaging findings, either of 2 neurosurgeons classified the clinical manifestations as TIA (n = 44), CS (n = 32), epilepsy (n = 1), intracranial hemorrhage (n = 1), other (n = 0), or asymptomatic (n = 0). “TIA” was defined as symptoms lasting <24 hours and CS, as symptoms lasting >24 hours. We used each patient's clinical manifestations as a categoric variable.
Evaluation of Angiographic and MR Imaging Findings at Diagnosis
On the basis of the Ministry Review Committee guidelines, 66 of the 78 patients were diagnosed by conventional angiographic findings and 12, by time-of-flight MR angiograms plus MR imaging without conventional angiography.10,12 Using angiographic staging as defined in the literature, we evaluated steno-occlusive ICA lesions (stages I-IV5) and PCA lesions (stages 1–44) on conventional or MR angiograms. The higher ICA stage was accompanied by more advanced steno-occlusive lesions in the ICA system, or the higher PCA stage was accompanied by more advanced the steno-occlusive lesions in the PCA system. PCA stage 1 represented no steno-occlusive changes in the artery. We used ICA and PCA stages evaluated in each hemisphere as ordinal variables in this study.
We determined the number of transdural collaterals supplying individual hemispheres bilaterally in 66 conventional carotid arteriograms and classified them into 3 groups by their originating arteries: ophthalmic artery and medial branches of the maxillary artery, anterior branches of the ECA, and posterior branches of the ECA. Anterior branches of the ECA included lateral branches of the maxillary artery, the anterior branch of the middle meningeal artery, and the frontal branch of the superficial temporal artery. The posterior branches of the ECA included the posterior branch of the middle meningeal artery, the parietal branch of the superficial temporal artery, the occipital artery, and the posterior meningeal artery. Each group of collaterals in 1 hemisphere included collaterals from the contralateral and ipsilateral originating arteries. We used the number of collaterals counted according to the arterial groups involved (4 grades, 0–3) in each hemisphere as an ordinal variable.
In all 78 patients, we reviewed MR images within 1 month of conventional or MR angiography. MR images included T1-weighted, T2-weighted, fluid-attenuated inversion recovery, and diffusion-weighted images. We divided the right and left cerebral hemispheres into the basal ganglia, the thalamus, and 6 corticosubcortical zones. The 6 corticosubcortical zones included the territory of the ACA, the ant-watershed, the ant-MCA, the post-MCA, the postwatershed, and the territory of the PCA from anterior to posterior in the hemisphere. The ant-MCA and post-MCA were divided at the central sulcus, and the temporal lobe was included in the post-MCA. We used the number of infarcted zones, including the basal ganglia and thalamus, and the distribution of the infarcted zones, excluding the basal ganglia and thalamus (corticosubcortical infarcted zones), as ordinal variables in each hemisphere.
Two radiologists (S.M. and S.H.), blinded to patient identity and clinical manifestations, evaluated MR images and either conventional or MR angiograms. MR images were interpreted without knowledge of either conventional or MR angiographic findings. When interpretations were inconsistent, final evaluation was reached by consensus. Initial interobserver agreement between the 2 radiologists was 84% in interpreting MR images and 90% in interpreting conventional or MR angiograms. The intraobserver agreement of the first radiologist was 90% in interpreting MR images and 88% in interpreting conventional or MR angiograms. The intraobserver agreement of the second radiologist was 85% in interpreting MR images and 90% in interpreting conventional or MR angiograms.
Age at Time of Diagnosis
Diagnostic age was defined as the age at diagnosis based on conventional or MR angiography findings. In an earlier study, Kim et al13 divided the age categories unevenly into 3 groups (younger than 3 years, 3–6 years, 7–16 years of age) without a clear explanation. In contrast, to avoid such arbitrary groups, we assigned our pediatric patients, whose ages ranged from 10 months to 15 years, into 1 of 4 diagnostic age groups of 4 years each before analysis: younger than 4 years (14 patients; median age, 2 years); 4–7 years (29 patients; median age, 6 years); 8–11 years (21 patients; median age, 9 years); and 12–15 years (14 patients; median age, 12 years). We used each patient's diagnostic age group as a categoric or ordinal variable.
Analysis and Statistics
We measured 7 variables–diagnostic age and clinical manifestations in each patient and 5 imaging findings in each hemisphere (ICA stage, PCA stage, number of transdural collaterals, number of infarcted zones, and distribution of corticosubcortical infarcted zones). Therefore, variables did not correspond 1 to 1. In addition, the corticosubcortical infarcted zones were multiple-choice variables (for example, ant-watershed and post-MCA in 1 hemisphere) and could be determined only in the limited hemispheres with corticosubcortical infarctions. To minimize the impact of such variations on statistical analysis and to determine the possible correspondence between age-related differences in clinical manifestations and imaging findings, we analyzed the 6 single factors, treating diagnostic age as an independent variable and the other 6 variables as dependent variables.
A recent study showed that CSs were more prevalent in the youngest patient age group (younger than 3 years of age) than in the next older age group (3–6 years) but not substantially different between patients 3–6 years and those 7–16 years of age.13 In this study, therefore, we focused mainly on the characteristics of the youngest age group (diagnosed before 4 years of age).
To avoid exploratory analysis as far as possible, we analyzed statistics according to the analyses plan in common among all the 6 single-factor analyses. First, among the 4 diagnostic age groups, we used the Fisher exact test to compare clinical manifestations and the Kruskal-Wallis test to compare the aforementioned 5 imaging findings. When we observed significant differences among the 4 diagnostic age groups, between patients diagnosed before 4 years of age and those diagnosed from 4 to 7 years, we used the Fisher exact test to assess the differences in clinical manifestations and the Wilcoxon rank sum test to assess the differences in imaging findings. Among the 3 oldest diagnostic age groups, we used the Fisher exact test to assess the differences in clinical manifestations, the Kruskal-Wallis test to assess the differences in imaging findings, and the Spearman rank correlation to test the correlation between diagnostic age and imaging findings.
Statistical significance was defined as 2-tailed P < .05. The Fisher exact test was performed by using R, Version 2.8.1 (http://cran.md.tsukuba.ac.jp/bin/windows/) and the Kruskal-Wallis and Wilcoxon rank sum tests, by using JMP 6.0 J software for Windows (SAS Institute, Cary, North Carolina).
Results
Among the 4 diagnostic age groups, we detected significant differences in all 6 dependent variables: clinical manifestations (Fig 1A, P = .0040), number of infarcted zones (Fig 1B, P = .0020), distribution of corticosubcortical infarcted zones (Fig 1C, P = .0258), ICA stage (Fig 1D, P = .0009), PCA stage (Fig 1E, P = .0132), and degree of transdural collaterals (Fig 1F, P = .0033).
Fig 1.
The relationship between the 4 diagnostic age groups and clinical manifestations (A), number of infarcted zones (B), distribution of corticosubcortical infarcted zones (C), ICA staging (D), PCA staging (E), and number of transdural collaterals (F). Asterisks indicate significant differences. Double asterisks indicate a significant difference and a positive relationship to diagnostic age among the 3 oldest diagnostic age groups.
Patients diagnosed before 4 years of age had the highest prevalence of CSs (85.7%, 12 of 14 patients) and the highest number of infarcted zones. CSs (85.7%) predominated over TIAs (14.3%, 2 of 14 patients), but TIAs (62.1%, 18 of 29 patients) predominated over CSs (34.5%, 10 of 29 patients) in patients diagnosed from 4 to 7 years of age.
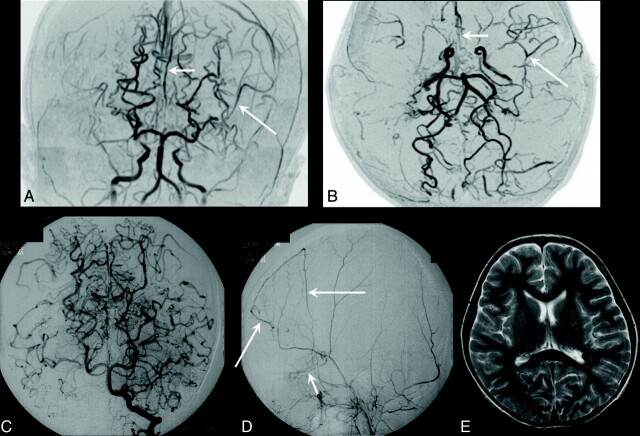
Clinical manifestations (P = .0028), number of infarcted zones (P = .0024), distribution of corticosubcortical infarcted zones (P = .0035), and PCA stages (P = .0222) differed significantly between patients diagnosed before 4 years of age and those diagnosed from 4 to 7 years, but ICA stage (P = .7081) and degree of transdural collaterals (P = .7820) did not. Figure 2 shows a typical case of a patient diagnosed before 4 years of age.
Fig 2.
A 15-month-old girl (diagnostic age group younger than 4 years of age) initially presented with CS with right upper motor paresis a month before and subsequently presented with contralateral hemiparesis CS. Moyamoya disease was diagnosed. Her clinical manifestation was CS. A and B, Coronal and axial time-of-flight MR angiograms show steno-occlusive bilateral lesions in the terminal part of the ICA and in the proximal parts of the ACA and MCA cerebral arteries (ICA stage II, bilaterally). The right PCA is almost completely occluded (short arrow), with well-developed dilated perforators around it (stage III); the proximal part of the left PCA is stenotic (long arrow, PCA stage II). Note that in this patient, the bilateral steno-occlusive changes involved the PCAs even in the hemispheres with less advanced ICA lesions (ICA stage II, bilaterally). C, Diffusion-weighted MR image shows hyperintense regions of recent infarction in the anterior and posterior watersheds and the boundary zone between the regions of the ACA and PCA in the right. Five infarcted zones are seen on the right. D, T2-weighted MR image additionally shows an old cortical infarction in the posterior MCA territory (arrow), seen on the left.
We observed infarctions in 77 (49.4%) of 156 hemispheres, which included 128 corticosubcortical zones and 4 basal ganglia. In patients diagnosed before 4 years of age, the prevalence of infarctions in ant-MCA (26.7%, 12 of 45 hemispheres with corticosubcortical infarcted zones) and post-MCA zones (33.3%, 15 of 45 hemispheres) was higher than the prevalence in those diagnosed from 4 to 7 years (17.1%, 7 of 41 hemispheres in the ant-MCA and 17.1%, 7 of 41 hemispheres in the post-MCA zone).
We observed infarctions in the basal ganglia in 4 hemispheres of 4 patients, 1 each from the age groups diagnosed before 4 years of age and at 8–11 years, and 2 from the group diagnosed from 4 to 7 years. Two hemispheres of 2 patients had infarctions in the basal ganglia without corticosubcortical infarction. In no patient did we observe infarction in the thalamus.
Among the 3 oldest diagnostic age groups, we observed neither significant differences with respect to clinical manifestations (P = .7751), number of infarcted zones (P = .6394), and distribution of corticosubcortical infarcted zones (P = .4561) nor a significant relationship to diagnostic age with respect to the number infarcted zones (P = .5059) and the distribution of corticosubcortical infarcted zones (P = .2229). In contrast, differences were significant for ICA stage (P = .0008), PCA stage (P = .0207), and degree of transdural collaterals (P = .0037); and the relationship to diagnostic age was significantly positive for ICA stage (P = .0005), PCA stage (P = .0243), and degree of transdural collaterals (P = .0007). Figure 3 shows a typical case of a patient diagnosed from 4 to 7 years; Fig 4 shows a case diagnosed from 12 to 15 years.
Fig 3.
A 7-year-old girl (diagnostic age group, 4–7 years) who had recurrent transient left hemiparesis for 5 years was diagnosed with Moyamoya disease, which manifested clinically as TIA. A and B, Coronal and axial time-of-flight MR angiograms show advanced steno-occlusive changes at or around the terminal part of the right ICA, with poorly visualized ACA and MCA branches (ICA stage III, right). Moderate steno-occlusive changes at or around the terminal part of the left ICA with relatively good visualization of the ACA (small arrows) and MCA (large arrows) cortical branches (ICA stage II) are seen on the left. No steno-occlusive lesions are seen bilaterally in the posterior cerebral artery (PCA stage 1, bilaterally). C, Anteroposterior view of the vertebral angiogram shows no steno-occlusive lesions bilaterally in the PCA and well-developed leptomeningial collateral circulation to the anterior circulation. D, Left lateral external carotid angiogram shows dilated anterior branches of the middle meningeal artery providing transdural collaterals to the contralateral frontal region (large arrows), with the medial branches of the maxillary artery providing transdural collaterals to the right anterior basal region (small arrow). Two transdural collaterals can be seen on the right, and none are seen on the left (anteroposterior view of the left external carotid angiogram, not shown). E, Axial T2-weighted MR image shows no infarction bilaterally (ie, the number of infarcted regions is zero on either side). Note that in this patient, the steno-occlusive changes do not involve the PCA, even in the right hemisphere, where ICA lesions are advanced (stage III).
Fig 4.
A 12-year-old boy (diagnostic age group, 12–15 years) with right and left transient motor paresis. His clinical manifestation was TIA. A, Axial time-of-flight MR angiogram shows advanced steno-occlusive changes bilaterally at or around the terminal parts of the ICAs, with no apparent ACA and MCA branches (ICA stage IV, bilaterally). Advanced steno-occlusive lesions bilaterally in the PCAs with well-developed Moyamoya vessels from the PCA are seen (PCA stage 3, bilaterally). B, Anteroposterior view of the vertebral angiogram shows advanced steno-occlusive lesions bilaterally in the PCAs (PCA stage III, bilaterally). C and D, Lateral view of the arterial (C) and capillary (D) phases of the right external carotid angiograms shows that the dilated posterior branch of the middle meningeal artery (large arrows) and the meningeal branch of the occipital artery (arrowhead) provide transdural collaterals mainly to the right posterior part of the cerebral hemispheres. The frontal branch of the superficial temporal artery (small arrow) and medial branches of the internal maxillary artery provide transdural collaterals to the frontobasal region. Three transdural collaterals are seen on the right. The right cerebral hemisphere is largely supplied through the transdural collaterals (D). Three transdural collaterals were seen on the left (left external carotid angiogram, not shown). E, Axial T2-weighted MR image shows an old small infarction in the left anterior MCA territory. No infarcted regions are seen on the right, but 1 is seen on the left. Note that this patient has severe steno-occlusive PCA changes that parallel the advanced ICA lesions. This progressive state should have provoked the development of transdural collaterals, which might have prevented a large infarction and CS.
Discussion
The prevalence of CSs and the number of infarcted zones were highest in patients diagnosed before 4 years of age, in whom CSs, infarctions, and PCA lesions were more prevalent than in patients diagnosed from 4 to 7 years, despite the absence of a significant difference between ICA stage and transdural collaterals between the 2 groups. Among the 3 oldest diagnostic age groups, clinical manifestations and number of infarcted zones were approximately constant, though ICA and PCA stages were higher.
The explanation for CSs being more frequent in patients diagnosed before 4 years of age is unclear, but the literature notes that children younger than 3 years of age cannot report TIA symptoms and thus appear symptomatic only when they develop CSs apparent to their caregivers.15 Although this hypothesis may be correct, it is unlikely the sole explanation for recent evidence that Moyamoya disease has a more progressive clinical course in children younger than 3 years of age than in older children, with additional CSs likely to occur even during the short interval between diagnosis and surgery.13
To explain the high prevalence of CSs prior to age 4, we propose 3 other hypotheses regarding age-related differences in PCA stage and transdural collaterals derived from the present study and in blood demand derived from the literature.
First, in the group diagnosed before 4 years of age, PCA stages were significantly more advanced than in those diagnosed from 4 to 7 years (P = .0222; Fig 1E), whereas differences in ICA stage were not significant (Fig 1D). This unique pattern of PCA involvement in patients diagnosed before 4 years of age prompted us to perform additional subgroup analysis in the 117 hemispheres with less advanced (stage I or II) ICA lesions (Fig 5). PCA stages in the group diagnosed before 4 years of age were significantly more advanced than in those diagnosed from 4 to 7 years (P = .0054). In contrast, there was neither significant difference (P = .9919) nor significant correlation (P = .9352) between diagnostic age and PCA stage among the 3 oldest diagnostic age groups. This finding indicated that in children diagnosed before 4 years of age, steno-occlusive changes involved the PCA aggressively, even in hemispheres with less advanced ICA lesions (stage I or II). In contrast, in older children, steno-occlusive changes may involve the PCA only after ICA lesions have advanced, in agreement with the positive relationship reported in the literature between the severity of ICA and PCA lesions.3,4 To our knowledge, ours are the first reported findings of differences in PCA involvement according to diagnostic age.
Fig 5.
The relationship between the 4 diagnostic age groups and PCA stage in the 117 hemispheres with less advanced steno-occlusive ICA lesions (ICA stage I or II). The asterisk indicates a significant difference between diagnostic age groups younger than 4 years and 4–7 years of age.
The distribution of corticosubcortical infarcted zones differed between the patients diagnosed before 4 years of age and those diagnosed from 4 to 7 years, but no significant difference was observed among the 3 oldest diagnostic age groups. This may be related to early involvement of the PCA, even with less advanced ICA lesions, in patients diagnosed before 4 years of age. In the group of children younger than 4 years at diagnosis, the high prevalence of infarctions, including the ant- and post-MCA zones, and the largest number of involved zones indicate the high prevalence of infarctions, particularly in the MCA territory, in this age group. In Moyamoya disease, the leptomeningeal collaterals from the uninvolved PCA generally compensate somewhat for the cerebral blood flow in the MCA territory.2,4 However, when steno-occlusive lesions also involve the PCA and decrease the leptomeningeal collaterals to the MCA territory, infarctions result in that territory.
Our second hypothesis, stemming from age-related differences we observed in the transdural collaterals, is that less developed transdural collaterals (Fig 1F) in patients diagnosed before 4 years of age may be related to the high prevalence of CSs in this age group and represent a need for further development of the collaterals. More developed collaterals found in the older groups, 8–11 years and 12–15 years of age, may be attributable to a longer interval of latency from the onset of the steno-occlusive disease process to clinical onset, which allowed the collaterals time to develop well. In contrast, in patients diagnosed before 4 years of age, the interval from the onset of the steno-occlusive disease process and clinical onset may have been too short for the full development of the transdural collaterals.
Our last hypothesis to explain the high prevalence of CSs in patients diagnosed before 4 years of age is the temporal serial changes in blood demanded by the developing brain. Previous evidence indicated that in the normally developing brain, cerebral blood flow increases beginning in the neonatal period, increases most rapidly before 4 years of age,16 reaches a maximum at approximately 6 years, and gradually decreases thereafter to the adult level.17,18 The serial changes in normal blood flow of the developing brain may derive from the increasing demand for blood characteristic of the brains of younger children and at least partially account for the highest prevalence of CSs in the very young group. This hypothesis may well explain why Moyamoya disease has a more progressive clinical course in children younger than 3 years of age.
It is unclear why the clinical manifestations and prevalence and distribution of infarction remain approximately constant among the 3 oldest diagnostic age groups despite advanced ICA and PCA stages. Our study findings indicate that the development of the transdural collaterals increase with diagnostic age, which may compensate for the decrease in cerebral blood flow from the ICA and PCA. In the normally developing brain, blood demand gradually decreases beginning at 6 years of age. Along with the development of the transdural collaterals, this serial alteration in demand may explain our observation of the relatively constant prevalence of CSs with infarctions in older children despite progressing ICA and PCA lesions.
Our study is limited by its retrospective nature and because the invasive nature of conventional arteriography precluded some patients from undergoing the procedure. In addition, to include all consecutive patients in the study, we evaluated ICA and PCA staging only on MR angiograms and did not evaluate the degree of transdural collaterals in such patients. As well, the number of transdural collaterals is an insufficient substitute for the degree of cerebral blood flow from such pathways.
Our study is further limited by our analytic methods. We only indirectly showed the relationship between age-related differences in clinical manifestations and angiographic differences. On the other hand, a direct relationship would be clarified by multivariable analysis by using hemispheric-based clinical symptoms or the number of infarcted zones as dependent variables and diagnostic age and angiographic findings as independent variables, adjusting for confounding among independent variables. In other words, such analysis would not clarify the age-related angiographic differences, including the PCA, which may explain the high prevalence of CS or infarctions in patients diagnosed before 4 years of age. Clarifying the age-related angiographical differences including PCA is the advantage of our adopting 6 single-factor analyses that treat diagnostic age as an independent variable and the other 6 variables as dependent variables.
Conclusions
Steno-occlusive PCA lesions are closely related to the presentation of CS with infarctions in children diagnosed with Moyamoya disease before 4 years of age. Despite the general neglect of the posterior circulation in the literature, our findings provide important information for therapeutic planning.
Acknowledgments
We thank Rosalyn A. Uhrig, MA, for her editorial assistance in the preparation of this manuscript.
Abbreviations
- ACA
anterior cerebral artery
- ant-MCA
anterior half of the territory of the MCA
- ant-watershed
the anterior watershed area of the ACA and MCA
- CS
completed stroke with permanent neurologic deficit
- ECA
external carotid artery
- ICA
internal carotid artery
- MCA
middle cerebral artery
- N.S.
no significant difference or no significant relationship with diagnostic age
- PCA
posterior cerebral artery
- post-MCA
posterior half of the territory of the MCA
- postwatershed
posterior watershed area of the MCA and PCA
- TIA
transient ischemic attack
References
- 1. Suzuki J, Takaku A. Cerebrovascular “Moyamoya” disease: disease showing abnormal net-like vessels in base of brain. Arch Neurol 1969;20:288–99 [DOI] [PubMed] [Google Scholar]
- 2. Miyamoto S, Kikuchi H, Karasawa J, et al. Study of the posterior circulation in Moyamoya disease: clinical and neuroradiological evaluation. J Neurosurg 1984;61:1032–37 [DOI] [PubMed] [Google Scholar]
- 3. Yamada I, Himeno Y, Suzuki S, et al. Posterior circulation in Moyamoya disease: angiographic study. Radiology 1995;197:239–46 [DOI] [PubMed] [Google Scholar]
- 4. Mugikura S, Takahashi S, Higano S, et al. The relationship between cerebral infarction and angiographic characteristics in childhood Moyamoya disease. AJNR Am J Neuroradiol 1999;20:336–43 [PMC free article] [PubMed] [Google Scholar]
- 5. Mugikura S, Takahashi S, Higano S, et al. Predominant involvement of ipsilateral anterior and posterior circulations in Moyamoya disease. Stroke 2002;33:1497–500 [DOI] [PubMed] [Google Scholar]
- 6. Suzuki J, Kodama N. Moyamoya disease: a review. Stroke 1983;14:104–09 [DOI] [PubMed] [Google Scholar]
- 7. Matsushima Y, Aoyagi M, Masaoka H, et al. Mental outcome following encephaloduroarteriosynangiosis in children with Moyamoya disease with the onset earlier than 5 years of age. Childs Nerv Syst 1990;6:440–43 [DOI] [PubMed] [Google Scholar]
- 8. Ishikawa T, Houkin K, Kamiyama H, et al. Effects of surgical revascularization on outcome of patients with pediatric Moyamoya disease. Stroke 1997;28:1170–73 [DOI] [PubMed] [Google Scholar]
- 9. Imaizumi T, Hayashi K, Saito K, et al. Long-term outcomes of pediatric Moyamoya disease monitored to adulthood. Pediatr Neurol 1998;18:321–25 [DOI] [PubMed] [Google Scholar]
- 10. Fukui M. Current state of study on Moyamoya disease in Japan. Surg Neurol 1997;47:138–43 [DOI] [PubMed] [Google Scholar]
- 11. Ikezaki K. Clinical manifestations: epidemiology, symptoms and signs, laboratory findings. In: Ikezaki K, Loftus CM. eds. Moyamoya Disease. Rolling Meadows, Illinois: American Association of Neurological Surgeons; 2001:43–54 [Google Scholar]
- 12. Kuroda S, Houkin K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol 2008;7:1056–66 [DOI] [PubMed] [Google Scholar]
- 13. Kim SK, Seol HJ, Cho BK, et al. Moyamoya disease among young patients: its aggressive clinical course and the role of active surgical treatment. Neurosurgery 2004;54:840–44 [DOI] [PubMed] [Google Scholar]
- 14. Scott RM, Smith JL, Robertson RL, et al. Long-term outcome in children with Moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg 2004;100(2 Suppl Pediatrics):142–49 [DOI] [PubMed] [Google Scholar]
- 15. Kim SK, Seol HJ, Cho BK, et al. Moyamoya disease among young patients: its aggressive clinical course and the role of active surgical treatment. Neurosurgery 2004;54:840–44, Discussion 844–46 [DOI] [PubMed] [Google Scholar]
- 16. Epstein HT. Stages of increased cerebral blood flow accompany stages of rapid brain growth. Brain Dev 1999;21:535–39 [DOI] [PubMed] [Google Scholar]
- 17. Takahashi T, Shirane R, Sato S, et al. Developmental changes of cerebral blood flow and oxygen metabolism in children. AJNR Am J Neuroradiol 1999;20:917–22 [PMC free article] [PubMed] [Google Scholar]
- 18. Ogawa A, Sakurai Y, Kayama T, et al. Regional cerebral blood flow with age: change in rCBF in childhood. Neurol Res 1989;11:173–76 [DOI] [PubMed] [Google Scholar]