Abstract
BACKGROUND AND PURPOSE:
Findings of standard MR imaging examinations are usually normal in primary CD. These findings are now increasingly challenged by studies using advanced neuroimaging techniques detecting abnormalities in brain areas that may be functionally involved in the pathophysiology of CD. Our purpose was to evaluate GM volumes in patients with CD at baseline and 5 years later.
MATERIALS AND METHODS:
We enrolled 19 patients (F/M = 15:4, mean age = 53.2 + 11.2 years), 12 of whom were studied at baseline and again approximately 5 years later. Twenty-eight healthy volunteers acted as controls (F/M = 17:11, mean age = 47.5 + 15.6 years). The subjects were imaged with a 1.5T scanner by using a 3D T1-weighted sequence on 150 contiguous axial 1-mm-thick sections to apply VBM.
RESULTS:
At entry, VBM analysis disclosed significantly lower GM volumes in the left caudate head and putamen and in the premotor and primary sensorimotor cortices bilaterally in patients than in controls. No correlation was found between decreased GM volumes and patient age, severity of dystonia, or disease duration. At the 5-year follow-up, GM volumes in the left primary sensorimotor cortex in patients had decreased significantly from baseline.
CONCLUSIONS:
The findings obtained at entry and after a 5-year follow-up consistently showed decreased caudate, putamen, and sensorimotor cortex GM volumes in patients with CD, and they probably play a pathophysiologic role in CD.
CD, one of the most common focal dystonias, is characterized by abnormal movements or postures of the neck and head.1 Although CD may be secondary to lesions in the basal ganglia or in other central nervous system structures,2 standard MR imaging generally detects no brain lesions in primary CD. These findings are now increasingly challenged by studies using advanced neuroimaging techniques detecting abnormalities in brain areas that may be functionally involved in the pathophysiology of dystonia.3,4
Cross-sectional studies using MR imaging–VBM, a technique that can evaluate intergroup and intragroup voxel-wise differences in GM volumes, have detected ultrastructural brain changes in apparently normal brain tissue in patients with CD.5–7 In these patients, regional GM abnormalities have been described in both subcortical and cortical structures, though controversial findings have been reported by the various authors. Whereas some found GM volume focally increased in the motor cortex, cerebellum, and GPi but decreased in the caudal SMA, dorsolateral frontal cortex, and visual cortex in patients with CD,5 others reported increased GM volumes in the thalamus, caudate head, superior temporal lobe, and left cerebellum.6 Yet others described an increased GM volume (though not statistically significant) in the GPi and SMA.7 These inconsistent results, the small numbers of patients with CD studied, and the lack of longitudinal VBM studies leave the topographic distribution of brain abnormalities in CD unclear. Additional information would help to understand the causes of CD.
We designed this MR imaging−VBM study primarily to evaluate the topographic distribution of brain abnormalities related to CD as measured by local VBM changes in GM volumes in patients with CD and healthy subjects. CD may worsen with time, and dystonia symptoms may spread to adjacent body parts.8 To map the progression of GM volume changes with time in the same patient group, we, therefore, investigated changes in GM values on entry to the study and again 5 years later. A further aim was to see whether changes in GM values with time correlated with possible changes in the clinical features of dystonia.
Materials and Methods
Patients
We prospectively enrolled 20 patients with primary CD between 2001 and 2003. Inclusion criteria were a diagnosis of primary CD according to published criteria9 and age older than 18 years. Exclusion criteria were other neurologic abnormalities and contraindications to performing MR imaging. Secondary forms of dystonia were excluded by medical history, neurologic examination, and neuroimaging studies. According to these criteria, 1 patient was excluded due to contraindications to performing MR imaging. The remaining 19 patients were included in the study. All patients were evaluated clinically, and the severity of symptoms was measured with the Tsui scale.10 The 19 patients had a mean age of 53.2 ± 11.2 years; there were 15 women (mean age = 52.5 ± 11.0) and 4 men (mean age = 55.5 ± 13.2). The mean duration of symptoms was 12.7 ± 6.5 years. Demographic and clinical characteristics of patients with CD are shown in On-line Table 1. All patients received botulinum toxin injections following standard treatment regimens and underwent the VBM study at the time of the maximal benefit (2–3 weeks after the injection). Of the 19 patients enrolled, 12 patients (3 men and 9 women, mean age 56.0 ± 13.0 years) were studied again, on average, 63 ± 5 months later. Twenty-eight healthy volunteers (mean age = 47.5 ± 15.6 years; 17 women, mean age = 47.6 ± 15.0; 11 men, mean age = 47.3 ± 15.8) with no known brain abnormalities and no neurologic symptoms were recruited as controls.
The study was approved by the Ethics Committee of the Sapienza University of Rome. Written informed consent was obtained from all the participants.
MR Imaging Acquisition
All subjects were imaged with a 1.5T scanner (Gyroscan NT 15; Philips Healthcare, Best, the Netherlands). The following sequences were acquired for all the subjects at the baseline and follow-up session: 1) axial dual-echo turbo spin-echo imaging (TR = 2150 ms, TE = 30/120 ms, matrix = 256 × 256, FOV = 250 mm square) on 48 contiguous 3-mm-thick sections, to exclude any focal brain alteration; 2) 3D T1-weighted imaging on 150 contiguous axial 1-mm-thick sections (TR = 40 ms, TE = 4 ms, flip angle = 30°, matrix = 512 × 512, FOV = 240 mm square) for subsequent analysis.
Volumetric Assessment
Imaging data were processed on a PC running Matlab 7.0 (Mathworks, Natick, Massachusetts) and the SPM5 software (Wellcome Department of Cognitive Neurology, London, United Kingdom; www.fil.ion.ucl.ac.uk/spm).
3D T1-weighted images underwent automated segmentation in SPM5 to yield GM, WM, and CSF images.11 Normalized GM images were modulated (ie, multiplied) by the local value derived from the deformation field,11 thereby preserving within-voxel volumes that may have been altered during nonlinear normalization. Data were smoothed by using a 12-mm full width at half maximum Gaussian kernel.
Because dystonia is considered a sensorimotor disorder, a mask was constructed on the automated anatomic labeling map,12 using MRIcro 1.38 software, (http://www.mricro.com)13 by selecting regions belonging to the sensorimotor network (primary sensory, primary motor, premotor cortices, SMA, thalamus, caudate nucleus, lentiform nucleus, and cerebellum) bilaterally.
Statistical Analysis
With SPM5, a 2-sample t test, corrected for age, considering only voxels included in the mask, was used for voxel-wise comparison of the GM volumes in patients and controls. A paired t test was used for voxel-wise comparison between data obtained at baseline and at follow-up in the 12 patients. The relationships between local GM volumes and demographic/clinical features were assessed by multiple regression. The statistical threshold was set at P corrected for multiple comparisons at both the cluster level (P < .05) and voxel level (FDR, P < .05).
Results
The mean Tsui score in the 19 patients with CD was 10 ± 3. No significant difference was found in the severity of dystonia in the 12 patients evaluated at baseline and 5 years later (Tsui score: 10.5 ± 3.3 versus 10.2 ± 3.7; P = .81, not significant).
MR imaging dual-echo turbo-spin-echo images showed no area of altered signal intensity. The voxel-wise VBM analysis of local GM volumes detected a significant GM volume reduction in the left caudate head and putamen as well as in the premotor and primary sensorimotor cortices bilaterally in patients compared with healthy subjects (On-line Table 2 and Fig 1). No areas of increased GM volumes were observed in patients with CD compared with healthy subjects. The multiple regression analysis found no correlation between the local GM volumes and age, Tsui scores, or duration of disease.
Fig 1.
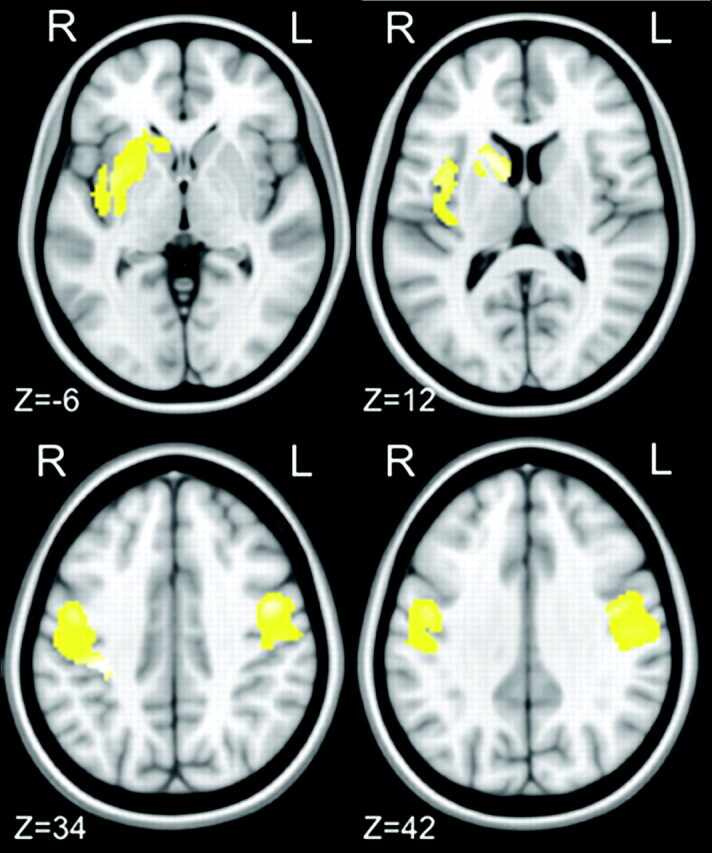
Images demonstrating focal decrease in GM volume between 19 patients with CD and 28 healthy controls. Clusters of decreased GM volume (in yellow) are overlapped with the T1-weighted mean image. Four axial sections passing at MNI z-coordinates of −6, 12, 34, 42 are shown. Clusters significant at P < .05 (2-sample t test; FDR corrected) are observed in the left caudate head and putamen and in the premotor and primary sensory motor cortices bilaterally. Images are displayed according to neurologic convention.
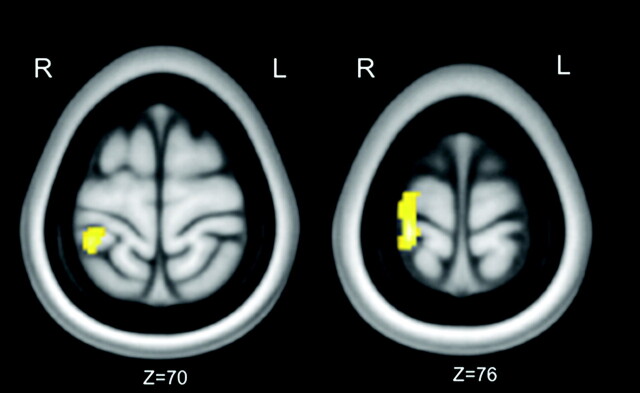
Longitudinal VBM analysis showed a significantly lower local GM volume in the left somatosensory cortex at the 5-year follow-up than at baseline in Broadmann areas 4, 1, 2, and 3 (P < .05, FDR-corrected) (Fig 2).
Fig 2.
Images demonstrating longitudinal changes in GM volume in 12 patients with CD during 5 years of follow-up. Clusters of decreased GM volume (in yellow) are overlapped with the T1-weighted mean image. Two axial sections passing at MNI z-coordinates of 70 and 76 are shown. Clusters significant at P < .05 (paired t test, FDR corrected) are observed in the left primary sensory motor cortex. Images are displayed according to neurologic convention.
Discussion
In this MR imaging−VBM study, we found that compared with healthy subjects, patients with CD had lower regional GM volumes in the left caudate head, putamen, and the bilateral premotor and primary sensorimotor cortices. We also found significantly lower GM volumes in the left sensorimotor cortex at the 5-year assessment than at baseline in the patients included in our prospective study. These findings provide new information on the topographic distribution of brain abnormalities related to CD.
The GM volume decrease detected in our MR imaging–VBM study in the primary sensorimotor cortex is a new finding in patients with CD, whereas the local GM decrease in the putamen has already been described.6 By contrast, unlike other authors who reported a GM increase in the thalamus, caudate nucleus, and left cerebellum,6 we found no GM volume increase in any sensorimotor network structure. We attribute the discrepancies to several factors: We studied a larger sample of patients with CD than those studied by Obermann et al6 and Egger et al7; our patients had a longer disease duration and were younger than those reported by others.6,7 Mapping the progression of changes in GM volume with time in the same patients showed that GM volumes in the left primary sensorimotor cortex decreased further during the subsequent 5-year interval. The GM changes we found in patients with CD suggest a disease-related loss of neuronal density in the caudate, putamen, and sensorimotor cortex. The follow-up data acquired 5 years later further imply that CD leads to progressive, albeit slow, neuronal loss within the sensorimotor cortex.
The reason that GM changes worsen in the longitudinal evaluation only in the sensorimotor cortex and not in the basal ganglia is unclear. The possibility that the changes in the cortical areas are compensatory and not primary effects of the disease is unlikely because our study showed a decrease, not an increase, in GM values. Because the cortical changes found in the present article do not correlate with a worsening of involuntary neck movements or disease duration, we believe that the GM changes reflect a progression of the disease not accompanied by a worsening in the clinical features. Last, we cannot exclude the possibility that cortical areas are affected to a greater extent than subcortical structures by the aging process.
The left-side predominance that we found deserves some consideration. In patients with writer's cramp, a form of focal dystonia with clear symptom lateralization,13 Delmaire et al14 reported GM decrease in the left primary sensorimotor cortex, contralateral to the clinically affected hand. Because the symptom lateralization is not so evident in patients with CD, we may hypothesize that neural structures on the left-brain side could be more susceptible to VBM changes. The GM decrease in the left sensorimotor cortex MR imaging–VBM analysis disclosed at follow-up supports the hypothesis of reduced cortical-subcortical fiber connections: The greater GM volume loss in the left caudate head and putamen at entry may have determined, through loss of fibers connecting the cerebral cortex to the basal ganglia, the focal atrophy in the left sensorimotor cortex.15,16
VBM studies similar to ours have detected ultrastructural changes in subcortical and cortical structures even in other focal dystonias. In patients with blepharospasm, the putaminal GM volume was increased17 or decreased,6 and the GM volume in the caudate and cerebellum was increased.6 In patients with focal hand dystonia, the GM volume in the sensorimotor cortex was either increased18 or decreased.14 The patients with focal hand dystonia who had decreased sensorimotor cortex GM volume also had decreased GM volume in the thalamus and cerebellum. Even though the types of regional abnormalities (increased or decreased GM values) found in the various focal dystonias differed in the various published studies, the consistent GM volume abnormalities involving the basal ganglia−cortical network also found in our study confirm that this network intervenes in the pathophysiology of primary dystonia.
The VBM findings reported here in CD receive support from recent neuroimaging studies using DTI, a technique that detects ultrastructural changes in intrinsic connectivity. In CD, DTI abnormalities in the putamen, caudate head, pallidum, prefrontal cortex, SMA, and corpus callosum have been previously demonstrated.19–22 In patients with focal hand dystonia, VBM data receive support from the recent finding of DTI abnormalities in the fiber tracts connecting the primary sensorimotor areas with subcortical structures.23 The reduced connectivity reported in the subcortical white matter of the sensorimotor area in DYT1 mutation carriers24 further supports the axonal loss in this subcortical white matter region and abnormalities in the neuronal density in the overlying cortex, which may contribute to the development of dystonia.
One limitation of our study is the lack of a longitudinal control group, which thus prevents us from excluding the possibility that our results on the longitudinal evaluation are affected by age-related changes. The GM volume decrease observed in healthy subjects during aging that diffusely affects the cerebral cortex may preferentially involve the associative neocortex, particularly the prefrontal and temporal regions25–28 and the medial frontal cortex.28
Although the GM changes detected in subcortical and cortical areas may be secondary to our patients' involuntary neck movements, this possibility seems unlikely. When they underwent the VBM studies, all the patients with CD were under optimal treatment with botulinum toxin, a therapy that significantly improves the severity of the involuntary neck movements.4,29,30
Conclusions
In patients with CD, our study clearly demonstrated that GM volume loss is detectable by VBM in the left caudate head and putamen and in the premotor and primary sensorimotor cortices bilaterally at baseline. The observation that abnormal GM changes worsen in the sensorimotor cortex in the same patients after a 5-year period strengthens the validity of our results. Last, these findings support the hypothesis that basal ganglia and sensory motor cortices play an important role in the pathophysiology of dystonia.
Supplementary Material
Abbreviations
- CD
cervical dystonia
- DTI
diffusion tensor imaging
- F
female
- FDR
false discovery rate
- GM
gray matter
- GPi
internal segment of globus pallidus
- k
voxel per cluster
- L
left
- M
male
- MNI
Montreal Neurological Institute
- mo
months
- MRI
MR imaging
- NA
not available
- R
right
- SMA
supplementary motor area
- SPM
Statistical Parametric Mapping
- VBM
voxel-based morphometry
- WM
white matter
- yr
years
Footnotes
Indicates article with supplemental on-line table.
References
- 1. Dauer WT, Burke RE, Greene P, et al. Current concepts on the clinical features, aetiology and management of idiopathic cervical dystonia. Brain 1998;121(pt 4):547–60 [DOI] [PubMed] [Google Scholar]
- 2. Bhatia KP, Marsden CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain 1994;117(pt 4):859–76 [DOI] [PubMed] [Google Scholar]
- 3. Berardelli A, Rothwell JC, Hallett M, et al. The pathophysiology of primary dystonia. Brain 1998;121(pt 7):1195–212 [DOI] [PubMed] [Google Scholar]
- 4. Gregori B, Agostino R, Bologna M, et al. Fast voluntary neck movements in patients with cervical dystonia: a kinematic study before and after therapy with botulinum toxin type A. Clin Neurophysiol 2008;119:273–80. Epub 2007 Dec 11 [DOI] [PubMed] [Google Scholar]
- 5. Draganski B, Thun-Hohenstein C, Bogdahn U, et al. “Motor circuit” gray matter changes in idiopathic cervical dystonia. Neurology 2003;61:1228–31 [DOI] [PubMed] [Google Scholar]
- 6. Obermann M, Yaldizli O, De Greiff A, et al. Morphometric changes of sensorimotor structures in focal dystonia. Mov Disord 2007;22:1117–23 [DOI] [PubMed] [Google Scholar]
- 7. Egger K, Mueller J, Schocke M, et al. Voxel based morphometry reveals specific gray matter changes in primary dystonia. Mov Disord 2007;22:1538–42 [DOI] [PubMed] [Google Scholar]
- 8. Weiss EM, Hershey T, Karimi M, et al. Relative risk of spread of symptoms among the focal onset primary dystonias. Mov Disord 2006;21:1175–81 [DOI] [PubMed] [Google Scholar]
- 9. Fahn S, Marsden C, Calne D. The treatment of dystonia. In: Fahn S, Jankovic J. eds. Principles and Practice Movement Disorders. London, UK: Butterworth and Co; 1987:332–58 [Google Scholar]
- 10. Tsui JK, Eisen A, Stoessl AJ, et al. Double-blind study of botulinum toxin in spasmodic torticollis. Lancet 1986;2:245–47 [DOI] [PubMed] [Google Scholar]
- 11. Ashburner J, Friston KJ. Voxel-based morphometry: the methods. Neuroimage 2000;11:805–21 [DOI] [PubMed] [Google Scholar]
- 12. Hallett M. Dystonia: a sensory and motor disorder of short latency inhibition. Ann Neurol 2009;66:125–27 [DOI] [PubMed] [Google Scholar]
- 13. Perlmutter JS, Thach WT. Writer's cramp: questions of causation. Neurology 2007;69:331–32 [DOI] [PubMed] [Google Scholar]
- 14. Delmaire C, Vidailhet M, Elbaz A, et al. Structural abnormalities in the cerebellum and sensorimotor circuit in writer's cramp. Neurology 2007;69:376–80 [DOI] [PubMed] [Google Scholar]
- 15. Hoffer ZS, Alloway KD. Organization of corticostriatal projections from the vibrissal representations in the primary motor and somatosensory cortical areas of rodents. J Comp Neurol 2001;439:87–103 [DOI] [PubMed] [Google Scholar]
- 16. Leh SE, Ptito A, Chakravarty MM, et al. Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neurosci Lett 2007;419:113–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Etgen T, Mühlau M, Gaser C, et al. Bilateral grey-matter increase in the putamen in primary blepharospasm. J Neurol Neurosurg Psychiatry 2006;77:1017–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garraux G, Bauer A, Hanakawa T, et al. Changes in brain anatomy in focal hand dystonia. Ann Neurol 2004;55:736–39 [DOI] [PubMed] [Google Scholar]
- 19. Colosimo C, Pantano P, Calistri V, et al. Diffusion tensor imaging in primary cervical dystonia. J Neurol Neurosurg Psychiatry 2005;76:1591–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blood AJ, Tuch DS, Makris N, et al. White matter abnormalities in dystonia normalize after botulinum toxin treatment. Neuroreport 2006;17:1251–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bonilha L, de Vries PM, Vincent DJ, et al. Structural white matter abnormalities in patients with idiopathic dystonia. Mov Disord 2007;22:1110–16 [DOI] [PubMed] [Google Scholar]
- 22. Fabbrini G, Pantano P, Totaro P, et al. Diffusion tensor imaging in patients with primary cervical dystonia and in patients with blepharospasm. Eur J Neurol 2008;15:185–89 [DOI] [PubMed] [Google Scholar]
- 23. Delmaire C, Vidailhet M, Wassermann D, et al. Diffusion abnormalities in the primary sensorimotor pathways in writer's cramp. Arch Neurol 2009;66:502–08 [DOI] [PubMed] [Google Scholar]
- 24. Carbon M, Kingsley PB, Su S, et al. Microstructural white matter changes in carriers of the DYT1 gene mutation. Ann Neurol 2004;56:283–86 [DOI] [PubMed] [Google Scholar]
- 25. Curiati PK, Tamashiro JH, Squarzoni P, et al. Brain structural variability due to aging and gender in cognitively healthy elders: results from the Sao Paulo Ageing and Health study. AJNR Am J Neuroradiol 2009;30:1850–56. Epub 2009 Aug 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gonoi W, Abe O, Yamasue H, et al. Age-related changes in regional brain volume evaluated by atlas-based method. Neuroradiology 2009. December 23. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27. Smith CD, Chebrolu H, Wekstein DR, et al. Age and gender effects on human brain anatomy: a voxel-based morphometric study in healthy elderly. Neurobiol Aging 2007;28:1075–87 [DOI] [PubMed] [Google Scholar]
- 28. Bergfield KL, Hanson KD, Chen K, et al. Age-related networks of regional covariance in MRI gray matter: reproducible multivariate patterns in healthy aging. Neuroimage 2010;49:1750–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry 2004;75:951–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ward AB, Molenaers G, Colosimo C, et al. Clinical value of botulinum toxin in neurological indications. Eur J Neurol 2006;13(suppl 4):20–26 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.