Abstract
BACKGROUND AND PURPOSE:
The “ivy” sign that is identified on FLAIR images in patients with Moyamoya disease is considered to be leptomeningeal collaterals. The aim of our study was to evaluate the correlation between postoperative decrease in ivy sign and cerebral hemodynamic status in the bypass-established hemisphere.
MATERIALS AND METHODS:
Twenty-two patients with Moyamoya disease were enrolled. Postoperative changes in the ivy sign on FLAIR images were examined in each patient after bypass surgery. The correlation between postoperative changes in the ivy sign and hemodynamic status was examined in 10 patients by using SPECT.
RESULTS:
Of the 22 preoperative ivy-positive patients, 21 showed decreased ivy signs on the operative side. Average intervals between the operation day and the date when the decreased or vanished ivy sign was first recognized were 157.6 days in patients who underwent direct bypass and 212.2 days in patients who underwent indirect bypass. A postoperative decrease in ivy signs was found to be significantly correlated with an improved hemodynamic status of the surgically treated hemisphere, resulting in a postoperative increase in regional vascular reserve and a decreased proportion of the misery perfusion area (P < .01).
CONCLUSIONS:
Postoperative changes in the ivy sign can be used as a marker for identifying improved hemodynamics and also for testing the effectiveness of cerebral revascularization.
The “ivy” sign refers to a diffuse leptomeningeal enhancement observed on postcontrast MR images of patients with Moyamoya disease in childhood.1 It can also be observed on FLAIR images of a child with Moyamoya disease.2 On FLAIR sequences, the ivy sign has been defined as a continuous linear or punctate leptomeningeal high signal intensity along the cortical sulci and subarachnoid space.3 This enhancement mechanism is considered to represent a retrograde slow flow of engorged pial vasculature toward an ischemic area via leptomeningeal collaterals.1–4 We recently reported that unilateral hemispheric ivy proliferation is highly correlated with an ipsilateral decrease in VR that is associated with the development of leptomeningeal collaterals in patients with Moyamoya disease.3 In addition, ivy proliferation was found to decrease in 10 of 18 patients (55.6%) who underwent bypass surgery during the follow-up period.3
The aim of our study was to evaluate postoperative changes in ivy signs on FLAIR after cerebral revascularization and to correlate a decrease in ivy signs with postoperative hemodynamic status in the bypass-established hemisphere, focusing especially on VR, which was measured by 123I-IMP SPECT with ACZ challenge in patients with Moyamoya disease.
Materials and Methods
Patients
During >2.5 years, 22 patients who presented with transient ischemic attacks (n = 17), headache (n = 2), involuntary movement (n = 1), or no symptoms (n = 2) were diagnosed with Moyamoya disease at our institution. Eleven female (average age, 25.8 years; range, 7–66 years) and 11 male (average age, 24 years; range, 7–61 years) patients were included in this study. In all patients, either indirect (encephaloduroarteriosynangiosis) or direct STA-MCA bypass was performed on the hemisphere where cerebral VR decreased without a major infarction. Ten patients (6 women and 4 men; average age, 42.4 years) had direct bypasses and 12 (5 females and 7 males; average age, 10.3 years) had indirect bypasses. The postoperative follow-up period ranged from 41 to 425 days (average, 184.9 days).
Imaging Examinations
Imaging examinations, including MR imaging and MRA, were performed in each patient pre- and postoperatively. Ten of 22 patients underwent quantitative SPECT with ACZ challenge pre- and postoperatively. Then, the correlation between a decrease in the ivy sign in the surgically treated hemisphere after cerebral revascularization and postoperative hemodynamic status was examined in 10 patients.
MR Imaging and MRA
The routine MR imaging protocol for cerebral ischemic diseases includes FLAIR imaging. MR imaging was performed by using a 3T MR imaging unit (MAGNETOM Trio, A Tim System; Siemens, Erlangen, Germany) without variability. FLAIR imaging was performed by using a fast FLAIR sequence with a TR of 9000 ms, a TEeff of 86 ms, a TI of 2500 ms, a 241-Hz/pixel bandwidth, and a flip angle of 150°. Image thickness, gap, and matrix for all sequences were 6.0 mm, 1.2 mm, and 384 × 372 pixels, respectively. 3D time-of-flight MRA was performed with a TR of 22 ms, a TE of 3.1 ms, a 250-Hz/pixel bandwidth, and a flip angle of 18°. Voxel size was 0.52 × 0.52 × 0.8 mm, and the acquisition matrix was 384 × 320 pixels.
An ivy sign on a FLAIR image was defined as a continuous linear or punctate leptomeningeal high signal intensity along the cortical sulci and subarachnoid space. Two neuroradiologists retrospectively reviewed MR images for ivy signs on FLAIR. A hemisphere was defined as ivy-positive when an ivy sign was observed in the MCA region (in any of the frontal, temporal, and parietal lobes). The degree of the ivy sign (ivy sign score) in the MCA region was classified into 3 grades (0–2): Grade zero indicated an absence of the ivy sign, grade 1 indicated that the ivy sign was seen on less than half of the cortical surface in the region, and grade 2 indicated that the ivy sign was seen on more than half of the cortical surface. The ivy sign was scored subjectively by reviewing all the FLAIR images traversing all the cerebral hemispheres. The average score for each region was defined as the ivy sign score for the individual hemisphere. The score of the ivy sign was compared pre- and postoperatively among patients with Moyamoya disease. Postoperative MR imaging was performed during the first 3 months after surgery and every 6 month after that.
Quantitative Estimation of CBF and VR
A SPECT Multispect 3 scanner (Siemens) and an ICON image processor (Siemens Medical Systems) were used, with a low-energy ultra-high-resolution fan-beam collimator (225 cpm/μCi). The conditions for acquisition were as follows: 20-minute acquisition (40 seconds/90 steps, 3° step; the step and shoot method), a matrix of 128 × 128 pixels (2.89 mm/pixel), and a rotation radius of 13.9 cm. A filtered back-projection method by using a Butterworth filter was used to reconstruct images from 20-minute data. A low-pass filter (cutoff, 0.3 cycles/cm; order, 5.0) was used as the 3D postfilter. Two SPECT images were summated to obtain a section thickness of 5.78 mm.
Ten patients underwent 2 SPECT sessions: a basal study and an ACZ-challenged study. Both rCBF and VR were quantified by autoradiographic processing of a single SPECT scan with a 1-point arterial blood sample (the IMP-ARG method).5 Patients were allowed to rest in a quiet dimly lit room and were injected with IV 123I-IMP, 166.5 MBq. Ten minutes after starting 123I-IMP infusion, an arterial blood sample was collected to calibrate the previously determined standard input function, and its whole-blood radioactivity concentration was counted by using a well counter that had been cross-calibrated with the SPECT scanner. After arterial blood sampling, a single SPECT scan was obtained at a midscan time of 10 minutes. Basal images were obtained 35 minutes after injecting the radiotracer. In 2-compartment 123I-IMP analysis, the distribution volume (ie, the ratio of influx constant and efflux constant) was set to a constant value (41.2 mL/mL). rCBF maps were calculated pixel by pixel from single SPECT data, and the standard input function was calibrated with a 1-point arterial blood sample.5
Several days later (3–5 days), the patients were consecutively placed in an environment similar to that of the first session and were injected with 1-g IV ACZ (Diamox) during a 1-minute period. Fifteen minutes later, the patients were injected with 123I-IMP and ACZ-challenged images were obtained in a manner similar to that followed in the basal study. An automated region-of-interest-setting method by using NEUROSTAT (http://128.95.65.28/∼Download) was performed without losing individual morphologic information in the SPECT images.6,7 To objectively classify the severity of hemodynamic ischemia according to the JET study protocol of patients with intracranial occlusive disease,8 we performed a quantitative analysis by using 3D stereotactic surface projections analytical software (3D-SSP; Nihon Medi-physics, Tokyo, Japan) and stereotactic extraction estimation display software (SEE-JET; Nihon Medi-physics).9 This method provides 3D displays of VR, the severity of hemodynamic brain ischemia, and CBF at rest and after Diamox challenge.
The measurements obtained with SPECT are not actually quantitative because true quantitative assessment of CBF requires the application of the Fick principle, as originally applied with the use of nitrous oxide by Kety and Schmidt10 and currently applied with PET and the use of 15O-labeled CO2 and water. rVR was defined as the ratio of the difference between Diamox-activated rCBF and resting rCBF to resting rCBF (Diamox-activated rCBF / resting rCBF − 1[×100]%). Resting rCBF and rVR of the MCA territory were evaluated in the hemispheres in all patients. The severity of hemodynamic brain ischemia was classified into 3 stages: stage 0 (resting CBF > 15 mL/100 g/min and VR > 30%), stage I (34 mL/100 g/min [80% of normal CBF] > resting CBF > 15 mL/100 g/min and 30% > VR > 10%), and stage II (34 mL/100 g/min > resting CBF > 15 mL/100 g/min and 10% > VR > −30%).8,9 The stage II area was identical to the misery perfusion area. Finally, the proportion of stage II area to hemisphere and mean rVRs in MCA territories were compared pre- and postoperatively between surgically treated and contralateral (nonsurgically treated) hemispheres.
Statistical Analysis
Statistical analysis was performed by using the paired t test to analyze the variance between the decreased ivy sign and contralateral hemispheres. A Student t test was performed to analyze the difference between direct and indirect bypass procedures. The difference was considered significant at P < .05.
Results
Postoperative Changes in the Presence of Ivy Signs in Moyamoya Disease
Ivy signs on FLAIR images of the affected side were observed in all patients with Moyamoya disease examined preoperatively. The preoperative ivy sign score in the affected MCA region was 1.60 ± 0.54. Of the 22 ivy-positive patients, 21 showed a postoperative decrease in the ivy sign on the operative side. The postoperative ivy sign score was 0.74 ± 0.41 in the region. A postoperative ivy sign was unchanged in 1 patient. Of the 21 patients in whom ivy signs decreased, 9 were treated with direct bypass and 12 were treated with indirect bypass. The average intervals between the operation day and the date when the decreased ivy sign was first recognized were 157.6 days (range, 47–303 days) in patients who underwent direct bypass and 212.2 days (range, 41–425 days) in patients who underwent indirect bypass. There was no significant difference between the 2 groups (P > .05). Postoperative courses of all patients were uneventful.
Ivy signs on FLAIR images of the nonaffected side were observed in 18 of 22 patients with Moyamoya disease examined preoperatively. A postoperative ivy sign was unchanged in all patients.
Postoperative Decrease in Ivy Signs and Cerebral Hemodynamics
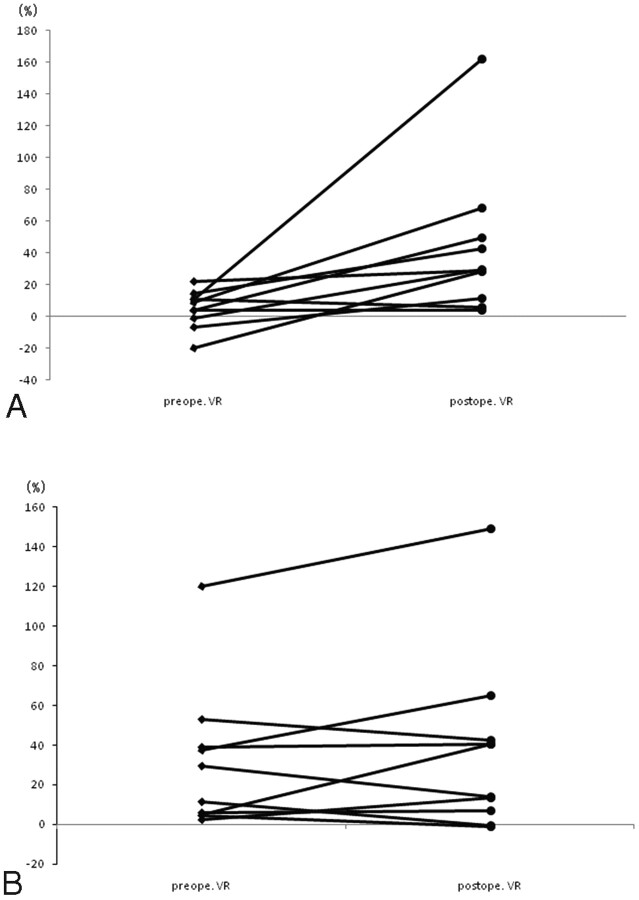
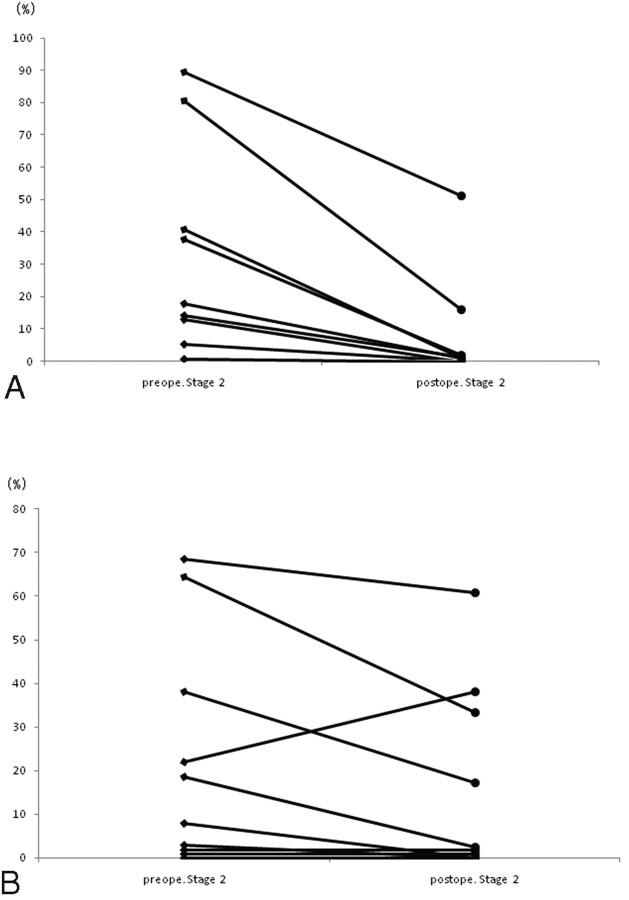
Of the 21 patients in whom ivy signs decreased or disappeared, 10 underwent a quantitative SPECT examination. Postoperative rVR increased in all patients in the hemisphere in which cerebral revascularization was established (Fig 1 A). The increased rVR rate was significantly higher in the bypass-established hemisphere than that in the contralateral hemisphere (P = .0018). The pre- and postoperative rVRs were 5.08 ± 11.82% and 43.27 ± 46.34% in the bypass-established hemisphere, respectively. Postoperative rVR increased in 6 patients and decreased in 4 patients on the contralateral hemisphere (Fig 1B). Pre- and postoperative rVRs were 31.06 ± 36.11% and 37.25 ± 44.99%, respectively. The proportion of the stage II area in the bypass-established hemisphere was significantly lower than that in the contralateral hemisphere (P = .0088, Fig 2 A, -B). Pre- and postoperative proportions of stage II areas in the bypass-established hemisphere were 30.04 ± 32.23% and 7.27 ± 16.19%, whereas those in the contralateral hemisphere were 22.66 ± 26.06% and 15.52 ± 21.55%, respectively. This was true for patients treated either by direct or indirect bypass (Figs 3 and 4).
Fig 1.
Postoperative change in rVR in the bypass-established hemisphere. The increased rVR rate is significantly higher on the bypass-established hemisphere (A) than that on the contralateral hemisphere (B) (P = .0018).
Fig 2.
Postoperative change in the misery perfusion area in the bypass-established hemisphere. The proportion of stage II area on the bypass-established hemisphere (A) is significantly lower than that on the contralateral hemisphere (B) (P = .0088).
Fig 3.
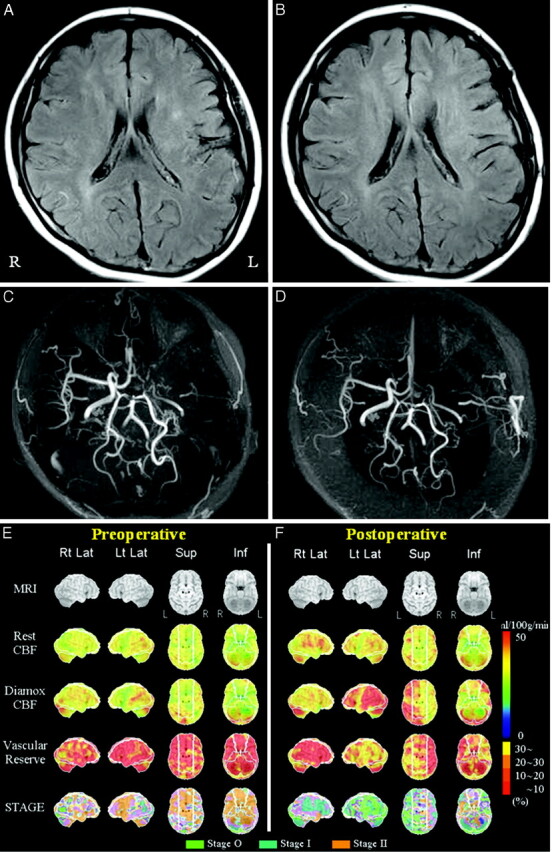
Adult Moyamoya disease. Data from a 25-year-old female patient who had a right transient ischemic attack. She underwent direct bypass surgery on the left cerebral hemisphere. MR imaging of the preoperative (A) and postoperative (B) views shows a decrease in the ivy sign in the left MCA territory after bypass surgery. MRA of the preoperative (C) and postoperative (D) views shows increased flow-related enhancement via left superficial temporal arteries to the left MCA region after surgery. Quantitative analysis of preoperative (E) and postoperative (F) basal/ACZ stress brain perfusion SPECT. The lower 4 columns show 3D stereotactic surface projection format view sets of resting CBF, Diamox CBF, VR, and staging by a JET study from the top. VR is impaired preoperatively in most of the left MCA territory (E). Both VR and staging in the left MCA territory have improved postoperatively (F).
Fig 4.
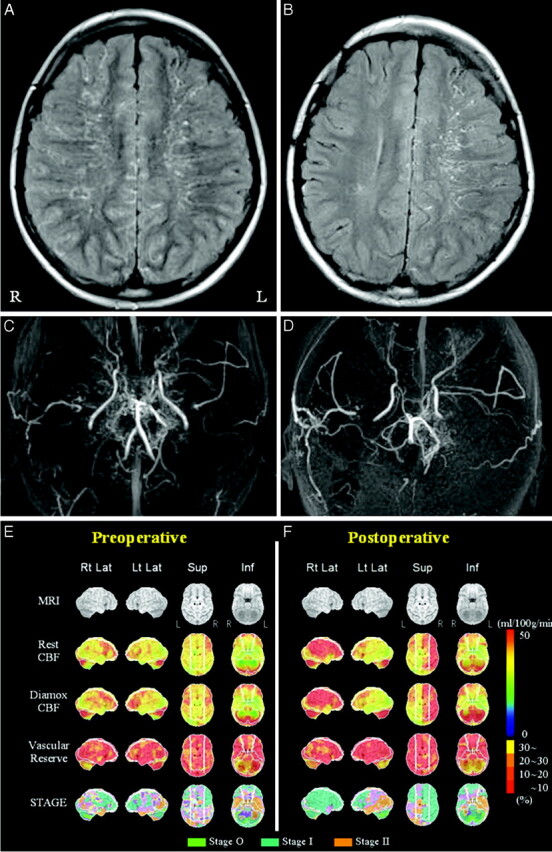
A child with Moyamoya disease. Data from an 11-year-old male patient who had left involuntary movement. He underwent indirect bypass surgery on the right cerebral hemisphere. MR imaging of the preoperative (A) and postoperative (B) views shows a decrease in the ivy sign on the right hemisphere after bypass surgery. MRA of the preoperative (C) and postoperative (D) views shows increased flow-related enhancement via the right temporal subcutaneous tissue to the right MCA region after surgery. Quantitative analysis of preoperative (E) and postoperative (F) basal/ACZ stress brain perfusion SPECT. VRs in bilateral MCA territories are impaired preoperatively (E). VR and staging in the right hemisphere are improved postoperatively (F).
Discussion
The present study had 2 major findings. First, the decrease in the ivy sign on the bypass-established hemisphere was associated with an improved hemodynamic status, resulting in an increased postoperative rVR and a decreased proportion of the misery perfusion area. Second, direct or indirect cerebral revascularization procedures were effective for decreasing the ivy sign in patients with Moyamoya disease.
Knowledge of secondary collaterals remains unclear, largely because of limitations in the methods for evaluating these diminutive CBF routes. Direct visualization of collaterals is limited to angiographic methods, including conventional angiography, transcranial Doppler, CT, angiography, and MRA. The ivy sign, which is identical to a hyperintense vessel on FLAIR images, is frequently observed in a hemisphere affected by an acute arterial occlusion.11–14 Stationary blood and anterograde or retrograde leptomeningeal collateral circulation have been suggested as possible explanations for the ivy sign.3,4,11,14,15 Because secondary collateral pathways are presumed to be recruited once primary collaterals at the circle of Willis have failed, the presence of secondary collateral pathways is possibly considered a marker for impaired cerebral hemodynamics.16,17 Furthermore, an ivy sign on FLAIR can be observed in patients with chronic vascular diseases. Two recent studies, 1 of which includes the study reported by our group, revealed that the existence of an ivy sign on a FLAIR image in patients with Moyamoya disease is associated with decreased cerebral VR and development of leptomeningeal collaterals.3,4 In Moyamoya disease, secondary collaterals are well-developed due to failure of primary collaterals. The recruitment of secondary collaterals is required to supply arterial blood to the ischemic area that has impaired cerebral hemodynamics.
In the present study, the presence of an ivy sign on the surgically treated hemisphere decreased after bypass surgery, supporting the idea that a secondary collateral pathway is possibly associated with impaired cerebral hemodynamics. In fact, rVR increased and the proportion of misery area decreased postoperatively in the surgically treated hemisphere, as shown by SPECT analysis. Postoperative rVR, increased in 6 patients and decreased in 4 patients on the contralateral hemisphere, may represent an indirect demonstration of the fact that CBF evaluation by SPECT is semiquantitative, showing a wide variability in the vascular capacity of the contralateral hemisphere, which is used as the normal control with this technique.
Ten of 21 patients underwent a SPECT examination. There was no selection bias. It was preferred that children and patients living a long distance from the hospital receive the 1-day method (semiquantitative SPECT analysis). So far, the postoperative hemodynamics of surgically treated hemispheres in patients with Moyamoya disease have been evaluated mainly by SPECT or PET. If a decrease in the ivy sign after bypass surgery implies improved hemodynamics, it would be easier for us to speculate on a patient's condition.
In our institution, a multiple combined indirect nonanastomotic procedure is recommended as an appropriate surgical option for treating children with Moyamoya disease, though the best treatment is STA-MCA anastomosis with encephalomyosynangiosis, if feasible.18 STA-MCA direct anastomosis is performed for most adult patients with Moyamoya disease because of suitable donor and recipient arteries; however, the appropriate technique to induce neovascularization is controversial.18,19 The present study revealed that the average intervals between the operation day and the date when the decreased or vanished ivy sign was first recognized in the 2 groups were not significantly different. It may take some time to revascularlize CBF by using an indirect bypass procedure. Direct or indirect cerebral revascularization can be an effective option for patients with Moyamoya disease and impaired hemodynamics.
Conclusions
A postoperative decrease in the ivy sign on a FLAIR image reflected an improvement in the impaired cerebral hemodynamic status in patients with Moyamoya disease. A change in the postoperative ivy sign, giving careful consideration to a patient's symptoms, can be a marker for identifying the effectiveness of cerebral revascularization.
Abbreviations
- ACZ
acetylzolamide
- CBF
cerebral blood flow
- FLAIR
fluid-attenuated inversion recovery
- Inf
inferior
- 123I-IMP
iodine-123-N-isopropyl-p-iodoamphetamine
- IV
intravenous
- JET
Japanese Extracranial-Intracranial Bypass Trial
- L
left
- Lt La
left lateral
- MCA
middle cerebral artery
- MRA
MR angiography
- MRI
MR imaging
- PET
positron-emission tomography
- postope.
postoperative
- preope.
preoperative
- R
right
- Rt Lat
right lateral
- STA-MCA
superficial temporal artery−middle cerebral artery
- rCBF
regional CBF
- Rest
resting
- rVR
regional VR
- SPECT
single-photon emission CT
- Sup
superior
- VR
vascular reserve
References
- 1. Ohta T, Tanaka H, Kuroiwa T. Diffuse leptomeningeal enhancement, “ivy sign,” in magnetic resonance images of Moyamoya disease in childhood: case report. Neurosurgery 1995; 37: 1009– 12 [DOI] [PubMed] [Google Scholar]
- 2. Maeda M, Tsuchida C. “Ivy sign” on fluid-attenuated inversion-recovery images in childhood Moyamoya disease. AJNR Am J Neuroradiol 1999; 20: 1836– 38 [PMC free article] [PubMed] [Google Scholar]
- 3. Kawashima M, Noguchi T, Takase Y, et al. Unilateral hemispheric proliferation of ivy sign on FLAIR image in Moyamoya disease correlates highly with ipsilateral hemispheric decrease of cerebrovascular reserve. AJNR Am J Neuroradiol 2009; 30: 1709– 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mori N, Mugikura S, Higano S, et al. The leptomeningeal “ivy sign” on fluid-attenuated inversion recovery MR imaging in Moyamoya disease: a sign of decreased cerebral vascular reserve? AJNR Am J Neuroradiol 2009; 30: 930– 35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iida H, Itoh H, Nakazawa M, et al. Quantitative mapping of regional cerebral blood flow using iodine-123-IMP and SPECT. J Nucl Med 1994; 35: 2019– 30 [PubMed] [Google Scholar]
- 6. Ogura T, Hida K, Masuzuka T, et al. An automated ROI setting method using NEUROSTAT on cerebral blood flow SPECT images. Ann Nucl Med 2009; 23: 33– 41. Epub 2009 Feb 11 [DOI] [PubMed] [Google Scholar]
- 7. Minoshima S, Berger KL, Lee KS, et al. An automated method for rotational correction and centering of three-dimensional functional brain images. J Nucl Med 1992; 33: 1579– 85 [PubMed] [Google Scholar]
- 8. JET Study Group. Japanese EC-IC Bypass Trial (JET study): study design and interim analysis. Surg Cereb Stroke 2002; 30: 97– 100 [Google Scholar]
- 9. Mizumura S, Nakagawara J, Takahashi M, et al. Three-dimensional display in staging hemodynamic brain ischemia for JET study: objective evaluation using SEE analysis and 3D-SSP display. Ann Nucl Med 2004; 18: 13– 21 [DOI] [PubMed] [Google Scholar]
- 10. Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. J Clin Invest 1948; 27: 476– 83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Noguchi K, Ogawa T, Inugami A, et al. MRI of acute cerebral infarction: a comparison of FLAIR and T2-weighted fast spin-echo imaging. Neuroradiology 1997; 39: 406– 10 [DOI] [PubMed] [Google Scholar]
- 12. Cosnard G, Duprez T, Grandin C, et al. Fast FLAIR sequence for detecting major vascular abnormalities during the hyperacute phase of stroke: a comparison with MR angiography. Neuroradiology 1999; 41: 342– 46 [DOI] [PubMed] [Google Scholar]
- 13. Gauvrit JY, Leclerc X, Girot M, et al. Fluid-attenuated inversion recovery (FLAIR) sequences for the assessment of acute stroke: inter observer and inter technique reproducibility. J Neurol 2006; 253: 631– 35. Epub 2005 Dec 13 [DOI] [PubMed] [Google Scholar]
- 14. Maeda M, Yamamoto T, Daimon S, et al. Arterial hyperintensity on fast fluid-attenuated inversion recovery images: a subtle finding for hyperacute stroke undetected by diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2001; 22: 632– 36 [PMC free article] [PubMed] [Google Scholar]
- 15. Sanossian N, Saver JL, Alger JR, et al. Angiography reveals that fluid-attenuated inversion recovery vascular hyperintensities are due to slow flow, not thrombus. AJNR Am J Neuroradiol 2009; 30: 564– 68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liebeskind DS. Collateral circulation. Stroke 2003; 34: 2279– 84 [DOI] [PubMed] [Google Scholar]
- 17. Girot M, Gauvrit JY, Cordonnier C, et al. Prognostic value of hyperintense vessel signals on fluid-attenuated inversion recovery sequences in acute cerebral ischemia. Eur Neurol 2007; 57: 75– 79 [DOI] [PubMed] [Google Scholar]
- 18. Matsushima T, Inoue T, Suzuki SO, et al. Surgical treatment of Moyamoya disease in pediatric patients: comparison between the results of indirect and direct revascularization procedures. Neurosurgery 1992; 31: 401– 05 [DOI] [PubMed] [Google Scholar]
- 19. Houkin K, Kuroda S, Ishikawa T, et al. Neovascularization (angiogenesis) after revascularization in Moyamoya disease: which technique is most useful for Moyamoya disease? Acta Neurochir (Wien) 2000; 142: 269– 76 [DOI] [PubMed] [Google Scholar]