SUMMARY:
“Giant” AGs (>1 cm) are uncommon and can be misdiagnosed as venous sinus pathology such as a neoplasm or thrombosis. Seventeen patients with a total of 19 venous sinus AGs of >1 cm were collected from contributing authors. MR imaging was available for all AGs; CT, for 5/19; and DSA, for 7/19. Intra-AG fluid was compared with CSF in subarachnoid spaces. Nonfluid AG tissue was compared with gray matter. Diagnosis was based on imaging findings. Fluid within giant AGs did not follow CSF signal intensity on at least 1 MR image in nearly 80% (15/19) of AGs. Nine of these 15 AGs had CSF-incongruent signal intensity on ≥2 MR images. CSF-incongruent signal intensity was seen in 8/8 AGs on FLAIR, 7/10 on precontrast T1WI, 13/19 on T2WI, and 8/14 on contrast-enhanced T1WI. Nonfluid signal intensity was present in 18/19 AGs and varied from absent/hypointense (intra-AG flow voids) to gray matter isointense (stromal tissue).
AGs are CSF-filled meningothelial-lined protrusions that extend into the venous sinuses through openings in the dura. These structures filter CSF across a lining of arachnoid cells and drain it into central venous circulation.1,2
Dural venous sinus AGs typically range from 2 to 8 mm.3–8 Occasionally AGs can exceed 1 cm in diameter. It is important to distinguish these benign “giant” AGs from more serious dural venous sinus pathology such as thrombosis and neoplasia to avoid unnecessary invasive procedures.9,10
The imaging diagnosis of AGs, regardless of size, is commonly established by identifying CSF-like signal intensity of intra-AG contents on all sequences.5,6 Leach et al6 recently noted in passing that giant AGs may be complex structures whose contents do not invariably parallel CSF signal intensity.
We present and analyze the imaging characteristics of 19 giant (>1 cm) AGs from 17 patients.
Materials and Methods
Case Material
A multi-institutional series of large AGs (prospectively defined as >1 cm) was gathered from case collections of the contributing authors. Cases with non-AG pathology such as neoplasms (meningioma, metastasis) and dural venous sinus thrombosis were excluded. Seventeen cases with a total of 19 giant AGs were submitted for analysis on the basis of independent evaluation by a CAQ-certified neuroradiologist (A.G.O.) by using the following criteria: 1) size >1 cm as proposed by Kan et al, 11 2) location within a dural venous sinus, and 3) exclusion of other pathology according to standard diagnostic criteria, including ovoid/round shape, lack of solid enhancement, and absence of blooming on T2* sequences.5,6
Imaging
Digital images were obtained by using multiple different 1.5T and 3T scanners and standard parameters for each sequence. Section thickness was 4–5 mm. T2WIs were available for all AGs. Precontrast T1WIs were available in 10/19. FLAIR was available for 8/19. Inversion recovery images and DWIs were both available for 1 AG. Contrast-enhanced T1WI images were obtained for 13/19 AGs. DWI was available for 1 patient. Six patients had CT. Three had CECT and 2 had noncontrast CT performed; 2 of the CTs had only bone windows available for analysis. Angiography was performed for 7 patients. One patient had CT venography, 3 had MR venography, and 3 had DSA studies performed. One patient also had intrasinus venous manometry.
Because images were submitted without original datasets, fluid and soft-tissue contents were evaluated separately by a CAQ-certified neuroradiologist (A.G.O.). Intra-AG fluid signal intensity on CT and MR imaging was assessed visually and compared directly with CSF in the ventricles and adjacent subarachnoid spaces. Intra-AG fluid was designated as isointense, hypointense, or hyperintense relative to CSF. Signal intensity for discrete nonfluid veins, septations, or undetermined soft tissue within AGs was compared with gray matter on a similar tripartite scale. The presence of contrast enhancement within AG soft tissue was identified.
Results
Case Material
Demographics and clinical history were available in 5 patients. Patients ranged from 45 to 75 years of age. Two patients were imaged for headaches; the other 3 were imaged for unrelated reasons. In compliance with the Health Insurance Portability and Accountability Act regulations, all identifying information, including patient age and sex, was discarded before image archiving in the authors' respective case collections and was not available for analysis for most patients.
Imaging
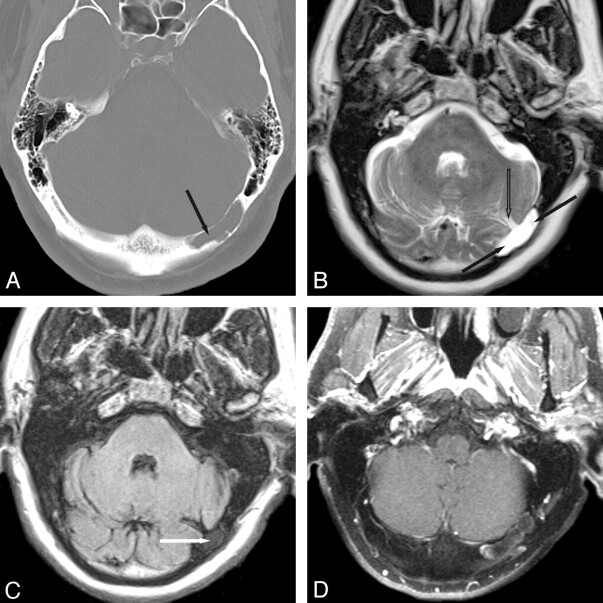
In our series, giant AGs were found only in the transverse and superior sagittal sinuses. Twelve of 19 giant AGs were located in the TSs. Eight of these were found in the right TS, and 4 were found in the left TS. One AG was found within the venous confluence (torcular herophili). In the 2/17 patients with multiple giant AGs, both had an AG in each TS. Six giant AGs were located in the superior sagittal sinus. One patient showed invasion and expansion of the diploic space by an AG in the left TS (Fig 1).
Fig 1.
Transverse sinus giant AG. A, Axial nonenhanced CT with a bone algorithm shows cystic expansion of the diploic space adjacent to the left TS (arrow). B, Axial T2WI shows a large AG in the left TS with 2 internal septations (arrows) and subarachnoid spaces converging at the base of the AG (open arrow). C, Axial FLAIR image demonstrates incomplete suppression of fluid within the margins of the intra-AG septations (arrow) and complete suppression of intra-AG fluid outside the septations. D, Axial fat-saturated postcontrast T1WI shows linear enhancement at the posteromedial margin of the AG, most likely representing an intra-AG vein. The soft-tissue septations themselves do not enhance.
In the 4 of 6 CT AGs with soft-tissue windows available for analysis, intra-AG fluid was isoattenuated to CSF in all (100%) AGs (Fig 2). For the 3 AGs with CECT imaging, all showed a nonenhancing CSF-like lesion within an otherwise strongly uniformly enhancing dural venous sinus.
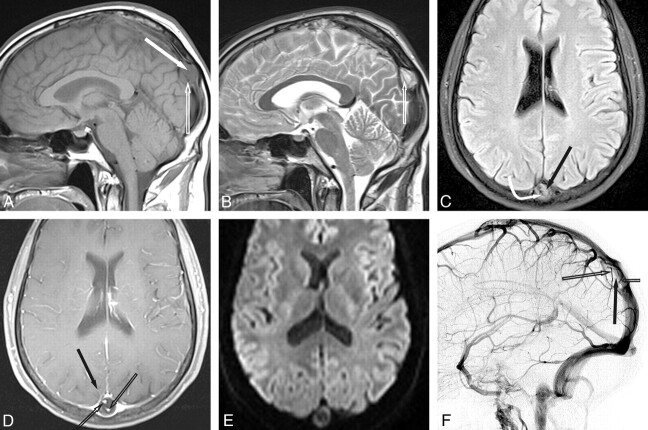
Fig 2.
Superior sagittal sinus giant AG. A, Sagittal T1WI shows a giant AG in the SSS. Note that fluid in the AG (arrow) is hyperintense to CSF. A distinct linear flow void (open arrow) is seen. B, Sagittal T2WI shows that fluid is mixed iso- and hypointense to CSF. A distinct intra-AG vein is present (open arrow). C, Axial FLAIR image shows that intra-AG fluid (arrow) is not suppressed. Phase dispersion (curved arrow) is present around the linear flow void entering the AG. D, Postcontrast fat-saturated T1WI shows an enhancing vein entering the AG (arrow) and enhancing veins (open arrows) within the AG itself. E, Axial DWI shows that fluid within the AG does not demonstrate restricted diffusion. F, Lateral DSA, venous phase, shows a filling defect in the SSS caused by the giant AG (arrow). Note veins (open arrows) within the AG.
Fluid signal intensity within the AGs on MR imaging was isointense to CSF on all sequences for only 4/19 AGs. Fifteen AGs (almost 80%) contained fluid that did not parallel that of CSF within the ventricles and cisterns on at least 1 MR image. Furthermore, fluid in 9 of the AGs did not parallel CSF on 3 sequences (47%). Five of these 9 AGs (26%) contained fluid that did not parallel CSF on all 4 standard MR images. Intra-AG fluid paralleled CSF in at least 1 sequence for all AGs.
T2WI showed CSF-incongruent signal intensity in the fluid of 13/19 AGs. Intra-AG fluid on T2WIs was hypointense to CSF in 6/19 AGs, isointense to CSF in 6/19 AGs, and mixed iso-and hypointense on 7/19 AGs. In the 10 AGs with precontrast T1WIs, intra-AG fluid was hyperintense to CSF in 5 AGs, isointense in 3, and mixed iso- and hypointense in 2.
Intra-AG fluid did not parallel CSF on 8/8 AGs with T1WI FLAIR imaging. AG fluid did not suppress and was hyperintense to CSF in 7/8 AGs. One AG showed hyperintensity within a loculated segment of the AG and signal intensity that was completely suppressed in the remainder of the AGs (Fig 1). In the single case with DWI, AG fluid did not demonstrate restricted diffusion (Fig 2). In the single case with T1WI inversion recovery, AG fluid was identical to CSF (Fig 3).
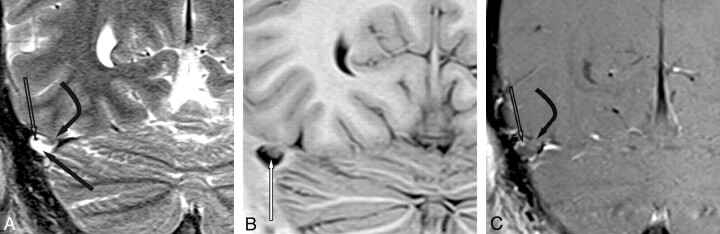
Fig 3.
TS giant AG with a soft-tissue mass. A, Coronal T2WI shows a 1-cm AG in the right TS. Fluid (arrow) is of CSF signal intensity; soft tissue (curved arrow) projects into the AG lumen through an opening in the dura. Note the flow void from a vein (open arrow) in the AG. B, Coronal inversion recovery image shows that of pedunculated soft tissue (arrow) at the base of the AG is isointense with adjacent gray matter, while the fluid is isointense with CSF. C, Coronal contrast-enhanced scan shows enhancement of a vein (open arrow) within the AG. Soft tissue (curved arrow) does not enhance.
CSF-like avascular filling defects within the opacified venous blood of the dural venous sinuses were identified in the single AG with CTV imaging, the 3 with MRV imaging, and the 3 with DSA imaging (Fig 2).
Veins, septations, or undetermined soft tissue were identified within 18/19 AGs. Twelve AGs demonstrated ≥1 intra-AG cortical vein crossing the subarachnoid space and entering the dural venous sinuses (Fig 2). MR imaging showed well-delineated linear flow voids for each of the 12 AGs with intra-AG veins. Veins were also identified by discrete intra-AG linear enhancement in 3 AGs with CECT imaging, 8 with contrast-enhanced T1WI imaging, 1 with CTV imaging, 3 with MRV imaging, and 2 with DSA imaging.
Soft-tissue stroma and/or septations were differentiated from CSF and intra-AG veins by nonenhancing gray matter signal intensity in 9 AGs. Of these, 3 showed nonenhancing linear tissue planes or septations spanning from the base to the apex of the AGs (Fig 1). Another 2 AGs from the same patient showed a thin layer of enhancement along tissue planes or septations following contrast administration on MR imaging. Distinct pedunculated soft-tissue nodules were seen at the base of 3 AGs (Fig 3).
Discussion
AGs are functionally and histologically related to AV, which are universally present and function in the filtration and resorption of CSF into the venous circulation. AV are formed by microscopic protrusions of arachnoid tissues into the venous sinuses via openings in the dura (Fig 4). It is thought that some AV hypertrophy is in response to increasing CSF volume and pressure, forming macroscopic lobulated AGs.1,8
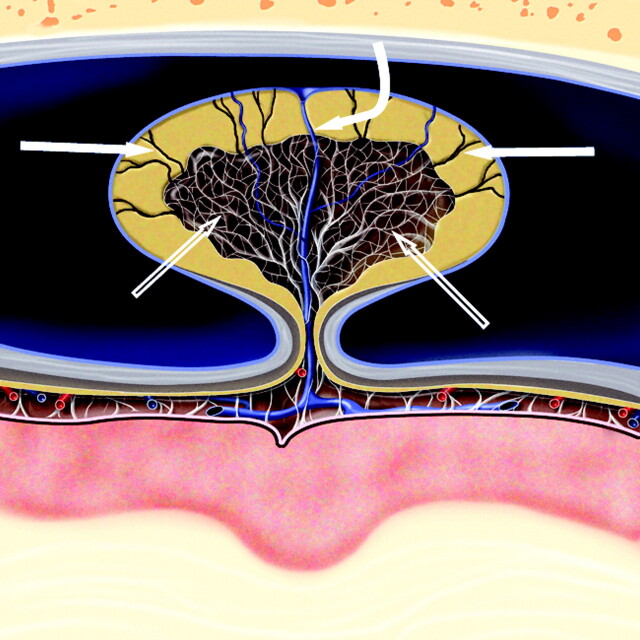
Fig 4.
Cross-sectional graphic of a giant venous sinus AG projecting into a dural venous sinus. A core of CSF-filled collagenous trabeculation (open arrows) extends from the subarachnoid space into the granulation and is covered by an apical cap of arachnoid cells. CSF channels (arrows) extend through the cap to the sinus endothelium and drain CSF into the venous circulation. A vein (curved arrow) also courses through the body of the AG, penetrates the arachnoid cap layer, and empties into the dural venous sinus. Graphic is used with permission from Amirsys Inc., Salt Lake City, Utah
AGs are present in approximately two-thirds of individuals in the population.3–7,8,13 They frequently occur in close relation to veins penetrating the dural venous sinuses, which are postulated to form weak areas in the dura through which perivascular arachnoid extrusion can occur.5,14 The dural covering at the base of the AG diminishes in thickness and regresses completely at its apex.15,16 The AG core is supported by trabeculated collagenous soft tissue and is filled with CSF from the contiguous subarachnoid space. CSF passes through channels in a “cap” of arachnoid cells which marginate the apex of the AG. It is thought that CSF is ultimately actively transported via vacuoles across a membrane of arachnoid cells at the periphery of the cap layer into the venous circulation.1,2 Compared with smaller AGs, larger granulations are more likely to contain fibrous soft tissue and internal veins.5,8,12
AGs have long been recognized on imaging studies. They were first identified on skull radiography as smoothly marginated impressions on the inner table of the calvaria and on the venous phase of cerebral angiograms as ovoid filling defects within the dural venous sinuses.9 Subsequently, CT and MR imaging signals of the intra-AG contents showed characteristics paralleling those of CSF.5,6 More recently, so-called giant AGs ranging from 1 to 2.4 cm have been reported.6,11,13,17–20
CSF-like attenuation on CT or fluid that parallels all MR images has been a conventional diagnostic criterion for AGs, though isolated exceptions to this general rule have been reported in the literature. Ikushima et al3 showed that 10% of AGs averaging 5.1 mm in diameter were slightly hyperintense to CSF on FLAIR imaging. Recently, Leach et al6 noted in passing that for AGs with an average size of 8.1 × 9.4 × 10.0 mm, intra-AG fluid may occasionally be FLAIR hyperintense. They commented that this appearance “may be due to pulsation artifact from the adjacent sinus and differing CSF flow characteristics within the granulation.”
The cause of CSF-incongruent MR imaging signal intensity within structures that clearly contain normal CSF will likely remain unknown because these structures are not biopsied, and analysis of actual intra-arachnoidal CSF is not performed. We postulate that spin dephasing due to disordered flow may account for the dissimilarity of the intra-AG fluid when compared with CSF in the adjacent subarachnoid spaces and ventricles. Altered CSF dynamics may be accentuated by stromal tissue frequently found in larger AGs.15,21 One AG showed lack of suppression of the fluid marginated by 2 intra-AG stromal tissue planes on FLAIR imaging (Fig 1). The same case showed complete suppression of fluid signal intensity within the remainder of the AG, suggesting the possibility that noncommunicating fluid within loculated cysts may also contribute to the dissimilarities between intra-AG fluid and CSF.
Vascular structures presumed to be veins were common in our series, supporting similar previously reported findings.6,12,12 These were identified as linear flow voids or focal contrast enhancement in regions both entering and within the AGs and were present in 63% of our 19 AGs.
Nonvascular soft tissue has also been reported in giant AGs and was variously interpreted as stromal collagenous tissue, hypertrophic arachnoid mesangial cell proliferation, or invaginated brain tissue.3,5–8,22–24 Nonvascular gray matter isointensities were identified in 9/19 of our AGs (47%). Of these, 5 showed linear tissue planes or septations, which may represent fibrous stromal tissue within the AG. Another 3 AGs demonstrated well-demarcated pedunculated soft-tissue nodules at the base of the AG, which may represent focal arachnoid cell proliferation or small meningoencephaloceles within the body of the AG.
Although most AGs communicate with the dural venous sinuses, a minority are located in regions of the temporal bone and do not communicate directly with venous circulation. These temporal bone and occipital bone AGs are thought to enlarge with time in response to CSF pulsations, which may lead to cephalocele formation and CSF leaks when located adjacent to pneumatized regions of the anterior skull base.25–29
Diffusion restriction, thought to be caused by intra-AG collagenous stromal tissue, has been reported in some larger AGs, though restricted diffusion was not seen in the single AG for which DWI imaging was available.5,8
Many investigators posit a broad differential diagnosis of giant AGs in the dural venous sinuses and include dural venous sinus thrombosis, calvarial osseous lesions, meningiomas, metastases, arachnoid cysts, dermoids, epidermoids, and extra-axial hemangiomas, including papillary endothelial hyperplasia (Masson vegetant hemangioendothelioma).9,10 With the exception of dural sinus thrombosis and meningioma, all these pathologies are rarely found in venous sinuses. Regardless of internal fluid and soft-tissue signals, all giant AGs are well-demarcated ovoid structures, differentiating them from dural venous sinus thrombus, which is typically elongated and sausage-shaped. Giant AGs do not enhance strongly and uniformly like typical neoplasms. MR imaging signal intensity within giant AGs is not fatlike, differentiating them from dermoids, and does not demonstrate restricted diffusion as do epidermoids.6
Although AGs are commonly differentiated from other pathologic entities by identifying intra-AG fluid paralleling CSF on all sequences, the present study suggests that fluid within most giant AGs does not consistently follow CSF on MR imaging. Because giant AGs contain fluid that does not always follow CSF and often contain vascular and stromal tissue, shape (round/ovoid), lack of solid contrast enhancement, and the absence of blooming artifacts are helpful features in differentiating giant AGs from more ominous pathology. Because we have demonstrated that MR imaging signal intensity in giant AGs is quite variable, we believe that the most definitive imaging study may be CT. In this small series, fluid in giant AGs measured CSF-like attenuation in all cases.
Our study is limited due to a nonrandom assortment of cases, lack of all conventional imaging sequences across the 17 patients, lack of original datasets for quantitative signal-intensity comparison, possible partial volume averaging artifacts, and lack of biopsy-proved results. Despite these limitations, our findings are, nevertheless, still striking. While all giant dural venous sinus AGs with CT imaging showed CSF-like attenuation, we found that nearly 80% of them did not follow CSF signal intensity on at least 1 MR image. Almost half had CSF-incongruent signal intensity on >1 series. FLAIR was the sequence that most commonly showed CSF-incongruent signal intensity (8/8, 100% of AGs), followed by precontrast T1WI (7/10, 70%), T2W1 (13/19, 68%), and postcontrast T1WI (8/14, 57%). Random sampling, quantitative region-of-interest signal-intensity analysis, and 1-mm sections in future studies would help confirm these findings.
The clinical significance of giant AGs is uncertain. While some large AGs may cause dural venous sinus pressure gradients and headaches, most are usually asymptomatic and incidental findings on imaging studies.17,18,20 They should be distinguished from other more ominous pathologies such as thrombus and neoplasm, and invasive studies such as biopsy should be assiduously avoided.
Conclusions
AGs occasionally exceed 1 cm in diameter and can be mistaken for pathologic processes in the dural venous sinuses. AGs are commonly diagnosed by identifying intra-AG fluid that is CSF-like on CT and parallels CSF signal intensity on all MR images. However, the present series demonstrates that approximately 80% of giant AGs contain CSF-incongruent fluid on at least 1 MR image and nearly half contain fluid that does not parallel CSF on at least 2 sequences. FLAIR is the least reliable, showing CSF-incongruent signal intensity in 100% of AGs in the present study.
Abbreviations
- AG
arachnoid granulation
- AV
arachnoid villus
- CAQ
Certificate of Added Qualification
- CECT
contrast-enhanced CT
- CTV
CT venography
- DSA
digital subtraction angiography
- DWI
diffusion-weighted imaging
- FLAIR
fluid-attenuated inversion recovery
- MRV
MR venography
- SSS
superior sagittal sinus
- T1WI
T1-weighted imaging
- T2WI
T2-weighted imaging
- TS
transverse sinus
Footnotes
Paper previously presented as a scientific poster at: 48th Annual Meeting of the American Society of Neuroradiology, May 17–20, 2010; Boston, Massachusetts.
References
- 1. le Gros Clark WE. On the Pacchionian bodies. J Anat 1920; 55 ( pt 1): 40– 48 [PMC free article] [PubMed] [Google Scholar]
- 2. Grzybowski DM, Holman DW, Katz SE, et al. In vitro model of cerebrospinal fluid outflow through human arachnoid granulations. Invest Ophthalmol Vis Sci 2006; 47: 3664– 72 [DOI] [PubMed] [Google Scholar]
- 3. Ikushima I, Korogi Y, Makita O, et al. MRI of arachnoid granulations within the dural sinuses using a FLAIR pulse sequence. Br J Radiol 1999; 72: 1046– 51 [DOI] [PubMed] [Google Scholar]
- 4. Koshikawa T, Naganawa S, Fukatsu H, et al. Arachnoid granulations on high-resolution MR images and diffusion-weighted MR images: normal appearance and frequency. Radiat Med 2000; 18: 187– 91 [PubMed] [Google Scholar]
- 5. Leach JL, Jones BV, Tomsick TA, et al. Normal appearance of arachnoid granulations on contrast-enhanced CT and MR of the brain: differentiation from dural sinus disease. AJNR Am J Neuroradiol 1996; 17: 1523– 32 [PMC free article] [PubMed] [Google Scholar]
- 6. Leach JL, Meyer K, Jones BV, et al. Large arachnoid granulations involving the dorsal superior sagittal sinus: findings on MR imaging and MR venography. AJNR Am J Neuroradiol 2008; 29: 1335– 39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liang L, Korogi Y, Sugahara T, et al. Normal structures in the intracranial dural sinuses: delineation with 3D contrast-enhanced magnetization prepared rapid acquisition gradient-echo imaging sequence. AJNR Am J Neuroradiol 2002; 23: 1739– 46 [PMC free article] [PubMed] [Google Scholar]
- 8. Roche J, Warner D. Arachnoid granulations in the transverse and sigmoid sinuses: CT, MR, and MR angiographic appearance of a normal anatomic variation. AJNR Am J Neuroradiol 1996; 17: 677– 83 [PMC free article] [PubMed] [Google Scholar]
- 9. Grossman CB, Potts DG. Arachnoid granulations: radiology and anatomy. Radiology 1974; 113: 95– 100 [DOI] [PubMed] [Google Scholar]
- 10. Peters SA, Frombach E, Heyer CM. Giant arachnoid granulation: differential diagnosis of acute headache. Australas Radiol 2007; 51 ( spec no.): B18– 20 [DOI] [PubMed] [Google Scholar]
- 11. Kan P, Stevens EA, Couldwell WT. Incidental giant arachnoid granulation. AJNR Am J Neuroradiol 2006; 27: 1491– 92 [PMC free article] [PubMed] [Google Scholar]
- 12. Farb RI. The dural venous sinuses: normal intraluminal architecture defined on contrast-enhanced MR venography. Neuroradiology 2007; 49: 727– 32 [DOI] [PubMed] [Google Scholar]
- 13. Haroun AA, Mahafza WS, Al Najar MS. Arachnoid granulations in the cerebral dural sinuses as demonstrated by contrast-enhanced 3D magnetic resonance venography. Surg Radiol Anat 2007; 29: 323– 28 [DOI] [PubMed] [Google Scholar]
- 14. Krisch B. Ultrastructure of the meninges at the site of penetration of veins through the dura mater, with particular reference to Pacchionian granulations: investigations in the rat and two species of New-World monkeys (Cebus apella, Callitrix jacchus). Cell Tissue Res 1988; 251: 621– 31 [DOI] [PubMed] [Google Scholar]
- 15. Wolpow ER, Schaumburg HH. Structure of the human arachnoid granulation. J Neurosurg 1972; 37: 724– 27 [DOI] [PubMed] [Google Scholar]
- 16. Yamashima T. Ultrastructural study of the final cerebrospinal fluid pathway in human arachnoid villi. Brain Res 1986; 384: 68– 76 [DOI] [PubMed] [Google Scholar]
- 17. Chin SC, Chen CY, Lee CC, et al. Giant arachnoid granulation mimicking dural sinus thrombosis in a boy with headache: MRI. Neuroradiology 1998; 40: 181– 83 [DOI] [PubMed] [Google Scholar]
- 18. Kiroglu Y, Yaqci B, Cirak B, et al. Giant arachnoid granulation in a patient with benign intracranial hypertension. Eur Radiol 2008; 18: 2329– 32. Epub 2008 May 6 [DOI] [PubMed] [Google Scholar]
- 19. Mamourian AC, Towfighi J. MR of giant arachnoid granulation: a normal variant presenting as a mass within the dural venous sinus. AJNR Am J Neuroradiol 1995; 16: 901– 04 [PMC free article] [PubMed] [Google Scholar]
- 20. Sunbulli M, Zak I, Chaturvedi S. Giant arachnoid granulations. Neurology 2005; 64: 2150 [DOI] [PubMed] [Google Scholar]
- 21. Upton ML, Weller RO. The morphology of cerebrospinal fluid drainage pathways in human arachnoid granulations. J Neurosurg 1985; 63: 867– 75 [DOI] [PubMed] [Google Scholar]
- 22. Celli P, Cervoni L, Quasho R. An asymptomatic hypertrophic Pacchionian granulation simulating osteolytic lesion of the calvaria. Neurosurg Rev 1999; 22: 149– 51 [DOI] [PubMed] [Google Scholar]
- 23. Kuroiwa T, Kajimoto Y, Ohta T, et al. Symptomatic hypertrophic pacchionian granulation mimicking bone tumor: case report. Neurosurgery 1996; 39: 860– 62 [DOI] [PubMed] [Google Scholar]
- 24. Kuroiwa T, Takeuchi E, Tsutsumi A. Ectopic arachnoid granulomatosis: a case report. Surg Neurol 2001; 55: 180– 86, discussion 186 [DOI] [PubMed] [Google Scholar]
- 25. Brodie HA. Prophylactic antibiotics for posttraumatic cerebrospinal fluid fistulae: a meta-analysis. Arch Otolaryngol Head Neck Surg 1997; 123: 749– 52 [DOI] [PubMed] [Google Scholar]
- 26. Gacek RR. Arachnoid granulation cerebrospinal fluid otorrhea. Ann Otol Rhinol Laryngol 1990; 99: 854– 62 [DOI] [PubMed] [Google Scholar]
- 27. Schuknecht B, Simmen D, Briner HR, et al. Nontraumatic skull base defects with spontaneous CSF rhinorrhea and arachnoid herniation: imaging findings and correlation with endoscopic sinus surgery in 27 patients. AJNR Am J Neuroradiol 2008; 29: 542– 49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stone JA, Castillo M, Neelon B, et al. Evaluation of CSF leaks: high-resolution CT compared with contrast-enhanced CT and radionuclide cisternography. AJNR Am J Neuroradiol 1999; 20: 706– 12 [PMC free article] [PubMed] [Google Scholar]
- 29. VandeVyver V, Lemmerling M, De Foer B, et al. Arachnoid granulations of the posterior temporal bone wall: imaging appearance and differential diagnosis. AJNR Am J Neuroradiol 2007; 28: 610– 12 [PMC free article] [PubMed] [Google Scholar]