Abstract
BACKGROUND AND PURPOSE:
Improved selection of patients with stroke for IV tPA treatment may enhance clinical outcomes. Given the limited availability of MR imaging in hospitals, we examined the cost-effectiveness of adding CTP to the usual CT-based methods for selecting patients on the basis of the presence and extent of penumbra.
MATERIALS AND METHODS:
A decision-analytic model estimated the costs and outcomes associated with penumbra-based CTP selection in a patient population similar to that enrolled in the IV tPA clinical trials. Model inputs were obtained from published literature, clinical trial data, standard US costing sources, and expert opinion. Cost per life-year saved and cost per QALY gained were estimated from a hospital perspective.
RESULTS:
Addition of penumbra-based CTP to standard unenhanced CT improved favorable outcome (mRS, ≤1) by 0.59% and reduced cost by $42 compared with selection based on unenhanced CT alone. Life-years and QALYs improved. Multivariate sensitivity analysis predicted cost-effectiveness (≤$50,000 per QALY) in 89.2% of simulation runs.
CONCLUSIONS:
Using penumbra-based CTP after routine CT to select patients with ischemic stroke for IV tPA is cost-effective compared with the usual CT-based methods for hospitals. With the ease of access of CTP, penumbra-based selection methods may be readily available to hospitals. Thus, this economic analysis may lend further support to the consideration of a paradigm shift in acute stroke evaluation.
Brain imaging with unenhanced CT is the standard diagnostic test used to distinguish hemorrhagic and nonhemorrhagic stroke so that appropriate thrombolytic evaluation can occur and treatment can be administered if appropriate.1,2 Penumbral brain MR imaging studies have shown a potential for identifying patients who may benefit from IV tPA treatment beyond 3 hours (eg, perfusion lesion greater than diffusion lesion volume by ≥20%).3,4 However, the use of MR imaging to perform penumbral imaging in addition to standard CT can be costly in terms of dollars and time, may require specialized technicians to be available to administer and interpret scans, and may not be readily available or in close proximity to the emergency department at many hospitals. CTP studies have been performed to understand which parameters best define infarct and penumbra.5 Thus, CTP might be considered a possible practical alternative in examining penumbra.
Treating acute ischemic stroke with IV tPA between 3 and 4.5 hours increases the potential pool of patients treated in this manner and has been shown to be beneficial.6 However, the question of optimal patient selection to reduce the risk of hemorrhage and increase the therapeutic index of tPA in this extended window is still relevant.
In a recent analysis, we found that penumbra-based MR imaging when added to unenhanced CT in selecting patients for IV tPA within the indicated 3-hour window was cost-effective from a general payer perspective.7 With the limited availability of MR imaging equipment and staff, the initial cost to set up a penumbra-based CTP program may be a more favorable alternative. We sought to determine if CTP-based methods of patient selection for thrombolysis would also be cost-effective.
Materials and Methods
Overview and Patient Population
A decision-analytic model was constructed on the basis of the previous MR imaging analysis7 to examine the cost and outcome associated with the treatment of patients with suspected stroke with either the usual acute stroke care or the usual acute stroke care with the addition of CTP to better select candidates for IV tPA treatment. Patients in the analysis were assumed to be similar to patients observed in the pooled analysis of ATLANTIS, ECASS, and the National Institute of Neurological Disorders and Stroke tPA trials,8 and Schellinger et al4 with baseline National Institutes of Health Stroke Scale scores that ranged from 11 to 14 and a mean age of 68 years, with most (85%) of the patients being white and non-Hispanic.
Comparators
Results from ECASS III reported an improved favorable outcome for patients treated with IV tPA over patients treated with a placebo between 3 and 4.5 hours. Thus, in the analysis presented in this article, we have included patients into the usual care arm of the model who underwent an unenhanced CT alone, patient history, and treatment with IV tPA within the expanded treatment time of 3–4.5 hours from onset of stroke symptoms, if the patient had no contraindications to IV tPA by ECASS III criteria.6 Other standard treatments, such as antiplatelet therapy or mechanical clot retrieval by FDA-approved devices, are assumed to be administered to patients beyond 4.5 hours. A secondary analysis was performed in which patients in the usual care arm were treated with IV tPA as indicated (ie, unenhanced CT alone, patient history, and treatment with IV tPA within the indicated 3 hours from onset of stroke symptoms) compared with adding a penumbra-based CTP.
Patients in the CTP arm of the model received unenhanced CT and patient history similar to those in the usual care arm. However, patients with acute ischemic stroke also received brain imaging via CTP, and IV tPA treatment was assumed to be administered to patients ≤6 hours after symptom onset if the patient had evidence of penumbra and no contraindications to IV tPA other than the time delay. If penumbra was not found in these patients, then other standard treatments were given on the basis of results from the unenhanced CT and patient history. Patient eligibility and dose of tPA administered were assumed to be per FDA labeling.9 On the basis of a study of 9 US clinics, 43.2% of patients arriving at the hospital <3 hours after onset of stroke symptoms would have contraindications for IV tPA.10
Model Structure
A decision tree model, programmed in Excel (Microsoft, Bothell, Washington), similar to that presented in a previous analysis,7 was developed to examine the cost-effectiveness of the use of CTP during the course of the patient's hospital stay (On-line Fig 1). Details of patient flow are presented in the On-Line Appendix.
Because stroke outcome is typically measured on the basis of a 90-day mRS of 0–6 and impact on outcome data before 90 days is limited in the literature, these data are used to proxy patient outcome at hospital discharge. Both adverse events and stroke outcome are assumed to influence a patient's length of stay in the hospital for the index event. After discharge from the hospital, a proportion of patients may experience hospitalization for recurrent stroke. The impact of extended time horizons of 1 and 5 years includes recurrent stroke and is examined in scenario analyses. Total hospital cost and outcome are estimated for each outcome state that may occur ≤90 days following the index stroke hospitalization.
The model assumes that all patients are admitted to a hospital where IV tPA can be administered. Thus, patients are not transferred to another facility for treatment. In analyses that extend beyond the index hospitalization, patients also are assumed to return to the same hospital as in the index stroke hospitalization for the recurrent stroke.
Input Parameters
Clinical Efficacy.
For patients in whom CTP was not performed and who received other standard treatments (ie, not IV tPA) within and beyond 4.5 hours, clinical outcomes were estimated from a pooled analysis of the IV tPA trials.8 In patients receiving IV tPA, favorable outcome (ie, mRS <2) was found to improve compared with patients not receiving IV tPA by an adjusted odds ratio of 2.81 (95% CI, 1.75–4.50; P value not reported) and 1.55 (95% CI, 1.12–2.15; P value not reported) in patients treated within 1.5 and between 1.5 and 3.0 hours after onset of stroke, respectively.8 The recent ECASS III clinical trial reported improvement of favorable outcome for patients receiving IV tPA within 3.0–4.5 hours with an odds ratio of 1.34 (95% CI, 1.02–1.76; P value = .04) compared with those not receiving IV tPA.6 Data from these publications were used to estimate the percentage of patients treated with the usual care in each mRS group. These percentages are presented in On-line Table 1.
The impact of the use of penumbra-based CTP on clinical outcome has not been directly quantified. As a result, the impact of CTP on stroke outcome is assumed to be equivalent to the estimates published for penumbra-based MR imaging regarding treatment in <3.0 hours and in 3.0–6.0 hours. Specifically, Schellinger et al4 reported odds ratios of favorable outcome (ie, mRS <2) among patients selected for IV tPA treatment via MR imaging of 1.467 (95% CI, 1.017–2.117; P value = .04) and 1.136 (95% CI, 0.841–1.534; P value > .05) and being treated >3 and <3 hours compared with unenhanced CT alone. These data were used to derive the percentage of patients with 90-day mRS for CTP IV tPA≤3.0 hours and CTP IV tPA>3.0 hours and are presented in On-line Table 1.
Adverse Events.
Major adverse events addressed in the model are SICH due to the administration of IV tPA and contrast-induced nephropathy due to the administration of contrast media during CTP. All other adverse events were assumed to have minimal impact on resource use while the patient was in the hospital.
SICH or transformation of infarction into a SICH was estimated from the IV tPA pooled analysis, ECASS III, and the clinical study of better selection via MR imaging.4,6,8 Hacke et al8 reported the percentage of SICHs occurring in patients treated with placebo or IV tPA within different time windows after symptom onset (On-line Table 1). For this analysis, we assume the incidence of SICH from the pooled analysis for patients treated with IV tPA and other treatments. The relative difference in SICH between patients treated with IV tPA and placebo in the pooled analysis was observed to be lower than that reported in ECASS III. As a result, this analysis is conservative in favor of the usual care. We examined the impact of these data in sensitivity analysis in which the incidence of SICH within 3.0–4.5 hours after onset of stroke for patients treated with IV tPA with no CTP was 5.9% (range: 2.1% [1.26 odds ratio from ECASS III × 1.7% placebo SICH]6,8 to 8.7% [upper bound on SICH observed in any time window]8).
If penumbra-based MR imaging selection was performed, SICH was found to be significantly reduced when 5.3%, 2.8%, and 4.4% of patients were treated with IV tPA in < 3 hours with unenhanced CT-selection only, with IV tPA in <3 hours with MR imaging selection, and with IV in tPA>3 hours with MR imaging selection, respectively.11 Mortality due to SICH was assumed to be 46.7% in patients treated with other standard treatments and 62.2% in patients treated with IV tPA.8 This percentage was assumed to be constant, regardless of selection technique.8 The incidence for SICH is presented in On-line Table 1.
Imaging.
Patterns of penumbra, defined as PWI>DWI by ≥20%, were assumed to exist in 61.7% of patients.12 If penumbra existed in patients, we assumed that the presence and extent could be determined 100% of the time if an MR imaging was performed. On the basis of the sensitivity, specificity, and accuracy of determining the presence and extent of penumbra via CTP versus MR imaging, CTP was assumed to accurately image penumbra 89.2% of time.5 Unenhanced CT and CTP diagnostics were assumed to be available 24 hours a day for 7 days a week (24/7), and patients were assumed not to have contraindications for CTP or CT scanning. Interpretation of scans in combination with patient history should diagnose hemorrhagic stroke with 100% accuracy. Extensive sensitivity analysis was performed around all parameters
Timing Data.
As in the previous MR imaging−based analysis,7 successful treatment of acute stroke is dependent on timely and proper administration of treatment.8,9 Total time from onset of stroke to acute stroke treatment was estimated by summing average times from stroke onset to arrival at the emergency department, time from arrival at the emergency department to determination of acute stroke treatment, and additional time due to administration and interpretation of CTP as reported in the published literature. An overall mean time was estimated at 436 minutes.13,14
Cost.
Costs in the model are those that would be incurred by a hospital and were obtained from standard US costing sources or published literature (On-line Table 2). Details are presented in the On-Line Appendix. All costs in the model were reported in 2008 US dollars, inflated by using the Medical Consumer Price Index15 and discounted at 3% per annum16 when appropriate.
Mortality.
Mortality due to stroke, SICH, and all causes was considered in the model. Ischemic stroke and SICH mortality rates were estimated from the clinical studies and are reported in On-line Table 1. Mortality due to hemorrhagic stroke was estimated from Earnshaw et al.17 In addition to stroke and SICH mortality, all-cause mortality was considered in scenario analyses in which the time horizon was extended to 1 and 5 years and was obtained from the US National Vital Statistics Reports.18 Age- and sex-specific life tables were obtained and adjusted by mRS-specific death hazard ratios reported by Samsa et al19 to estimate life expectancy for stroke survivors in these scenario analyses. Life expectancy was allowed to decrease as patients age. Mortality due to stroke and SICH are presented in On-line Table 1. Death hazard ratios for adjusting patient life expectancy are presented in On-line Table 2.
Utility Weights.
Utility weights, by mRS group status, were obtained from a study by Samsa et al.19 These all-stroke utility weights are presented in On-line Table 2. Life-years and QALYs were discounted at 3% per annum when appropriate.
Model Calculations.
The model is designed to calculate costs, life-years, QALYs, percentage of patients with ischemic stroke treated, percentage of patients with 90-day mRS score 0–6, and percentage of patients with favorable outcome (mRS, <2). Incremental cost-effectiveness ratios calculated include the following: incremental cost per life-year gained, incremental cost per QALY gained, and incremental cost to avoid major disability (mRS, ≥4).
Sensitivity Analysis.
To test the robustness of the assumptions and specific parameters of the model, we examined the effect of changing several parameters in 1-way, scenario, and probabilistic analyses. The effect of varying individual parameters was examined by using plausible ranges of values from the literature (On-line Tables 1 and 2), 95% CIs, or by varying the estimates by ≤20% in each direction. One-way analysis is presented in the form of a tornado diagram.
In probabilistic sensitivity analysis (second-order Monte Carlo simulation), all parameters were varied simultaneously. The analysis was run 10,000 times to capture stability in the results and uncertainty represented in a scatterplot.
Results
Base-Case Analysis
From the immediate hospital perspective, our analysis suggests that fewer patients with ischemic stroke would be treated with IV tPA when adding CTP due to the ability to select out less favorable patients (eg, those who may be at higher risk for SICH in the 3 to <4.5 hours) and to include patients who could benefit from IV tPA treatment at >4.5 hours (Table 1). Although fewer patients are treated, overall favorable outcome is improved slightly. Thus, CTP improves life-years by 0.0004 and QALYs by 0.0005. Even though patients undergoing CTP receive additional diagnostic costs, these costs are largely offset because of the reduction in use of IV tPA, which is an expensive medication. Thus, adding CTP reduces the overall cost to the hospital and was found to be cost-saving (ie, more efficacious and less costly).
Table 1:
Costs and outcomes associated with selecting patients for IV tPA treatment with CTP versus CT scanning base-case results
| Outcome (per patient) | Usual Care | CTP |
|---|---|---|
| % of patients with ischemic stroke treated | 28.30% | 20.94% |
| % of all patients with stroke treated | 24.62% | 18.22% |
| % of all patients with stroke who receive CTP | 0.00% | 49.42% |
| Favorable outcome (mRS, 0.1) | 39.44% | 40.03% |
| mRS outcomes for patients with ischemic stroke | ||
| % with 90-day mRS 0 | 18.41% | 18.23% |
| % with 90-day mRS 1 | 21.03% | 21.80% |
| % with 90-day mRS 2 | 12.56% | 11.96% |
| % with 90-day mRS 3 | 13.14% | 13.27% |
| % with 90-day mRS 4 | 15.75% | 16.13% |
| % with 90-day mRS 5 | 7.13% | 6.78% |
| % dead at 90 days | 11.98% | 11.83% |
| Life-years | 0.2115 | 0.2119 |
| QALYs | 0.1241 | 0.1246 |
| Hospitalization costs (including SICH) (in US $) | $11,400 | $11,358 |
| Novel diagnostic costs (in US $) | $0 | $236 |
| Drug costs (in US $) | $1,070 | $792 |
| Total costs (in US $) | $12,470 | $12,386 |
| Incremental cost per life-year gained (in US $) | – | Cost-saving |
| Incremental cost per QALY gained (in US $) | – | Cost-saving |
| Incremental cost per major disability avoided (mRS, ≥3) (in US $) | Cost-saving |
When extending the time horizon of the analysis from 90 days to 1 and 5 years, we observe greater benefit and lower cost difference because the true benefit of CTP selection and IV tPA to patients extends beyond the time horizon of the index hospitalization. Thus, CTP remains a cost-saving strategy at both a 1- and 5-year time horizon.
In a secondary analysis, when we compared CTP selection with the usual care where IV tPA is administered only within the indicated 3 hours, hospitals may still expect to treat fewer patients with IV tPA. The addition of CTP results in similar costs and outcomes compared with the usual care.
Sensitivity Analyses
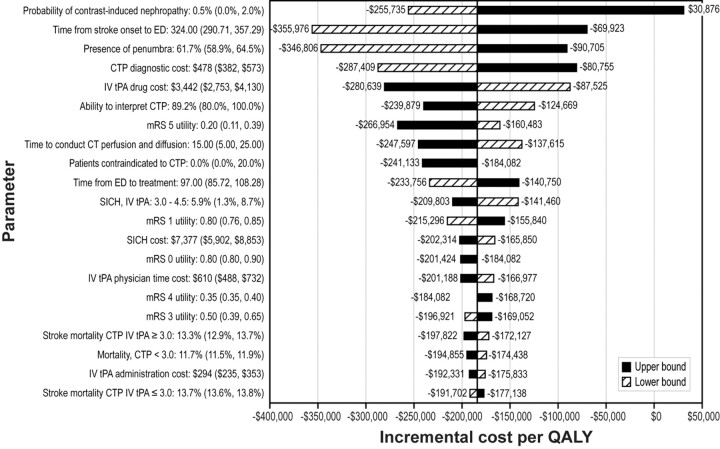
One-way sensitivity analysis evaluates the effect of varying input parameters on the incremental cost per QALY of CTP versus CT-based selection (Fig 1). Overall, the results were most sensitive to changes in the probability of contrast-induced nephropathy; however, even at the upper bound of its acceptable range, the incremental cost per QALY is cost-effective (ie, incremental cost per QALY < $50,000).
Fig 1.
One-way sensitivity analysis: effect of parameter variation on the incremental cost per QALY for patient selection using penumbra-based CTP compared with CT. Dark-shaded bars represent the upper bound. Striped bars represent the lower bound. Baseline incremental cost / QALY = −$184,082.
Two parameters, which are not shown in the tornado diagram, had a significant impact on the incremental cost per QALY. These parameters are the odds of favorable outcome for patients receiving CTP imaging and IV tPA≤3.0 hours and the odds of favorable outcome for patients receiving CTP imaging and IV tPA>3.0 hours. When the odds of favorable outcome for patients receiving CTP imaging and IV tPA were set at their lower bound for either parameter (ie, CTP imaging has less impact on efficacy), CTP is less costly and less efficacious. As expected, when the odds of favorable outcome for patients receiving CTP imaging and IV tPA were set at their upper bound, the results remained cost-saving compared with CT alone.
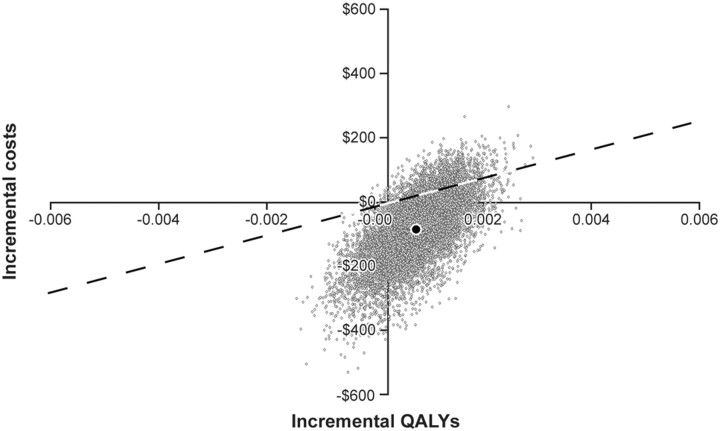
Results of the 10,000-iteration probabilistic sensitivity analysis (Fig 2) showed that in 59.4% of simulation runs, CTP had lower costs and greater QALYs than CT-based selection alone. CTP selection was cost-effective in 89.15% of the simulation runs (ie, cost-saving or incremental cost per QALY ≤ $50,000); it remained cost-saving in 78.4% of those runs.
Fig 2.
Results of probabilistic sensitivity analysis: incremental cost-effectiveness scatterplot of patient selection using CTP versus CT for 90 days following primary stroke. Dotted line represents an incremental cost / QALY = $50,000. Points to right of the dotted the line are considered cost-effective. Gray dots represent simulations. The black dot represents the base-case result.
Discussion
CTP provides an alternative diagnostic approach for hospitals in examining the presence and extent of penumbra. Despite the increase in imaging costs and a potential for delay in treating patients (eg, due to increased time taken to perform the CTP if IV tPA is not given immediately after the unenhanced CT), penumbra-based CTP selection was shown to decrease mortality and improve functional outcome in patients on discharge from the hospital. Overall, costs to the hospital were comparable. When extending the IV tPA treatment to 4.5 hours, selection with CTP results in a cost-saving approach to the hospital. Sensitivity analyses showed these results to be insensitive to changes in most parameters, with the exception of changes in the odds of achieving favorable outcome for CTP tPA≤3 and CTP tPA>3. In these scenarios, the reduced costs of better selection of patients could not be overcome by the odds of favorable outcome if the probability of a favorable outcome was estimated at its lowest boundary. Probabilistic sensitivity analysis confirmed that CTP selection would be cost-effective in 89% of situations. These models all assume that there is no alternative method for penumbral detection (eg, MR imaging) that is readily available.
In this analysis, we observed that CTP would reduce the number of patients (by 0.8%) who receive IV tPA when considering treatment within 3.0–4.5 hours, while improving overall outcomes. This reduction occurred by selecting fewer patients within the first 3 hours. If we assume that all patients within 3 hours were treated and we examined only the cost-effectiveness of the use of CTP in patients 3–4.5 hours after stroke onset, we observe an incremental cost per QALY >$100,000. However, note that this only accounts for the cost-effectiveness in the short term (ie, hospital perspective). In an analysis that considers the full benefits of improved outcomes due to the selection of patients by using CTP, we would observe an incremental cost per QALY of well under $50,000 because the additional initial $500 cost of undergoing CTP is offset by reduced downstream medical costs.
We recognize that this model has a number of limitations. A key limitation is that there is no direct evidence supporting the assumption that the use of CTP leads to improved functional outcome. We also recognize that CTP cannot provide the MR imaging–specific information such as diffusion imaging–weighting infarct volumes and detection of microbleeds that is useful for potentially reducing the risk of bleeding. For this analysis, better selection by penumbral detection was drawn by inference from the MR imaging literature, with a reduction in selection ability4 based on the lower expected accuracy and whole-brain coverage of CTP compared with MR imaging. Even with this reduction, CTP demonstrated promising cost-effectiveness compared with the usual care of unenhanced CT. Our data should not be interpreted as scientific evidence that penumbral detection by CTP should now be used to withhold IV tPA from otherwise eligible patients who present within 3 hours but rather that if data do accumulate that support this hypothesis, then CTP will be increasingly cost-effective.
Sensitivity analysis showed that the results are sensitive to the clinical impact that CTP might truly demonstrate. Specifically, we saw that when the odds of favorable outcome for patients undergoing CTP imaging were at their lower bounds (ie, close to no effect in the case of treating beyond 3 hours and a negative effect when treating within 3 hours), the use of CTP became less efficacious. When running a scenario in which penumbra-based CTP provides no benefit in terms of favorable outcome and reduction in SICH, we also see that CTP is less efficacious because fewer patients will be treated as a result of the additional time it takes to perform the CTP. Thus, patients become ineligible for IV tPA because they now fall outside the treatment time window. As a result, additional research will be important to further support these results.
Another limitation of the analysis is the ability to accurately estimate the cost of implementing and performing CTP 24/7 in a hospital that does not already have this service. This additional cost might include that for specialized training of other CT technologists and having them in the hospital 24/7 so as not to limit the ability of other x-ray–based technologists to help support acute stroke response requirements. Increased cost due to additional wear and tear on CT machines, increased data storage needs, and the need for physician supervision of contrast infusion should also be considered. However, because most hospitals already have CT, there is little cost to upgrade to CTP. To examine the impact of this assumption in the analysis, we extended the upper bound of the cost of CTP to account for these additional costs. When increasing the cost by 45%, the incremental cost per QALY is $50,000. Increasing the cost by 54% increases the incremental cost per QALY to $100,000. Thus, implementation costs may have a great effect. Complete implementation cost analyses within specific hospital settings will be important to help fully estimate the financial impact.
Another potential limitation of this analysis is that the use of a modeling approach of a decision tree was chosen over the more traditionally used Markov model. Because we set out to examine the impact that IV tPA selection methods have on costs and outcomes from a hospital perspective, we thought that the most appropriate modeling to use was a decision tree approach.29 The advantage is that we can capture the details of timing and different clinical pathway decisions that occur in the short time horizon much better in a decision tree approach. For the longer term scenario analyses we performed, we extended the decision to account for the extended time rather than run patients through a Markov component. Although Markov methods are typically thought of as being more appropriate for modeling longer term time horizons, we also believe that use of a Markov component would not greatly alter the results. More important from a hospital perspective, it is unknown whether patients with recurrent stroke will return to the same hospital for treatment. Therefore, the use of longer term horizons for an analysis undertaken from the individual hospital perspective requires careful consideration.
In this analysis, we developed a model to compare the use of penumbra-based CTP selection with the usual unenhanced CT–based methods. Because we did not extend this analysis to examine how CTP-based methods compare with MR imaging–based methods, we cannot comment on the relative effectiveness compared with MR imaging selection. Furthermore, it could be argued that if CTP is cost-effective then perhaps CT angiography may be cost-effective as well. These are all important points. Overall, we observed that the use of additional diagnostics to assist in determining appropriate treatment for acute stroke may have a positive cost impact. As a result, it will be important to perform additional analyses to understand how these penumbra-based and anatomic methods compare with one another.
Conclusions
In summary, diagnostic imaging with CTP may provide hospitals and clinicians with greater access and a more cost-efficient alternative to improve stroke outcomes with IV tPA based on penumbral selection. These effects might be synergistic with advantages gleaned from performing CT angiography to identify patients with proximal vessel occlusions. Even in the short term, we observed that using penumbra-based CTP selection after routine CT would be cost-effective for hospitals. Thus, this economic analysis may lend further support to the consideration of a paradigm shift in acute stroke evaluation.
Supplementary Material
Abbreviations
- CI
confidence interval
- CTP
perfusion CT
- DWI
diffusion-weighted imaging
- ECASS
European Cooperative Acute Stroke Study
- ED
emergency department
- FDA
US Food and Drug Administration
- Hrs
hours
- ICD-9
international classification of diseases, 9th revision
- ICH
intracranial hemorrhage
- IV
intravenous
- IV tPA
intervenous tissue plasminogen activator
- mRS
modified Rankin Scale
- PWI
perfusion-weighted imaging
- QALY
quality-adjusted life-year
- SAH
subarachnoid hemorrhage
- SICH
symptomatic intracerebral hemorrhage
Footnotes
This work was funded by GE Healthcare.
Drs. Earnshaw and Farkouh are employees of RTI Health Solutions, an independent contract research organization that has received research funding for this and other studies from GE Healthcare and pharmaceutical companies that market medical devices, diagnostics, and drugs for use in the treatment of patients with stroke and other medical conditions. Mr. Jackson is an employee of GE Healthcare, a medical device and diagnostics manufacturer. Dr. Schwamm is Vice Chairman of the Department of Neurology at Massachusetts General Hospital and Associate Professor of Neurology at the Harvard Medical School. He is a paid consultant for RTI Health Solutions. He serves on the advisory board of Coaxia, a maker of medical devices for acute stroke treatment, and serves on the international steering committee for the DIAS3/4 trial, a trial of extended window thrombolysis using desmoteplase; he occasionally provides expert legal testimony in court cases involving acute stroke management.
Indicates article with supplemental on-line appendix and tables.
Indicates article with supplemental on-line figure.
References
- 1. Adams HP, Jr, del Zoppo G, Alberts MJ, et al. , for the American Heart Association, American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups—the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 2007; 38: 1655– 711 [DOI] [PubMed] [Google Scholar]
- 2. Intercollegiate Stroke Working Party. National Clinical Guidelines for Stroke. London: Royal College of Physicians; 2004 [Google Scholar]
- 3. Thomalla G, Schwark C, Sobesky J, et al. Outcome and symptomatic bleeding complications of intravenous thrombolysis within 6 hours in MRI-selected stroke patients: comparison of a German multicenter study with the pooled data of ATLANTIS, ECASS, and NINDS tPA trials. Stroke 2006; 37: 852– 58 [DOI] [PubMed] [Google Scholar]
- 4. Schellinger PD, Thomalla G, Fiehler J, et al. MRI-based and CT-based thrombolytic therapy in acute stroke within and beyond established time windows: an analysis of 1210 patients. Stroke 2007; 38: 2640– 45 [DOI] [PubMed] [Google Scholar]
- 5. Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-CT assessment of infarct core and penumbra receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 2006; 37: 979– 85 [DOI] [PubMed] [Google Scholar]
- 6. Hacke W, Kaste M, Bluhmki E, et al. , for the ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317– 29 [DOI] [PubMed] [Google Scholar]
- 7. Earnshaw SR, Jackson D, Farkouh R, et al. Cost-effectiveness of patient selection using penumbral-based MRI for intravenous thrombolysis. Stroke 2009; 40: 1710– 20 [DOI] [PubMed] [Google Scholar]
- 8. Hacke W, Donnan G, Fieschi C, et al. , and the ATLANTIS Trials Investigators, ECASS Trials Investigators, and NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004; 363: 768– 74 [DOI] [PubMed] [Google Scholar]
- 9. Activase (alteplase) [package insert]. San Francisco, California: Genentech; 2005 [Google Scholar]
- 10. Katzan IL, Hammer MD, Hixson ED, et al. Cleveland Clinic Health System Stroke Quality Improvement Team: utilization of intravenous tissue plasminogen activator for acute ischemic stroke. Arch Neurol 2004; 61: 346– 50 [DOI] [PubMed] [Google Scholar]
- 11. Schellinger PD, Fiebach JB, Hacke W. Imaging-based decision making in thrombolytic therapy for ischemic stroke: present status. Stroke 2003; 34: 575– 83 [PubMed] [Google Scholar]
- 12. Darby DG, Barber PA, Gerraty RP, et al. Pathophysiological topography of acute ischemia by combined diffusion-weighted and perfusion MRI. Stroke 1999; 30: 2043– 52 [DOI] [PubMed] [Google Scholar]
- 13. Morris DL, Rosamond W, Madden K, et al. Prehospital and emergency department delays after acute stroke: the Genentech Stroke Presentation Survey. Stroke 2000; 31: 2585– 90 [DOI] [PubMed] [Google Scholar]
- 14. Smith RW, Scott PA, Grant RJ, et al. Emergency physician treatment of acute stroke with recombinant tissue plasminogen activator: a retrospective analysis. Acad Emerg Med 1999; 6: 618– 25 [DOI] [PubMed] [Google Scholar]
- 15. Medical Consumer Price Index. 2008. http://data.bls.gov/PDQ/outside.jsp?survey=cu. Accessed June 30, 2008
- 16. Gold MR, Siegel JE, Russell LB, et al. Cost-effectiveness in Health and Medicine. New York: Oxford University Press; 1996 [Google Scholar]
- 17. Earnshaw SR, Joshi AV, Wilson MR, et al. Cost-effectiveness of recombinant activated factor VII in the treatment of intracerebral hemorrhage. Stroke 2006; 37: 2751– 58 [DOI] [PubMed] [Google Scholar]
- 18. Arias E. United States life tables, 2003. Natl Vital Stat Rep 2006; 54: 1– 40 [PubMed] [Google Scholar]
- 19. Samsa GP, Reutter RA, Parmigiani G, et al. Performing cost-effectiveness analysis by integrating randomized trial data with a comprehensive decision model: application to treatment of acute ischemic stroke. J Clin Epidemiol 1999; 52: 259– 71 [DOI] [PubMed] [Google Scholar]
- 20. Lloyd-Jones D, Adams R, Carnethon M, et al. , for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2009 update—a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119: 480– 86 [DOI] [PubMed] [Google Scholar]
- 21. Nationwide Inpatient Sample. Healthcare Cost and Utilization Project. 2000–2001. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed May 20, 2009
- 22. Fagan SC, Morgenstern LB, Petitta A, et al. Cost-effectiveness of tissue plasminogen activator for acute ischemic stroke: NINDS rtPA Stroke Study Group. Neurology 1996; 50: 883– 90 [DOI] [PubMed] [Google Scholar]
- 23. Aspelin P, Aubry P, Fransson SG, et al. Cost-effectiveness of iodixanol in patients at high risk of contrast-induced nephropathy. Am Heart J 2005; 149: 298– 303 [DOI] [PubMed] [Google Scholar]
- 24. Gleeson TG, Bulugahapitiya S. Contrast-induced nephropathy. AJR Am J Roentgenol 2005; 185: 1079 [DOI] [PubMed] [Google Scholar]
- 25. Shield CF, Jacobs RJ, Wyant S. A cost-effectiveness analysis of OKT3 induction therapy in cadaveric kidney transplantation. Am J Kidney Dis 1996; 28: 958 [DOI] [PubMed] [Google Scholar]
- 26. Beebe A, Dalton JA, Espronceda M. CPT 2009 Standard Edition. Chicago: American Medical Association; 2009 [Google Scholar]
- 27. Ingenix. The Essential Resource-Based Relative Value Scale: A Comprehensive Listing of RBRVS Values for CPT and HCPCS Codes. Eden Prairie, Minnesota: St. Anthony Publishing; 2008 [Google Scholar]
- 28. Red Book for Windows [computer program]. Version 61127, Volume 41. Montvale, New Jersey: Thomson PDR; 2006 [Google Scholar]
- 29. Earnshaw SR, Wilson M, Mauskopf J, et al. Model-based cost-effectiveness analyses for the treatment of acute stroke events: a review and summary of challenges. Value Health 2009; 12: 507– 20. Epub 2008 Nov 10 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.