SUMMARY:
In the foreseeable future, the MI field could greatly assist neuroradiologists. Reporter molecules provide information on specific molecular or cellular events that could not only aid diagnosis but potentially differentiate stages of disorders and treatments. To accomplish this, reporter molecules literally need to pass a barrier, the BBB, which is designed to repel nonessential molecules from the brain. Although this is not a trivial task, several transport systems could be tricked into guiding molecules into the brain. The noninvasive nature in conjunction with a wide availability makes MR imaging particularly suitable for longitudinal neurologic imaging studies. This review explains the principles of MR imaging contrast, delineates different types of reporter molecules, and describes strategies to transport reporters into the brain. It also discusses recent advances in MR imaging hardware, pulse sequences, the development of targeted reporter probes, and future directions of the MR neuroimaging field.
Anatomic images have always been the center of gravity in the daily work of radiologists. They provide the basis of many diagnoses supplemented by physiologic MR imaging data or metabolic profiling if necessary. Despite the sophistication of these techniques and the wealth of information that can be obtained, the diagnostic information often remains nonspecific, and evidence regarding the nature of the underlying disease commonly remains circumstantial. In contrast to generic contrast agents used in the clinic, the MI field uses reporter molecules tailored for in vivo detection of specific molecular or cellular events. Formally, MI encompasses techniques that directly or indirectly monitor and record the spatiotemporal distribution of molecular or cellular processes for biochemical, biologic, diagnostic, or therapeutic applications (Table 1). 1,2 The technique is widely used in preclinical research and is on the verge of entering the clinical arena. It enables radiologists to add molecular or cellular information to their array of diagnostic tools, which will have a tremendous effect on the diagnosis of neurologic disorders, where invasive diagnostic techniques like biopsies can rarely be used.3,4 Radiologists may even be able to detect “predisease” or “presymptomatic” states when molecular and cellular changes arise before they lead to anatomic or functional disturbances. Following an early diagnosis, MI could closely monitor the effectiveness of therapeutic interventions.
Table 1:
Definitions
| Term | Definition |
|---|---|
| Molecular imaging | In vivo imaging of the spatiotemporal distribution of molecular or cellular processes |
| Cellular imaging | In vivo imaging of the spatiotemporal distribution of cellular processes |
| Contrast agent, label | Chemical functional group that allows visualization by an appropriate imaging technique: For example, Gd chelates or iron oxides are MR imaging contrast agents, 18F atoms are PET contrast agents, and fluorophores are optical contrast agents |
| Nanoparticle | Molecules in the 10- to 1000-nm range that serve as an imaging platform; examples include SPIO particles, liposomes, dendrimers, and quantum dots |
| Reporter probe, reporter molecule | A molecule or nanoparticle that is used to image particular biological processes; the molecule or nanoparticle is a composite of a contrast agent and targeting moiety |
| Reporter cell | A cell that contains a contrast agent |
| Reporter gene | A gene that encodes for a protein that (directly or indirectly) is easy to assay; reporter genes are linked to genes of interest to study expression levels |
MR imaging is already the technique of choice for neuroradiologists, and MR imaging systems are widely available. In principle, one could collect anatomic and physiologic information and report the location of reporter molecules in a patient during a single MR imaging session. For these reasons, this review focuses on MR imaging−based MI of the brain, explaining the principles of MR reporter molecules, describing strategies to target them to the brain, and reviewing the state of the art in CNS MI.
Brain Targeting
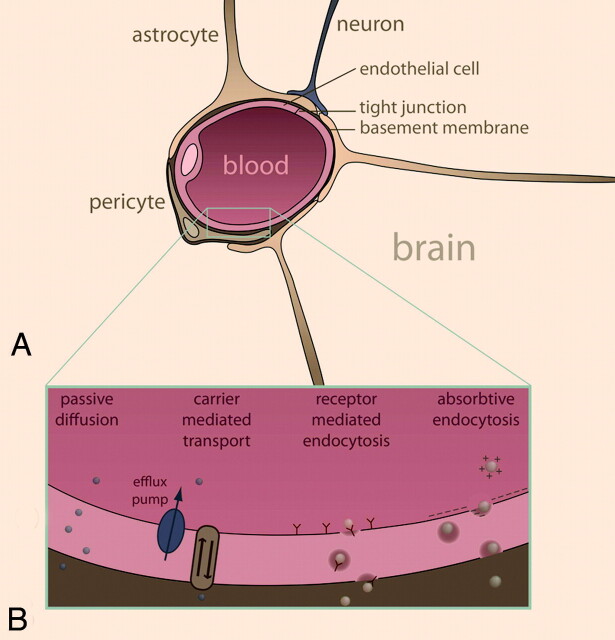
An important feature of the brain that sets it apart from other organs in terms of MI is the presence of the BBB, a selective barrier to the CNS that impedes the influx of most compounds from blood to brain (Fig 1A). It permits the passage of metabolic compounds and ions to maintain neuronal function, while shielding off possible harmful compounds. The effectiveness of the barrier results from the selective permeability of tight junctions between endothelial cells, though the underlying layers and cell types also exhibit great influence on its function and permeability. The endothelial cells in the cerebral vasculature differ from normal endothelial cells in having low pinocytotic activity, abundant mitochondria, fewer fenestrations, and specialized junctions to adjacent cells; all these features play a role in the impermeability of the BBB.5
Fig 1.
Schematic representation of the BBB (A) and transport mechanisms for BBB passage (B) (see text for details).
The BBB is generally regarded as a bottleneck for MI of the brain because it severely hinders the delivery of reporter probes to the brain. Designing reporter molecules so that they may cross the barrier is not trivial, though a good understanding of BBB physiology has resulted in several delivery strategies.6 With paracellular transport sealed off, transmembrane transport through endothelial cells is the only way to gain access to the brain. Several strategies to transport MI probes across the BBB can be followed, exploiting different endogenous transport systems (Fig 1B): passive diffusion, carrier-mediated transport, receptor-mediated endocytosis, and adsorptive endocytosis.7–10
Small molecules such as oxygen and carbon dioxide readily diffuse into the brain, but the BBB is quite restrictive for other compounds. Nevertheless, small noncharged lipophilic compounds may be engineered to passively enter the brain.11 However, lipophilic compounds have major drawbacks, including enhanced uptake and retention by peripheral tissues, complicating the compound biodistribution and pharmacokinetics.12 In practical terms, lipophilic compounds are generally not soluble in aqueous solutions. Organic solvents are usually excluded from in vivo experiments for toxicity reasons, while others that are in clinical use, such as DMSO,13 reduce the integrity of the BBB, which clearly is not desirable for noninvasive imaging purposes.
Metabolites such as glucose, amino acids, nucleosides, and neurotransmitters are transported into the brain by carrier-mediated transport through proteins in the plasma membrane of endothelial cells that catalyze bidirectional transport (blood to brain and vice versa).14 These pumps operate on both sides of the cell to maintain a nutritional balance. Reporter molecules that mimic the structure of a nutrient could trick the transport system into gaining entrance to the brain, though efflux pumps on the luminal (blood) side may impede this effort.15 PET has used this strategy extensively (eg, by using FDG as a glucose analog).16
Larger reporter probes may target internalizing receptors, resulting in receptor-mediated endocytosis: Following complex formation of the probe and the receptor, the complex is internalized, transported to the abluminal (brain) side of endothelial cells, and released into the brain. The insulin receptor and transferrin receptor are well-known examples.7 This transport mechanism is suitable for the translocation of macromolecules and nanoparticles and is, therefore, particularly interesting for MI.9 A prerequisite for this mechanism is a receptor-binding ligand, such as a molecular mimic of an endogenous ligand or an antibody against the receptor of interest. Evidently, the ligand should be conjugated to a contrast agent or an MR imaging−detectable nanoparticle to be able to visualize it using MR imaging.
Compounds may also be internalized by nonspecific interactions with the cell surface. Cationized albumin, for example, interacts with the anionic cell surface of the endothelial cells and is then internalized via adsorptive endocytosis.7 This is a general internalization mechanism that is not specific for endothelial cells, so with regard to MI, this strategy is not very useful because reporter probes would be internalized by a range of cell types, resulting in a high nonspecific background signal intensity.
It is possible to disrupt the BBB temporarily to gain access to the brain—that is, through osmotic pressure (mannitol). A method that appears relatively safe is the injection of microbubbles into the bloodstream, followed by focused local sonographic exposure causing cavitation, leading to BBB disruption.17 Such methods may be used for preclinical studies but will not be suitable for clinical use of targeted probes, except perhaps for image-guided drug delivery.
Generating MR Imaging Contrast
Whatever the object of interest is, the reporter system used to visualize it should contain an MR imaging−visible contrast agent. An overview of these agents is provided below and in Table 2.
Table 2:
MR imaging contrast
| MR Imaging Contrast Agent | Examples | Predominant Effect | MR Imaging Sensitivity | Notes |
|---|---|---|---|---|
| T1 agent | Gd-DTPA (magnevist), Gd-DOTA (dotarem), Mn2+ | T1 | ++ | Gd is chelated for toxicity reasons |
| T2 agent | SPIO, USPIO, MION, CLIO | T2 | ++++ | Large range of sizes |
| CEST, PARACEST | Amino acids, sugars; Eu-DOTA | Saturation transfer | ++ | Preclinical stage |
| Heteronuclei | 19F, 31P | Hot spot imaging | + | Negligible background signal |
Note:—Plus signs indicate the relative sensitivity of the different contrast mechanisms.
Iron oxide particles (SPIO, CLIO, and MION) are composed of iron oxide crystals with polymer coatings and are often biodegradable. They are synthesized in different forms and sizes, ranging from 300 nm to 1.6 μm in diameter, and each size category exhibits different pharmacodynamic behavior and relaxation effects.18 All iron oxide particles possess relatively large (negative) magnetic susceptibilities, thereby reducing T2 and T2* relaxation times, resulting in signal-intensity voids or hypointense regions in T2-weighted or T2*-weighted MR images. The fact that SPIOs cause “negative contrast” could make them difficult to distinguish from imaging artifacts or other susceptibility sources. Imaging sequences are being developed that produce positive contrast by using SPIOs to overcome this disadvantage,19 though the generated contrast is still nonspecific and cannot be exclusively attributed to the presence of iron oxide particles. Some SPIOs are approved by the US Food and Drug Administration, making them popular choices for cellular imaging and MI, though their relatively large size makes them less useful for targets that are not easily accessible, as in neurologic applications.
Paramagnetic agents (eg, Gd) have small positive magnetic susceptibilities that cause a modest decrease of relaxation times, particularly T1, resulting in “positive contrast” on T1-weighted images.20 The positive contrast effect of the paramagnetic contrast agents is much weaker than the negative contrast effect of SPIOs. Sensitivity could be enhanced by conjugating multiple Gd-containing chelates to a single probe, such as a dendrimer or protein, but the design of large complexes is always a trade-off between sensitivity and molecular weight. The larger (and brighter) the contrast agents that are synthesized, the more difficulties they will encounter in crossing the BBB. A special class of paramagnetic contrast agents is encompassed by “smart” or “responsive” contrast agents. Their relaxation properties significantly change in response to local physiologic changes (eg, in pH, temperature, or enzyme activity).21,22 Paramagnetic agents are small and generally easy to conjugate to probes of interest; these features make them suitable for neuroimaging, though they remain relatively insensitive.
CEST is a contrast mechanism that has been developed during the past decade and is based on magnetization transfer through proton exchange.23 CEST agents contain exchangeable protons with a resonance frequency different from bulk water. On selective irradiation of this frequency, magnetization is transferred to bulk water through chemical exchange, causing a decrease in the signal intensity of bulk water. The contrast can thus be switched on (by presaturation of the CEST protons) or off (no presaturation). The efficacy of CEST agents depends on the exchange rate and frequency difference between the exchangeable CEST protons and bulk water and can be improved by the incorporation of lanthanide ions (PARACEST).24 CEST probes can be engineered to work at different excitation frequencies, which potentially allows us to study interactions of cells labeled with different CEST agents.25
Contrast agents described thus far are detected indirectly, in the sense that they affect the relaxation properties of water protons. Their effect is registered in the final proton image. A more direct approach is heteronuclear MR imaging. In principle, any nucleus with a nonzero magnetic spin and a large enough magnetic sensitivity could be used. 19Fluorine is a popular choice in the MI field because it exhibits favorable MR imaging characteristics with a spin-1/2, a magnetic sensitivity close to that of protons, and 100% natural abundance. Moreover, there are hardly any endogenous fluorine-containing molecules, so fluorine-based reporter molecules do not have background signal intensity. Compared with proton imaging, however, the fluorine signal intensity will be rather low; fluorine probes accumulate at most in the millimolar range, whereas the water concentration in vivo is roughly 40 mol/L. The design of fluorine-based probes is very variable, ranging from low-MW molecules with covalently bound fluorine atoms26 to perfluorocarbon molecules measuring several hundred nanometers.27,28 Low-MW fluorine probes may be designed to accumulate in the brain, where they could take full advantage of the “hot spot imaging” principle if they locally agglomerate to sufficiently high concentrations.
Reporter Systems
MR imaging−based MI is used to detect a wide range of biologic events, which can be classified in 3 categories (with some overlap among them). First, the presence of specific molecules, such as receptors, can be imaged (see “Detecting Molecules”). Second, specific cells, such as stem cells after transplantation (see “Detecting Cells”), can be traced; and third, the expression of specific genes (see “Reporter Genes”) can be visualized.
Detecting Molecules
Molecular reporter probes come in many shapes and sizes, but their principal components are a targeting molecule and a contrast agent. The probe should be targeting a unique hallmark of the biologic process of interest. Popular target choices are receptors, enzymes, and cytokines, which are often expressed to much higher levels in pathologies. Once a target is chosen, one needs to select a complementary ligand that binds the target with high affinity and specificity. Natural ligands, such as receptor agonists, can be used, or specific ligands, such as antibodies or peptides, can be developed.
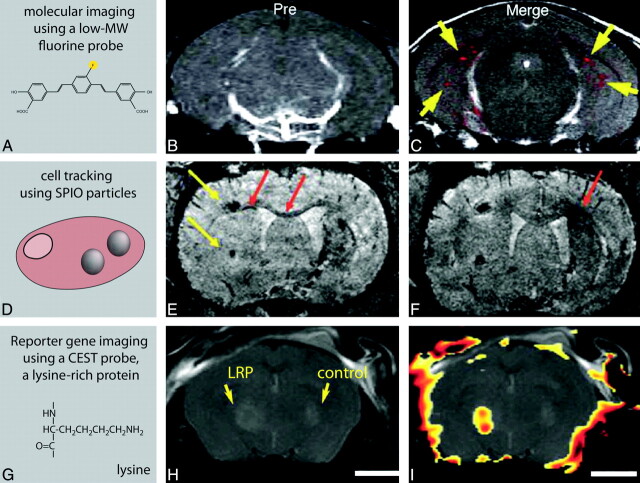
Reporter probes are synthesized in a wide range of sizes (Fig 2) and can be divided in low-MW probes and large-MW probes or nanoparticles. Low-MW probes are based on drugs, metabolites, or inhibitors, in which single atoms or small functional groups are substituted with a contrast moiety. For neurologic MI applications, low-MW probes most likely find their way to the brain via passive diffusion or carrier-mediated transport. A beautiful example is the small fluorine-containing reporter probe developed by Higuchi et al.26 This molecule targets amyloid plaques, one of the hallmarks of AD (Fig 3A−C) and was intravenously injected into transgenic AD mice. Using a combination of 19F and 1H-MR imaging, Higuchi showed the presence of this probe in the brain of these mice, presumably bound to amyloid plaques.
Fig 2.
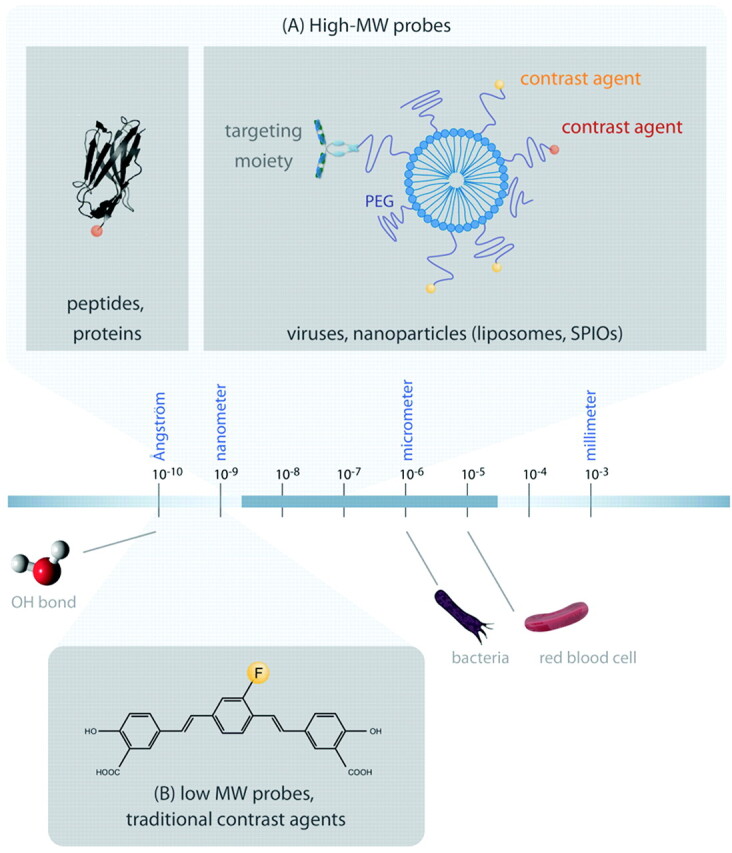
Reporter probes and orders of magnitude. The actual size of reporter molecules ranges from several ångstroms (10[−10]m) to hundreds of nanometers. A, High-MW biomolecules (not drawn to scale as it is long and not informative), such as peptides or antibodies, that have target affinity could be labeled directly with a contrast agent. Reporter molecules based on liposomes or SPIO particles, tens to hundreds of nanometers, are conjugated to targeting moieties and contrast agents. B, Low-MW reporter probes are small molecules that contain contrast-generating labels. They fall in the low nanometer range. The figure shows a reporter probe that is able to cross the BBB and bind to amyloid plaques. It is labeled with 19F for MR imaging.26
Fig 3.
In vivo examples of MI studies of the brain by using MR imaging. A−C, MR images of transgenic mice displaying symptoms of AD. A, A fluorine-containing reporter probe with affinity for senile plaques was intravenously injected and imaged by 19F and 1H-MR imaging. B, Proton MR image before injection of the reporter probe. C, Merged proton and fluorine image after injection of the reporter probe, indicating the anatomic locations of the reporter molecule in the brain.26 D−F, Cellular imaging of migrating stem cells in a mouse model of ischemic stroke. E, Stem cells are labeled with ultrasmall SPIO particles and transplanted into the contralateral hemisphere. F, They migrate from the implantation sites (yellow arrows) along the corpus callosum toward the ischemic borderzone (migration shown as red arrows).37 G−I, In vivo reporter gene imaging by using CEST reporter proteins. The left hemisphere of a mouse brain was injected with glioma cells expressing LRP (a CEST agent); the right hemisphere was injected with control tumor cells. H, Anatomic MR image of the brain. I, CEST signal-intensity-difference map superimposed on the anatomic image, indicating the LRP-expressing tumorxenograft.34
High-MW reporter probes (with MWs of >5 kDa and diameters ranging from a few to several hundred nanometers) are based on proteins or nanoparticles to which contrast agents and ligands are conjugated. Some nanoparticles, such as SPIOs or perfluorocarbon particles, generate contrast themselves (see “Generating MR Imaging Contrast”), while others (such as liposomes and dendrimers) simply supply a crosslinking platform. There is a trend in the field to construct nanoparticles that contain multiple types of contrast agents, which can be visualized by different imaging modalities.29 This concept, referred to as multimodality imaging, combines the strengths of different imaging techniques, such as sensitive PET tracers, with high-resolution MR images. For example, Kircher et al30 set out to aid neurosurgeons in visualizing brain tumor margins. They synthesized a long-circulating multimodality reporter molecule, composed of an iron-oxide nanoparticle and a near-infrared fluorochrome, and injected this into rats bearing gliosarcoma that expressed green fluorescent protein. MR imaging confirmed that microglia had sequestered the nanoparticle and displayed the tumor as hypointense regions in T2-weighted images. Subsequent optical imaging allowed discrimination of tumors from brain tissue, which ultimately could be used by surgeons during surgery.
Detecting Cells
Instead of visualizing reporter molecules, it is also possible to label intact cells, allowing cell tracking and gaining knowledge on cell behavior, such as migration patterns of immune cells following immunotherapy, or stem cell survival following transplantation.
Evidently, cells should remain viable and functional after the labeling procedure. Cellular contrast agents should ideally remain within the desired cell type and not dilute with cell division, to enable reliable longitudinal studies.31,32 SPIO particles are by far the most used MR imaging contrast agents for MR imaging cell tracking. However, a particular confound is that the MR image cannot distinguish viable cells from nonviable cells. Additionally, due to susceptibility effects, hypointense regions in MR images are significantly larger than the actual cluster of labeled cells, which could lead to misinterpretations of the iron source. MR imaging−based cell tracking does not exclusively lean on SPIO particles; Gd-based approaches benefiting from positive contrast have also been demonstrated,33 though substantial amounts of lanthanide-based contrast agents are required to affect MR images, which increases toxicity issues. Phosphorous and fluorine imaging have the advantage of a low background and can often be performed in a quantitative manner, but they too have sensitivity problems. Finally, CEST imaging is emerging as a very useful technique, especially in reporter gene imaging.34
Cell labeling can be performed in vitro, after which the labeled cells are implanted. Alternatively the contrast agent may be injected systemically, which is then taken up by phagocytotic cells.35 Many cell types readily take up contrast agents via phagocytosis, but nonphagocytic cells (including stem cells) can be labeled via transfection agents, fluid phase pinocytosis, encapsulation, receptor-mediated uptake, or magnetoelectroporation.31,32 Cells that have been labeled for CNS application include neural stem cells, oligodendrocyte precursors, and macrophages.36–38 Tracking neuronal stem cells empowers us to study the best way to administer the cells (intravenous-versus-intraparenchymal transplantation), estimate the dose (how many cells to use), assess the survival rate, evaluate their migration patterns, check if they find their target site and home, and optimize the therapeutic time window.31,39 To illustrate this, Hoehn et al37 transplanted SPIO-labeled stem cells into the contralateral hemisphere of a mouse displaying ischemic stroke. T2*-weighted MR images showed the migration of these cells across the brain from the implantation site to the edge of an ischemic lesion (Fig 3D−F). This example demonstrates the possibility of following cells in real time, which may be used to study the therapeutic potential of stem cells in the future.37
Reporter Genes
The previous 2 sections focused on reporting specific molecules or cells; this section addresses imaging gene function, which, in contrast to cell tracking, exclusively reports on viable cells. Reporter gene imaging is a technique in which gene products (ie, reporter proteins) are imaged in vivo.40,41 Essentially, a reporter gene is transcribed to messenger RNA, which in turn is translated into a reporter protein (which are far more abundant in the cell than DNA or RNA). A good reporter protein must be easy to assay and must not normally be expressed in the cells of interest or, when encoding for endogenous proteins, must be expressed at much higher levels than normal.
The gene of interest is unlikely to encode an MR imaging−visible protein, though the protein of interest may interact with exogenous reporter molecules.42 Often, the gene of interest is teamed up with a reporter gene. These genes can be engineered so that they are both driven by the same promotor. On activation of this promotor (which can be conditional or tissue-specific), the expression of both genes is simultaneously enhanced; imaging the reporter protein thus “reports” on the expression of the gene of interest. The reporter protein may produce endogenous contrast or may be imaged via exogenous reporter molecules.42
Reporter gene imaging is currently in a preclinical stage, but its potential is enormous. One could monitor gene expression, track cells in normal and abnormal development, map dynamic protein interactions, and check cell transplantation therapy. Taking this 1 step further, one could follow the effects of gene therapy, in which cells are genetically modified to produce a therapeutic effect.41,42 Reporter gene imaging is commonly used in the nuclear and optical imaging field, with green fluorescent protein43 and luciferase44 as prominent examples, but MR imaging has started claiming a place on the stage as well, by virtue of its noninvasive nature and whole-body coverage.
Needless to say, for MR reporter gene imaging, the reporter protein needs to be MR imaging−detectable, and common strategies are outlined in recent reviews.41,42,45 Reporter genes may encode for artificial proteins, which are detectable by CEST imaging (Fig 3G−I),34 but most MR imaging reporter systems are based on the accumulation of iron, circumventing the administration of exogenous contrast agents. When reporter genes overexpress proteins such as ferritin,46,47 the transferrin receptor,48 or MagA49 at high levels, they cause a local buildup of iron, which leads to enhanced negative contrast in T2- or T2*-weighted images. This principle has been demonstrated by Genove et al,47 who visualized gene expression by using genetically modified replication-defective adenoviruses carrying the genes for the light-chain or heavy-chain subunits of ferritin. They injected the adenoviruses into the striatum of a living mouse, which led to the local overexpression of ferritin and the accumulation of endogenous iron. This resulted in enhanced negative contrast in T2-weighted MR images, which was visible for weeks.
The field of MR reporter gene imaging is developing at a steady pace, but before it becomes a mainstream clinical tool, it has to overcome several issues. The efficiency of gene transfer is currently very modest, which, in combination with low MR imaging sensitivity, makes MR reporter imaging a challenge. Reporter gene imaging (in combination with gene therapy) often uses viral-based vectors to package genes and a delivery device to pass them to the target organ. In this respect, the method is not strictly noninvasive. Current applications of reporter gene imaging are, therefore, mostly confined to small animal models, though clinical applications are anticipated in the future, most likely as tools to visualize gene therapy.
Current Developments
The field of MI is developing in several directions. Imaging modalities perform better, molecular biologists construct ingenious reporter genes and transgenic mice, while chemists improve contrast agents20,50 and create multimodal reporter molecules.
There is a continuous effort to increase the field strengths of human scanners, and these high-field magnets are making their way into the clinic. The most commonly used clinical scanners operate at field strengths of 1.5T, while 3T is regarded as high-field; but ultra-high-field magnets at 7T are also used for patient studies. Because the MR imaging signal intensity is proportional to the magnetic field strength, using high-field scanners improves the sensitivity and enhances the signal intensity–to-noise ratio, improving anatomic and functional imaging.51 A higher magnetic field also increases the spectral resolution, which is an additional benefit for 1H-MR spectroscopy and CEST-based imaging. Unfortunately, high-field magnets do not come without expense: Increased magnetic susceptibility effects, field inhomogeneity, and energy deposition pose technical challenges, but these are partly overcome by using optimized coils, fast and parallel imaging, and refocusing flip angles.52,53
With regard to MI, high-field scanners can detect lower concentrations of reporter molecules, which decrease toxicity issues. This is particularly the case for iron oxide−based particles, heteronuclear contrast agents, and CEST agents. On the other hand, the current lanthanide-based contrast agents do not perform as well at high fields, and their relaxation behavior should be significantly improved.20 Regardless of the field strength there is a continuous effort to tailor and improve pulse sequences, such as ultrafast imaging sequences and creating positive contrast for SPIO particles.19
Outlook
The road to MR imaging−based MI has been a long one. MR imaging−based contrast agents are inherently far less sensitive than radiotracers; this difference makes them more likely to fail due to sensitivity or toxicity problems. Nevertheless, the advantage of providing anatomic, physiologic, and molecular or cellular information during a single examination is so great that research in this area is booming. In the near future, we anticipate that fluorine-based reporter molecules in conjunction with MR imaging at higher fields holds great promise, combining hot-spot imaging and sensitivity. In contrast to fluorine-based radiotracers, MR reporter probes are relatively long-lived and could be imaged on a regular basis while avoiding excessive ionizing radiation. While BBB research is in fifth gear, MI of the brain is already in use for pathologies with a possible compromised BBB, such as cerebrovascular diseases, inflammatory conditions, and gliomas. Without the need to cross the BBB, this approach could take full advantage of current developments in the field.
Also, alternative therapies for neurologic disorders, such as gene therapy and stem cell transplantation, are active areas of research, and we expect that MI will play an essential role in the development of these therapies, for example, by tracing labeled stem cells or by developing a common platform for treatment and diagnostics. Examples of this common approach are already known from cancer research, in which liposomal particles that target tumor sites and contain anticancer drugs and MR imaging–visible contrast agents to monitor the treatment response have been used.
Many radiologists may regard MI as science fiction, but they should be aware that this field develops at a fast pace. Although currently most research is applied in animal models, the first radiologic studies in humans have already been performed. It is generally believed that MI based on radiologic techniques will be introduced in the clinical arena within the next few years. However, the clinical implementation of MR imaging−based MI will only occur when academic radiologists are actively involved in research programs in which they master data collection and interpretation of MI images. This could be achieved by providing MI courses to residents, organizing postgraduate courses for radiologists, and by creating MI fellowships. Radiologists could also assist chemists and biochemists in the development of reporter probes to advance clinical applications. Seeing that MI is on the brink of leaping to the clinic, radiologists should prepare themselves now to join that jump in the near future.
Abbreviations
- AD
Alzheimer disease
- BBB
blood-brain barrier
- CA
contrast agent
- CEST
chemical exchange saturation transfer
- CLIO
cross-linked iron oxide
- CNS
central nervous system
- DMSO
dimethyl sulfoxide
- Eu-DOTA
europium tetra-azacyclododecane tetraacetic acid
- 18F
fluorine-18
- 19F
fluorine-19
- FDG
fluorodeoxyglucose
- Gd
gadolinium
- Gd3+
gadolinium 3+
- 1H-MR imaging
proton MR imaging
- LRP
lysine rich protein
- MI
molecular imaging
- MION
monocrystalline iron oxide nanoparticle
- Mn2+
manganese 2+
- MRS
magnetic resonance spectroscopy
- MW
molecular weight
- 31P
phosphorus-31
- PARACEST
chemical exchange saturation transfer using paramagnetic ions
- PEG
polyethylene glycol
- PET
positron-emission tomography
- RNA
ribonucleic acid
- SPIO
superparamagnetic iron oxide
- T1
longitudinal relaxation time
- T2
transverse relaxation time due to spin-spin interactions (irreversible effect)
- T2*
transverse relaxation time due to spin-spin interactions and local inhomogeneities (partly reversible)
- USPIO
ultrasmall superparamagnetic iron oxide
Footnotes
M.E.d.B. and R.J.N. were supported by the Center for Translational Molecular Medicine. L.v.d.W. was partially supported by the Netherlands Science Organization (NWO-Veni 700.56.407 and NWO-Athena700.58.801).
References
- 1. Thakur M, Lentle BC. Report of a summit on molecular imaging. Radiology 2005; 236: 753– 55 [DOI] [PubMed] [Google Scholar]
- 2. Weissleder R, Mahmood U. Molecular imaging. Radiology 2001; 219: 316– 33 [DOI] [PubMed] [Google Scholar]
- 3. Hammoud DA, Hoffman JM, Pomper MG. Molecular neuroimaging: from conventional to emerging techniques. Radiology 2007; 245: 21– 42 [DOI] [PubMed] [Google Scholar]
- 4. Herholz K, Coope D, Jackson A. Metabolic and molecular imaging in neuro-oncology. Lancet Neurol 2007; 6: 711– 24 [DOI] [PubMed] [Google Scholar]
- 5. Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 2005; 57: 173– 85 [DOI] [PubMed] [Google Scholar]
- 6. Neuwelt E, Abbott NJ, Abrey L, et al. Strategies to advance translational research into brain barriers. Lancet Neurol 2008; 7: 84– 96 [DOI] [PubMed] [Google Scholar]
- 7. de Boer AG, Gaillard PJ. Drug targeting to the brain. Annu Rev Pharmacol Toxicol 2007; 47: 323– 55 [DOI] [PubMed] [Google Scholar]
- 8. Pardridge WM. Blood-brain barrier delivery. Drug Discov Today 2007; 12: 54– 61 [DOI] [PubMed] [Google Scholar]
- 9. Barbu E, Molnàr E, Tsibouklis J, et al. The potential for nanoparticle-based drug delivery to the brain: overcoming the blood-brain barrier. Expert Opin Drug Deliv 2009; 6: 553– 65 [DOI] [PubMed] [Google Scholar]
- 10. Tosi G, Costantino L, Ruozi B, et al. Polymeric nanoparticles for the drug delivery to the central nervous system. Expert Opin Drug Deliv 2008; 5: 155– 74 [DOI] [PubMed] [Google Scholar]
- 11. Abraham MH. The factors that influence permeation across the blood-brain barrier. Eur J Med Chem 2004; 39: 235– 40 [DOI] [PubMed] [Google Scholar]
- 12. Banks WA. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol 2009; 9 ( suppl 1): S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Broadwell RD, Salcman M, Kaplan RS. Morphologic effect of dimethyl sulfoxide on the blood-brain barrier. Science 1982; 217: 164– 66 [DOI] [PubMed] [Google Scholar]
- 14. Ohtsuki S, Terasaki T. Contribution of carrier-mediated transport systems to the blood-brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm Res 2007; 24: 1745– 58 [DOI] [PubMed] [Google Scholar]
- 15. Tsuji A. Small molecular drug transfer across the blood-brain barrier via carrier-mediated transport systems. NeuroRx 2005; 2: 54– 62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reivich M, Kuhl D, Wolf A, et al. The [18F]fluorodeoxyglucose method for the measurement of local cerebral glucose utilization in man. Circ Res 1979; 44: 127– 37 [DOI] [PubMed] [Google Scholar]
- 17. Kinoshita M, McDannold N, Jolesz FA, et al. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun 2006; 340: 1085– 90 [DOI] [PubMed] [Google Scholar]
- 18. Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed 2004; 17: 484– 99 [DOI] [PubMed] [Google Scholar]
- 19. Cunningham CH, Arai T, Yang PC, et al. Positive contrast magnetic resonance imaging of cells labeled with magnetic nanoparticles. Magn Reson Med 2005; 53: 999– 1005 [DOI] [PubMed] [Google Scholar]
- 20. Caravan P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem Soc Rev 2006; 35: 512– 23 [DOI] [PubMed] [Google Scholar]
- 21. Sosnovik DE, Weissleder R. Emerging concepts in molecular MRI. Curr Opin Biotechnol 2007; 18: 4– 10 [DOI] [PubMed] [Google Scholar]
- 22. Querol M, Bogdanov A. Amplification strategies in MR imaging: activation and accumulation of sensing contrast agents (SCAs). J Magn Reson Imaging 2006; 24: 971– 82 [DOI] [PubMed] [Google Scholar]
- 23. Ward K, Aletras A, Balaban R. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J Magn Reson 2000; 143: 79– 87 [DOI] [PubMed] [Google Scholar]
- 24. Woods M, Woessner DE, Sherry AD. Paramagnetic lanthanide complexes as PARACEST agents for medical imaging. Chem Soc Rev 2006; 35: 500– 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McMahon MT, Gilad AA, DeLiso MA, et al. New “multicolor” polypeptide diamagnetic chemical exchange saturation transfer (DIACEST) contrast agents for MRI. Magn Reson Med 2008; 60: 803– 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higuchi M, Iwata N, Matsuba Y, et al. 19F and 1H MRI detection of amyloid beta plaques in vivo. Nat Neurosci 2005; 8: 527– 33 [DOI] [PubMed] [Google Scholar]
- 27. Ruiz-Cabello J, Walczak P, Kedziorek DA, et al. In vivo “hot spot” MR imaging of neural stem cells using fluorinated nanoparticles. Magn Reson Med 2008; 60: 1506– 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ahrens ET, Flores R, Xu H, et al. In vivo imaging platform for tracking immunotherapeutic cells. Nat Biotechnol 2005; 23: 983– 87 [DOI] [PubMed] [Google Scholar]
- 29. McCarthy JR, Weissleder R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv Drug Deliv Rev 2008; 60: 1241– 51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kircher MF, Mahmood U, King RS, et al. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res 2003; 63: 8122– 25 [PubMed] [Google Scholar]
- 31. Politi LS, Bacigaluppi M, Brambilla E, et al. Magnetic-resonance-based tracking and quantification of intravenously injected neural stem cell accumulation in the brains of mice with experimental multiple sclerosis. Stem Cells 2007; 25: 2583– 92 [DOI] [PubMed] [Google Scholar]
- 32. Long CM, Bulte JW. In vivo tracking of cellular therapeutics using magnetic resonance imaging. Expert Opin Biol Ther 2009; 9: 293– 306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Modo M, Cash D, Mellodew K, et al. Tracking transplanted stem cell migration using bifunctional, contrast agent-enhanced, magnetic resonance imaging. Neuroimage 2002; 17: 803– 11 [PubMed] [Google Scholar]
- 34. Gilad AA, McMahon MT, Walczak P, et al. Artificial reporter gene providing MRI contrast based on proton exchange. Nat Biotechnol 2007; 25: 217– 19. Epub 2007 Jan 28 [DOI] [PubMed] [Google Scholar]
- 35. Hoehn M, Himmelreich U, Kruttwig K, et al. Molecular and cellular MR imaging: potentials and challenges for neurological applications. J Magn Reson Imaging 2008; 27: 941– 54 [DOI] [PubMed] [Google Scholar]
- 36. Bulte JW, Zhang S, van Gelderen P, et al. Neurotransplantation of magnetically labeled oligodendrocyte progenitors: magnetic resonance tracking of cell migration and myelination. Proc Natl Acad Sci U S A 1999; 96: 15256– 61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoehn M, Wiedermann D, Justicia C, et al. Cell tracking using magnetic resonance imaging. J Physiol 2007; 584 ( pt 1): 25– 30. Epub 2007 Aug 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Syková E, Jendelová P. Migration, fate and in vivo imaging of adult stem cells in the CNS. Cell Death Differ 2007; 14: 1336– 42 [DOI] [PubMed] [Google Scholar]
- 39. Kraitchman DL, Gilson WD, Lorenz CH. Stem cell therapy: MRI guidance and monitoring. J Magn Reson Imaging 2008; 27: 299– 310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Massoud TF, Singh A, Gambhir SS. Noninvasive molecular neuroimaging using reporter genes: part I, principles revisited. AJNR Am J Neuroradiol 2008; 29: 229– 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gilad AA, Winnard PT, van Zijl PCM, et al. Developing MR reporter genes: promises and pitfalls. NMR Biomed 2007; 20: 275– 90 [DOI] [PubMed] [Google Scholar]
- 42. So PW, Parkes HG, Bell JD. Application of magnetic resonance methods to studies of gene therapy. Prog Nucl Magn Reson Spectr 2007; 51: 49– 62 [Google Scholar]
- 43. Yeh E, Gustafson K, Boulianne GL. Green fluorescent protein as a vital marker and reporter of gene expression in Drosophila. Proc Natl Acad Sci U S A 1995; 92: 7036– 40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gould SJ, Subramani S. Firefly luciferase as a tool in molecular and cell biology. Anal Biochem 1988; 175: 5– 13 [DOI] [PubMed] [Google Scholar]
- 45. Gilad AA, Ziv K, McMahon MT, et al. MRI reporter genes. J Nucl Med 2008; 49: 1905– 08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cohen B, Ziv K, Plaks V, et al. MRI detection of transcriptional regulation of gene expression in transgenic mice. Nat Med 2007; 13: 498– 503 [DOI] [PubMed] [Google Scholar]
- 47. Genove G, DeMarco U, Xu H, et al. A new transgene reporter for in vivo magnetic resonance imaging. Nat Med 2005; 11: 450– 54 [DOI] [PubMed] [Google Scholar]
- 48. Weissleder R, Moore A, Mahmood U, et al. In vivo magnetic resonance imaging of transgene expression. Nat Med 2000; 6: 351– 55 [DOI] [PubMed] [Google Scholar]
- 49. Zurkiya O, Chan AWS, Hu X. MagA is sufficient for producing magnetic nanoparticles in mammalian cells, making it an MRI reporter. Magn Reson Med 2008; 59: 1225– 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aime S, Castelli DD, Crich SG, et al. Pushing the sensitivity envelope of lanthanide-based MRI contrast agents for molecular imaging applications. Acc Chem Res 2009; 42: 822– 31 [DOI] [PubMed] [Google Scholar]
- 51. Willinek WA, Kuhl CK. 3.0 T neuroimaging: technical considerations and clinical applications. Neuroimaging Clin N Am 2006; 16: 217– 28, ix [DOI] [PubMed] [Google Scholar]
- 52. Trattnig S, Pinker K, Ba-Ssalamah A, et al. The optimal use of contrast agents at high field MRI. Eur Radiol 2006; 16: 1280– 87 [DOI] [PubMed] [Google Scholar]
- 53. Nakada T. Clinical application of high and ultra high-field MRI. Brain Dev 2007; 29: 325– 35 [DOI] [PubMed] [Google Scholar]