Abstract
BACKGROUND AND PURPOSE:
Perfusion and angiographic imaging using intravenous contrast application to evaluate stroke patients is now technically feasible by flat detector CT performed by the angiographic system. The aim of this pilot study was to show the feasibility and qualitative comparability of a novel flat detector CT dynamic perfusion and angiographic imaging protocol in comparison with a multimodal stroke MR imaging protocol.
MATERIALS AND METHODS:
In 12 patients with acute stroke, MR imaging and the novel flat detector CT protocol were performed before endovascular treatment. Perfusion parameter maps (MTT, TTP, CBV, CBF) and MIP/volume-rendering technique images obtained by using both modalities (MR imaging and flat detector CT) were compared.
RESULTS:
Comparison of MIP/volume-rendering technique images demonstrated equivalent visibility of the occlusion site. Qualitative comparison of perfusion parameter maps by using ASPECTS revealed high Pearson correlation coefficients for parameters CBF, MTT, and TTP (0.95–0.98), while for CBV, the coefficient was lower (0.49).
CONCLUSIONS:
We have shown the feasibility of a novel dynamic flat detector CT perfusion and angiographic protocol for the diagnosis and triage of patients with acute ischemic stroke. In a qualitative comparison, the parameter maps and MIP/volume-rendering technique images compared well with MR imaging. In our opinion, this flat detector CT application may substitute for multisection CT imaging in selected patients with acute stroke so that in the future, patients with acute stroke may be directly referred to the angiography suite, thereby avoiding transportation and saving time.
Flat detector CT (FD-CT) equipped angiographic systems are now widely used in neurointerventional institutions. Recently, an application to perform imaging of the brain parenchyma (FD-CT), cerebral vasculature (flat detector CT dynamic perfusion and angiographic imaging [FD-CTA]), and cerebral blood volume has been described and was evaluated in patients with acute middle cerebral artery occlusions.1–3 However, this application was limited due to the inferior FD-CT soft-tissue resolution of the brain parenchyma in comparison with multisection CT (MS-CT) or MR imaging. Additionally, there was a lack of temporal resolution so that calculation of dynamic (time-dependent) perfusion parameters was not possible.
Assessment of the impact of an ischemic stroke is best performed with physiologic criteria because especially in the acute phase, morphologic changes are only minimal and may be difficult to recognize by using MS-CT imaging.4 The use of perfusion and angiographic imaging increases the sensitivity of MS-CT and MR imaging in the acute phase of ischemic stroke. Thus, MS-CT angiography and MS-CT perfusion imaging are used to assess patients within a 0- to 4.5-hour time window of ischemic stroke. On the basis of the mismatch concept beyond 4.5 hours, multimodal MR imaging by using FLAIR, MR angiography, diffusion-weighted, and MR perfusion imaging (MRP) is used in many centers to identify patients eligible for recanalization therapies.5 MS-CT and MR imaging applications allow visualization of brain parenchyma and vessel occlusion (MS-CT angiography, MRA); and calculation of the dynamic perfusion parameter maps (time-to-peak, mean transit time, cerebral blood flow, and cerebral blood volume) to assess the viability of the brain.6–9 The ability to obtain dynamic perfusion maps (FD-CTP) and angiographic images by using an intravenous contrast application within the angiography suite would seem to create a single ideal venue for both diagnosis and treatment of patients with an acute ischemic stroke. A FD-CT application replacing MS-CT or MR imaging would optimize the work flow, avoid transportation of the patient from one imaging location to the other, save time, and may allow periodic monitoring of brain viability during the endovascular treatment.
To date, dynamic perfusion imaging with the C-arm angiographic system has been limited by the slow gantry rotation time. However, recent studies in canines and swine models have now demonstrated the feasibility of dynamic perfusion imaging with the use of a flat detector angiographic system.10–12 The aim of this pilot study was to test the feasibility of this novel application by comparing FD-CTP and FD-CTA with a multimodal MR imaging protocol in patients with acute ischemic stroke. Additionally, the effective patient dose was evaluated.
Materials and Methods
Under an institutionally approved protocol (ethics committee approval was obtained), 12 patients were included in this study. According to institutional guidelines, patients seen beyond 4.5 hours after symptom onset are examined primarily by a multimodal stroke MR imaging protocol. If large-vessel occlusion and diffusion/perfusion mismatch are identified, intravenous thrombolytic therapy is initiated and patients are transferred to the angiography suite for subsequent endovascular treatment. In our practice, FD-CT is frequently used prior to and after endovascular treatment. This enables ruling out hemorrhage due to intravenous thrombolytic therapy prior to and iatrogenic hemorrhage after endovascular treatment. In this study, initial MRP and MRA images were compared with a novel FD-CT application that was applied before endovascular treatment.
Imaging Protocol
MR Imaging.
In all patients, initial imaging was performed by using MR imaging (Magnetom Aera 1.5T; Siemens, Erlangen, Germany). We used standard applications including FLAIR, DWI, MRA (turbo time-of-flight), and MR perfusion imaging. MRP was performed by scanning the entire brain (gradient echo-planar imaging; 25 sections; 5-mm section thickness; TR, 2230 ms; TE, 50 ms; flip angle, 90°; FOV, 230 mm; 50 acquisitions) after injection of paramagnetic contrast material (gadobutrol, Gadavist; Bayer Schering Pharma, Berlin, Germany) by using a dual-syringe power injector (Accutron HP-D; Medtron, Saarbrücken, Germany). MRA was performed by using a time-of-flight technique (5 slabs; 0.5-mm section thickness; TR, 24 ms; TE, 7 ms; FOV, 200 mm). Postprocessing was performed by using a dedicated workstation and standard perfusion software (syngo MMWP; Siemens). We reconstructed TTP, MTT, CBF, and CBV parameter maps (5-mm section thickness and spacing). Additional volume-rendering technique and maximum intensity projection images (5-mm section thickness, 2.5-mm spacing) of the MRA were reconstructed.
FD-CT.
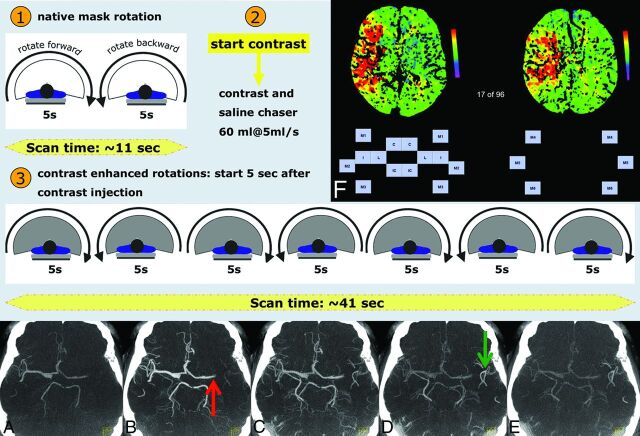
A biplane flat detector angiographic system (Axiom Artis dBA; Siemens) was used both for FD-CT imaging and endovascular treatment. In FD-CT imaging, contrast was injected into a peripheral vein with the use of a dual-syringe angiographic power injector (Accutron HP-D). We used a contrast application protocol identical to that for MS-CT perfusion imaging (60 mL of contrast material, iomeprol, Iomeron 350; Bracco, Milan, Italy; injected at a rate of 5 mL/s followed by a 60-mL saline flush).11 Technical details of this novel FD-CT perfusion application have been described and discussed in detail elsewhere.11 Briefly, before the start of the contrast injection, 1 forward and 1 reverse high-speed baseline scan (rotation) were performed to obtain a baseline set of nonenhanced (mask) images. Five seconds after the start of the peripheral contrast injection, a bidirectional series of 7 high-speed FD-CT rotational acquisitions was initiated. Each rotation was 5 seconds in duration and covered a rotation angle of 200°. There was a 1-second pause between the rotations (4 forward rotations and 3 reverse rotations). During each rotation, the angiographic system acquires 160 projections (angulation step, 0.8°/frame). Each projection was acquired at 77 kV(peak) and 0.36-μGy/frame dose level. This acquisition protocol resulted in a sampling rate of approximately 6 seconds, with a total acquisition duration of 41 seconds (Fig 1).
Fig 1.
The FD-CT imaging protocol. The application is initiated (1) by 2 bidirectional mask runs. The contrast injection is started (2), and with a delay of 5 seconds, the acquisition of the dynamic fill runs (3) is initiated. Each fill run may be reconstructed as an FD-CTA (A–E), serving as “dynamic” contrast-enhanced angiography (4D CTA). In this example, 5 of 7 fill runs are displayed as MIP images (5-mm section thickness). Early arterial inflow is visible in A. During the arterial phase (B), left MCA occlusion (red arrow) is obvious. The late arterial phase (C) shows developing contrast attenuation increase in the left peripheral MCA branches, while during the venous phase (D), they are well-seen (green arrow). Filling is due to collateral flow. During the late venous phase (E), left peripheral MCA branches are still visible. Matlab software was used to display the parameter maps (F). Scaling is provided (color bar). Each ASPECTS region was represented by a button. By simply clicking on the desired button, the side and region of the assumed perfusion abnormality were recorded. C = caudate; L = lentiform; IC = internal capsule; I = insular ribbon; M1 = anterior MCA cortex; M2 = MCA cortex lateral to insular ribbon; M3 = posterior MCA cortex; M4, M5, and M6 are anterior, lateral, and posterior MCA territories immediately superior to M1, M2, and M3, rostral to the basal ganglia.
Prototype perfusion software was used to calculate the FD-CTP parameter maps. The basic reconstruction represents a modified version of the algorithm reported previously, which uses the widely accepted deconvolution approach to calculate the dynamic perfusion parameters.11,12 The arterial input function was manually selected at the anterior cerebral artery. We reconstructed all parameters maps (eg, TTP, MTT, CBV, and CBF) by using a 5-mm section thickness and spacing. With standard FD-CT software (InSpace DynaCT, Siemens), it is possible to perform further reconstructions by using this FD-CTP dataset. A first reconstruction by using data obtained before contrast injection (mask images) was performed to visualize the brain parenchyma and was substituted for the FD-CT performed as a regular procedure in all our patients. Second, the 7 runs after contrast injection were reconstructed with standard parameters as described before to visualize the arterial vessels (ie, FD-CTA) (Fig 1A–E).1 Briefly, reconstruction of the fill runs was performed by using the reconstruction-mode “native fill,” kernel-type “HU,” image-impression “normal,” and an FOV of 14 cm. Reconstruction of raw data resulted in 7 volume datasets with a batch of approximately 400 sections with a single section thickness of 0.2 mm. From the 7 datasets obtained after contrast injection, the one in the arterial phase was chosen for comparison with MRA. We used a matched volume-rendering technique and MIP reconstructions (section thickness, 5 mm; 2.5-mm spacing; axial orientation) to compare MRA with FD-CTA.1
Dose Evaluation
We used a phantom-based method as described elsewhere to measure the effective dose to patients in this novel application.13
Image Analysis
Two experienced neuroradiologists blinded to clinical information randomly reviewed the perfusion studies separately. The Alberta Stroke Program Early CT Score was used for qualitative comparison of FD-CTP and MRP.14–17 For ASPECTS, the territory of the middle cerebral artery is allotted 10 points. One point is subtracted for any area of perfusion abnormality for each of the defined regions (Fig 1F). The standard ASPECTS section position at and immediately superior to the ganglionic level was identified for all datasets. These 2 images were displayed in anonymous random order by using Matlab software (MathWorks, Natick, Massachusetts) (Fig 1F). The type of the map (eg, TTP, MTT, CBV, or CBF) and the scaling (color bar) were displayed. There was no information on the technique used to obtain each single displayed map. The reviewers had to identify whether any perfusion abnormality was obvious on the 2 images. By a simple click on the region assumed to be affected by ischemic stroke, the side and ASPECTS region of the brain were recorded.
All vascular images (volume-rendering technique/MIP reconstructions of MRA and FD-CTA) were stored in anonymous random order on a dedicated workstation. Image evaluation was performed by the 2 neuroradiologists in consensus. Review was performed with the reviewers recording the presence of any vascular occlusion (eg, internal carotid or middle cerebral artery occlusion).
Statistical analysis was performed by using the Statistical Package for the Social Sciences, Version 14.0 (IBM, Armonk, New York). The Pearson correlation coefficient was calculated for each reviewer in the comparison of the 2 different perfusion modalities. Additionally, interobserver correlation of the FD-CTP and MRP was calculated.
Results
Between July 2012 and December 2013, 12 patients (5 men, 7 women; mean age, 72 ± 8 years) admitted for acute ischemic stroke were included. In all patients, the novel FD-CT application could be applied. In all patients, the time between MR imaging and FD-CT imaging was within 30 minutes, so we assume that relevant differences in the parameter maps were not present.
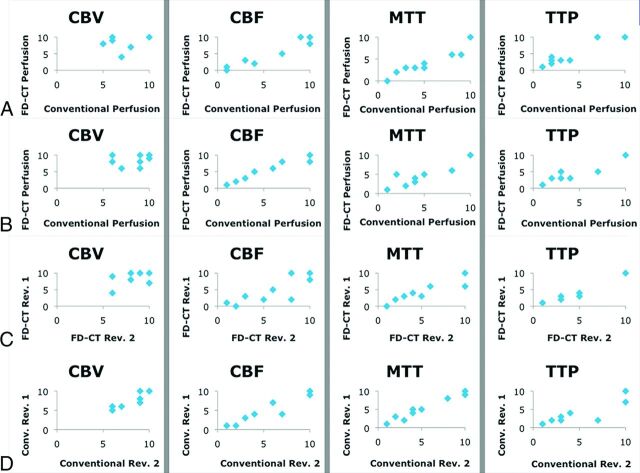
All 4 different parameter maps could be processed for both FD-CTP and MRP. Eight pairs of images per patient (initial MRP and FD-CTP before treatment), altogether 96 pairs, had to be evaluated. The ASPECTS correlation, FD-CTP versus MRP, of the 2 reviewers (reviewer A and B) revealed a high Pearson correlation coefficient in both reviewers for CBF, MTT, and TTP (0.95 to 0.98). For CBV, the correlation coefficient was lower (0.49) (Fig 2A, -B). The interobserver correlation of the FD-CTP maps also revealed a high Pearson correlation coefficient for CBF, MTT, and TTP (0.87–0.99), while for CBV, it was 0.59 (Fig 2C). The interobserver correlation of the MRP maps showed a high correlation (0.91–0.99) (Fig 2D). In summary, the perfusion abnormalities could be recognized identically in FD-CTP and MRP parameter maps (Fig 3).
Fig 2.
Pearson correlation coefficient calculation to compare FD-CTP with MRP (conventional perfusion). The correlations of the 2 reviewers (reviewers A and B) are given in A (Pearson: CBV, 0.49; CBF, 0.97; MTT, 0.96; TTP, 0.96) and B (Pearson: CBV, 0.40; CBF, 0.98; MTT, 0.95; TTP, 0.97). The correlation in CBV is limited in both reviewers. We believe this limitation is because most CBV lesions were small. Thus, the data points appear in the upper right part of the diagrams (A–D, left column). The correlation of the results of the 2 reviewers concerning FD-CTP (C, Pearson: CBV, 0.59; CBF, 0.87; MTT, 0.94; TTP, 0.99) and MR perfusion imaging (D, Pearson: CBV, 0.93; CBF, 0.96; MTT, 0.99; TTP. 0.91) was high. Scaling: x and y axis zero to a maximum of 10 ASPECTS points.
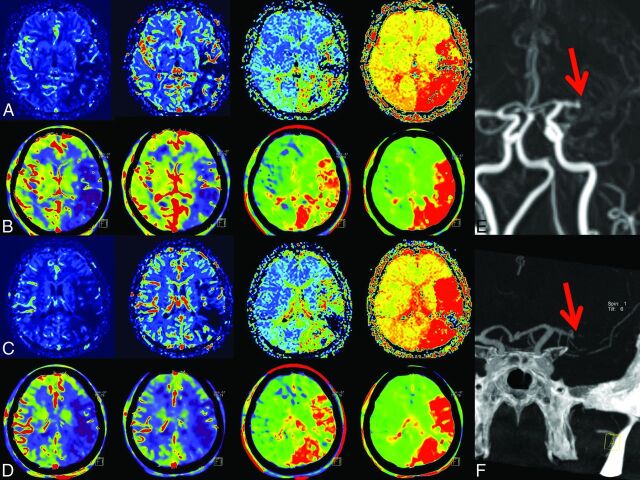
Fig 3.
1) Case example. In rows A and C, MRP; in B and D, FD-CTP parameter maps (left to right: CBF, CBV, MTT, and TTP) are displayed. A and B and C and D are corresponding MRP and FD-CTP images. Due to an MCA occlusion, there is a significant perfusion deficit in all parameter maps. The MCA occlusion was visible in either MRA (E, red arrow) or in FD-CTA (F, red arrow) volume-rendering technique reconstruction.
Review of the volume-rendering technique/MIP reconstructions of the vascular images from both FD-CTA and MRA revealed that all occlusions seen on MRA images could also be recognized in FD-CTA reconstructions. The reviewers identified MCA occlusions (5 right, 7 left MCA occlusions, all TICI 3) in all cases (Figs 3F and 4F).
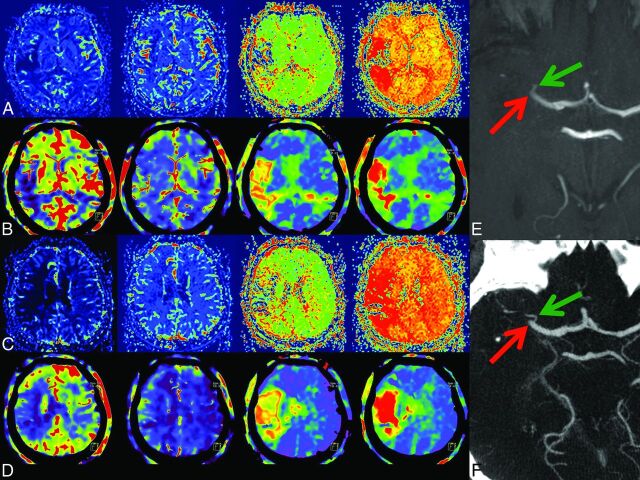
Fig 4.
2) Case example. In rows A and C, MRP; in B and D, corresponding FD-CTP parameters maps (left to right: CBF, CBV, MTT, and TTP) are displayed. A and B and C and D are corresponding MRP and FD-CTP images. Due to an MCA occlusion, there is a significant perfusion deficit in the CBF, MTT, and TTP maps. There is no significant CBV lesion in this patient. The MCA occlusion was visible in either the MRA (E, red arrow) or in FD-CTA (F, red arrow) MIP reconstruction. The green arrow indicates a small temporal branch.
Considering patient safety, knowledge of the effective patient dose is necessary. According to the measurement method as described elsewhere, the effective dose of this novel application was 4.6 mSv.13
Discussion
The aim of this study was to evaluate the feasibility of dynamic perfusion imaging by using a novel FD-CT application within the angiography suite. First, we described a scanning scheme analogous to MS-CT perfusion imaging (Fig 1). Contrary to MS-CT perfusion imaging, the number of acquired datasets and the duration of the entire scanning time are limited. However, the previously described prototype software allowed us to calculate and display all dynamic parameter maps. Using a qualitative evaluation method, we demonstrated the comparability of the parameter maps obtained by MRP and FD-CTP. Because MRP does not provide the ability to calculate absolute values of the different parameters, absolute perfusion values were not considered. This does not represent a major drawback because in most institutions, qualitative evaluation of the maps is performed rather than measurement of absolute values in different regions of the affected tissue. It was our aim to show that the maps obtained by either MRP or FD-CTP may provide similar qualitative information. The analysis revealed high Pearson correlation coefficients except for CBV maps. We believe this limited Pearson correlation of CBV maps to be a bias caused by the fact that the CBV lesions in the patients examined were mostly small or absent (Fig 4). Visible in the diagrams, the average absolute ASPECTS numbers in the CBV evaluation were high (Fig 2). According to current literature, we only consider revascularization treatment beyond 4.5 hours if significant mismatch (small DWI and CBV lesion) is present.18 This “selection bias” is, in our opinion, why, in our series, the CBV lesion size was small. The patient example demonstrating the largest CBV lesion in our series is presented in Fig 3.
We performed additional postprocessing of the dataset by using standard software to reconstruct images to display the intracranial vessels.1 We found that these reconstructions allowed us to visualize the intracranial arteries and occlusions with high image quality (Fig 3F and 4F). All occlusions were recognized as identical to those on MRA and FD-CTA images. This novel application performs a series of 7 bidirectional acquisitions, meaning that a time-resolved “dynamic” FD-CTA may be reconstructed (Fig 1A–E). Recently, some articles described identical reconstructions conducted from MS-CT perfusion datasets (4D MS-CT angiography) to assess collaterals or thrombus length. As demonstrated in Fig 1A–E, this novel FD-CT application may also provide useful information concerning collateral flow and clot burden like that described for 4D MS-CT angiography.6–8,19 However, this evaluation may be subject to further assessment and was not the scope of this work.
Information on the effective dose of a novel application is crucial. In addition, we performed a dose measurement by using an Alderson Rando phantom, resulting in an effective dose of 4.6 mSv. The effective dose of this new FD-CT application is in line with MS-CT perfusion applications. In the literature, dose values of 3.6–5 mSv for MS-CT perfusion imaging are published. Additionally, as a second acquisition, a 3- to 5.5-mSv dose for angiographic MS-CT imaging is necessary, resulting in a total dose of approximately 8–9 mSv for the entire MS-CT evaluation.20–22 We believe that the new FD-CT application providing perfusion and angiographic evaluation with a single simultaneous acquisition and contrast is advantageous because contrast material volume and dose are less than those in MS-CT imaging.
There are some limitations. First, the number of patients included was small but sufficient to show the feasibility of this novel application. Only a qualitative comparison of the parameter maps was performed. A subject of further studies should be to quantify absolute values in comparison with CT perfusion imaging. In all patients, general anesthesia was used. Movement may impair image quality. As a feasibility study, we did not evaluate the clinical outcome of our patients. This novel application is also limited by the total scanning time of 41 seconds and the temporal resolution of 5 seconds. A longer acquisition may further improve measurement of perfusion parameters (most CT and MR imaging applications use acquisition times of approximately 60 seconds). Additionally, a higher temporal resolution (faster rotation time than 5 seconds and less pause time between rotations) is desirable. This may improve the parameter maps and the evaluation of the dynamic FD-CTA to recognize the location of the occlusion and appearance of collateral flow. As described before, the image quality of the FD-CT needs further improvement to approach the quality level of regular MS-CT; therefore, detailed analysis of the FD-CT reconstructions was not performed. Ideally the time between the 2 imaging procedures should be equal to zero. We assume that there was no relevant change within 30 minutes.
The aim of this study was to show the feasibility and qualitative comparability of a new dynamic FD-CT perfusion and angiographic application with a standard method. Despite the necessity of further evaluation and improvement of this novel application, we have the impression that this FD-CT application is approaching MS-CT quality in terms of perfusion and angiographic imaging. Our idea is that in the future, this new application may modify the work flow of those caring for patients with stroke. To avoid time-consuming transportation of a patient from one imaging location to the angiography suite, in which the treatment is performed, direct referral will save time. In the identical environment, diagnostic imaging and treatment becomes feasible. Additional monitoring during the treatment may be possible. Further evaluation of this work flow with all its operational consequences is necessary but is beyond the scope of this work. We believe that physiologic data may be best to recognize the viability of brain tissue. Therefore, we believe that this new application is of high value. On the other hand, FD-CT quality in the visualization of brain tissue does, in our experience, not yet reach the level of MS-CT. Here further effort is necessary and ongoing so that FD-CT scanning may replace MS-CT.
Conclusions
We have shown the feasibility of a novel dynamic FD-CT perfusion and angiographic application in patients with acute stroke by using intravenous contrast application. The parameter maps and MIP/volume-rendering technique images compared well qualitatively with those of MR imaging. This FD-CT application may be substituted for MS-CT imaging in selected patients with acute stroke so that in the future, patients may be directly referred to the angiography suite to save time and avoid transportation.
ABBREVIATIONS:
- FD-CT
flat detector CT
- FD-CTA
flat detector CT dynamic perfusion and angiographic imaging
- FD-CTP
flat detector CT dynamic perfusion
- MRP
MR perfusion imaging
- MS-CT
multisection CT
Footnotes
Disclosures: Tobias Struffert—RELATED: Grant: The authors gratefully acknowledge the funding of the Medical Valley National Leading Edge Cluster, Erlangen, Germany, diagnostic imaging network, subproject BD 16, research grant no. 13EX1212G.* Yu Deuerling-Zheng—OTHER RELATIONSHIPS: employment by Siemens. Michael Manhart—RELATED: Grant: Medical Valley National Leading Edge Cluster, Erlangen. Charles M. Strother—UNRELATED: Grants/Grants Pending: Research Support from Siemens.* *Money paid to the institution.
This work was supported by the Medical Valley National Leading Edge Cluster, Erlangen, Germany, diagnostic imaging network, subproject BD 16, research grant no. 13EX1212G.
REFERENCES
- 1. Struffert T, Deuerling-Zheng Y, Kloska S, et al. Flat detector CT in the evaluation of brain parenchyma, intracranial vasculature, and cerebral blood volume: a pilot study in patients with acute symptoms of cerebral ischemia. AJNR Am J Neuroradiol 2010;31:1462–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Struffert T, Deuerling-Zheng Y, Engelhorn T, et al. Feasibility of cerebral blood volume mapping by flat panel detector CT in the angiography suite: first experience in patients with acute middle cerebral artery occlusions. AJNR Am J Neuroradiol 2012;33:618–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Struffert T, Deuerling-Zheng Y, Kloska S, et al. Cerebral blood volume imaging by flat detector computed tomography in comparison to conventional multislice perfusion CT. Eur Radiol 2011;21:882–89 [DOI] [PubMed] [Google Scholar]
- 4. Lees KR, Hankey GJ, Hacke W. Design of future acute-stroke treatment trials. Lancet Neurol 2003;2:54–61 [DOI] [PubMed] [Google Scholar]
- 5. Grigoryan M, Tung CE, Albers GW. Role of diffusion and perfusion MRI in selecting patients for reperfusion therapies. Neuroimaging Clin N Am 2011;21:247–57, ix-x [DOI] [PubMed] [Google Scholar]
- 6. Saake M, Goelitz P, Struffert T, et al. Comparison of conventional CTA and volume perfusion CTA in evaluation of cerebral arterial vasculature in acute stroke. AJNR Am J Neuroradiol 2012;33:2068–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frölich AM, Wolff SL, Psychogios MN, et al. Time-resolved assessment of collateral flow using 4D CT angiography in large-vessel occlusion stroke. Eur Radiol 2014;24:390–96 [DOI] [PubMed] [Google Scholar]
- 8. Frölich AM, Schrader D, Klotz E, et al. 4D CT angiography more closely defines intracranial thrombus burden than single-phase CT angiography. AJNR Am J Neuroradiol 2013;34:1908–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schramm P, Schellinger PD, Klotz E, et al. Comparison of perfusion computed tomography and computed tomography angiography source images with perfusion-weighted imaging and diffusion-weighted imaging in patients with acute stroke of less than 6 hours' duration. Stroke 2004;35:1652–58 [DOI] [PubMed] [Google Scholar]
- 10. Royalty K, Manhart M, Pulfer K, et al. C-arm CT measurement of cerebral blood volume and cerebral blood flow using a novel high-speed acquisition and a single intravenous contrast injection. AJNR Am J Neuroradiol 2013;34:2131–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ganguly A, Fieselmann A, Marks M, et al. Cerebral CT perfusion using an interventional C-arm imaging system: cerebral blood flow measurements. AJNR Am J Neuroradiol 2011;32:1525–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fieselmann A, Ganguly A, Deuerling-Zheng Y, et al. Interventional 4-D C-arm CT perfusion imaging using interleaved scanning and partial reconstruction interpolation. IEEE Trans Med Imaging 2012;31:892–906 [DOI] [PubMed] [Google Scholar]
- 13. Struffert T, Hauer M, Banckwitz R, et al. Effective dose to patient measurements in flat-detector and multislice computed tomography: a comparison of applications in neuroradiology. Eur Radiol 2014;24:1257–65 [DOI] [PubMed] [Google Scholar]
- 14. Aviv RI, Mandelcorn J, Chakraborty S, et al. Alberta Stroke Program Early CT Scoring of CT perfusion in early stroke visualization and assessment. AJNR Am J Neuroradiol 2007;28:1975–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin K, Rapalino O, Law M, et al. Accuracy of the Alberta Stroke Program Early CT Score during the first 3 hours of middle cerebral artery stroke: comparison of noncontrast CT, CT angiography source images, and CT perfusion. AJNR Am J Neuroradiol 2008;29:931–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sillanpaa N, Saarinen JT, Rusanen H, et al. CT perfusion ASPECTS in the evaluation of acute ischemic stroke: thrombolytic therapy perspective. Cerebrovasc Dis Extra 2011;1:6–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Psychogios MN, Schramm P, Frölich AM, et al. Alberta Stroke Program Early CT Scale evaluation of multimodal computed tomography in predicting clinical outcomes of stroke patients treated with aspiration thrombectomy. Stroke 2013;44:2188–93 [DOI] [PubMed] [Google Scholar]
- 18. Saake M, Breuer L, Gölitz P, et al. Clinical/perfusion CT CBV mismatch as prognostic factor in intraarterial thrombectomy in acute anterior circulation stroke. Clin Neurol Neurosurg 2014;121:39–45 [DOI] [PubMed] [Google Scholar]
- 19. Klingebiel R, Siebert E, Diekmann S, et al. 4-D imaging in cerebrovascular disorders by using 320-slice CT: feasibility and preliminary clinical experience. Acad Radiol 2009;16:123–29 [DOI] [PubMed] [Google Scholar]
- 20. Cohnen M, Wittsack HJ, Assadi S, et al. Radiation exposure of patients in comprehensive computed tomography of the head in acute stroke. AJNR Am J Neuroradiol 2006;27:1741–45 [PMC free article] [PubMed] [Google Scholar]
- 21. Mnyusiwalla A, Aviv RI, Symons SP. Radiation dose from multidetector row CT imaging for acute stroke. Neuroradiology 2009;51:635–40 [DOI] [PubMed] [Google Scholar]
- 22. Diekmann S, Siebert E, Juran R, et al. Dose exposure of patients undergoing comprehensive stroke imaging by multidetector-row CT: comparison of 320-detector row and 64-detector row CT scanners. AJNR Am J Neuroradiol 2010;31:1003–09 [DOI] [PMC free article] [PubMed] [Google Scholar]