Abstract
BACKGROUND AND PURPOSE:
Timing-invariant (or delay-insensitive) CT angiography derived from CT perfusion data may obviate a separate cranial CTA in acute stroke, thus enhancing patient safety by reducing total examination time, radiation dose, and volume of contrast material. We assessed the diagnostic accuracy of timing-invariant CTA for detecting intracranial artery occlusion in acute ischemic stroke, to examine whether standard CTA can be omitted.
MATERIALS AND METHODS:
Patients with suspected ischemic stroke were prospectively enrolled and underwent CTA and CTP imaging at admission. Timing-invariant CTA was derived from the CTP data. Five neuroradiologic observers assessed all images for the presence and location of intracranial artery occlusion in a blinded and randomized manner. Sensitivity and specificity of timing-invariant CTA and standard CTA were calculated by using an independent expert panel as the reference standard. Interrater agreement was determined by using κ statistics.
RESULTS:
We included 108 patients with 47 vessel occlusions. Overall, standard CTA and timing-invariant CTA provided similar high diagnostic accuracy for occlusion detection with a sensitivity of 96% (95% CI, 90%–100%) and a specificity of 100% (99%–100%) for standard CTA and a sensitivity of 98% (95% CI, 94%–100%) and a specificity of 100% (95% CI, 100%–100%) for timing-invariant CTA. For proximal large-vessel occlusions, defined as occlusions of the ICA, basilar artery, and M1, the sensitivity and specificity were 100% (95% CI, 100%–100%) for both techniques. Interrater agreement was good for both techniques (mean κ value, 0.75 and 0.76).
CONCLUSIONS:
Timing-invariant CTA derived from CTP data provides diagnostic accuracy similar to that of standard CTA for the detection of artery occlusions in acute stroke.
Stroke imaging research currently focuses on prediction of patient outcome and identifying patients who are suitable for neurointerventional treatment.1,2 For these purposes, advanced stroke imaging protocols typically add CT perfusion imaging or diffusion-weighted MR imaging to the traditional work-up, consisting of noncontrast CT and CT angiography.2,3 Noncontrast CT is used to differentiate hemorrhagic stroke from ischemic stroke and to assess early signs of ischemia. CTA is used to localize arterial occlusions and to identify proximal large-vessel occlusions that may be suitable for endovascular treatment. CT perfusion imaging and DWI are used to assess the extent and severity of hypoperfusion and particularly increase the sensitivity of imaging in the early stages of ischemic stroke.4 The practical advantages of CT perfusion imaging are that it is widely available and does not delay treatment decisions because it is fast and most patients already undergo CT scanning.3
Currently, CTA can be derived from CT perfusion data. Such an approach allows the enhancement of patient safety by reducing the total scanning time, radiation dose, and amount of contrast material needed.5 In CT perfusion imaging, multiple scans after intravenous injection of contrast material are obtained with time, generating a 4D dataset, which is used to derive cerebral perfusion maps such as the cerebral blood flow, cerebral blood volume, and arrival times. When imaging is performed on a CT scanner with large spatial coverage, however, this 4D data can also be used to provide CT angiographic information, referred to as 4D-CTA or dynamic CTA. Previous studies have assessed whether 4D-CTA can be used for detection of vascular occlusion in a stroke setting but found that image quality was moderate and diagnostic performance for stroke assessment was limited because large-vessel occlusions may be missed.5–8 Recently, a different approach to obtain CTA from CT perfusion source data was presented that combines the whole 4D-CTA dataset into 1 high-quality 3D-CTA dataset by displaying maximum contrast enhancement with time.5 This technique is referred to as “timing-invariant CTA” because it is insensitive to delayed contrast arrival and was shown to provide similar-to-superior image quality compared with standard CTA.5
The aim of our study was to test the diagnostic performance of timing-invariant CTA for stroke evaluation, to assess whether standard CTA can be omitted when CT perfusion imaging has been performed.
Materials and Methods
Patients
Institution review board approval and informed consent were obtained. Consecutive patients admitted to our hospital with suspected ischemic stroke were prospectively enrolled. They underwent standard CTA and CT perfusion imaging if they fulfilled the following criteria: 1) admission at <9 hours after onset of neurologic deficit (including patients who woke up with stroke symptoms if the time between going to sleep and admission was <9 hours), 2) NIHSS score of at least 2, 3) no signs of hemorrhage on noncontrast CT, and 4) no known contrast allergy or kidney failure. For this study, we selected all patients who were scanned on our 256-section CT scanner (iCT; Philips Healthcare, Best, the Netherlands). Scans were performed according to a previously reported protocol: CT perfusion imaging was performed by using 40 mL of intravenous contrast material, 80 kV(peak), and 150 mAs every 2 seconds during 48 seconds (volume CT dose index = 5.8 mGy per acquisition, dose-length product = 1157 mGy × cm), and CT angiography was performed by using 50 mL of intravenous contrast material, 120 kVp, and 150 mAs (volume CT dose index = 19.5 mGy, dose-length product = 775 mGy cm).5 CT perfusion imaging and CTA were performed subsequently, and both, before tPA administration. For the purpose of this study, the volume covered by standard CTA was manually clipped to correspond with the volume of CT perfusion. For all imaging studies, the intracranial arteries being included in the CT perfusion volume were verified.
Timing-invariant CTA was automatically derived from the CT perfusion data on a scientific workstation (iX Viewer; ISI, Utrecht, the Netherlands). Timing-invariant CTA provides angiographic images by displaying maximal enhancement with time (temporal maximum intensity projection) with a prior noise-reducing filter in the temporal domain.5,9 This temporal noise-reducing filter improves image quality without loss of spatial resolution. Due to the choice of the temporal maximum, this technique is timing-invariant (ie, the maximal enhancement of a vessel is displayed independent of its contrast arrival time).9 In this study, timing-invariant CTA was implemented as a 1D Gaussian low-pass filter across all time points of the CTP sequence (parameters: SD = 1.5 seconds, kernel size = 5) followed by determining the maximal CT number with time (temporal MIP), which is the output CT number displayed on the timing-invariant CTA.
Procedures
Timing-invariant CTA and standard CTA were assessed for the presence and location of arterial occlusion.
All scans were assessed by 5 experienced radiologic observers (2 neuroradiologists, 1 radiologist, and 2 radiology residents, with 9 [I.v.d.S.], 7 [F.J.A.M.], 7 [A.D.H.], 5 [J.W.D.] and 3 [E.J.S.] years of experience in evaluating cerebral CTA examinations, respectively). These observers were individually presented with a random sequence of scans and were blinded to patient information and imaging technique. To reduce potential sources of bias, we presented all sequences with different randomization.
Images were scored for the presence of occlusion in the following arteries: 1) intracranial part of the internal carotid artery, 2) middle cerebral artery segment 1 (M1), 3) distal MCA segment 2 (M2+), 3) anterior cerebral artery segment 1 (A1), 4) anterior cerebral artery segment 2 (A2), 5) basilar artery, and 6) posterior cerebral artery. Arteries were separately scored for both hemispheres. Scans were presented with equal display settings, and observers could change these settings in accordance with clinical practice (arbitrary planes, slab thickness, and window leveling).
The final diagnosis (reference standard) was made by an independent expert panel consisting of a neurointerventional radiologist and a neuroradiologist (with >15 and 10 years of experience in evaluating cerebral CTA examinations, respectively) who were provided with all available imaging and relevant clinical information (including follow-up). This expert panel was blinded to the observer scores and reviewed all cases in which differences were found between the observer scores on standard CTA and timing-invariant CTA.
Statistical Analysis
To assess diagnostic accuracy, we determined the sensitivity and specificity of standard CTA and timing-invariant CTA with the diagnosis of the expert panel serving as the reference test. The diagnosis per CTA technique was the one made by the majority of the observers. The sensitivity and specificity were calculated per artery segment separately and for the major cerebral arteries combined. These values were expressed with their 95% confidence intervals.
To assess reproducibility, we determined interobserver agreement by using κ statistics for both timing-invariant CTA and standard CTA. These parameters were expressed as average values together with their range among observers.
Results
From October 2009 to October 2011, 167 consecutive patients underwent imaging at our hospital for suspected ischemic stroke. One hundred fifteen patients were eligible for this diagnostic study because they were scanned on a 256-section CT scanner and CT perfusion source data were available for analysis. Of these, 6 patients were excluded because imaging was unsuccessful due to severe patient motion (4 on CT perfusion imaging and 2 on standard CTA), and 1 patient was excluded because the CT perfusion images did not include the circle of Willis. The final analysis included 108 patients (58 women; mean age, 68 ± 13 years). The median NIHSS score at admission was 6 (interquartile range, 3–13), and the median time from onset of symptoms to CT was 107 minutes (interquartile range, 40–200 minutes).
Thirty-five patients (32%) had an arterial occlusion, in which 9 occlusions were located in the internal carotid artery, 33 in the middle cerebral artery (25 in the M1 segment and 8 in M2+ segment), 2 in the anterior cerebral artery (both in segment A1), 1 in the basilar artery, and 2 in the posterior cerebral artery.
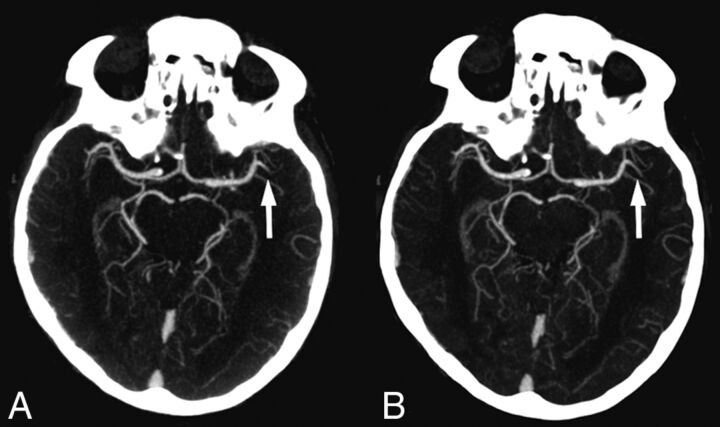
An overview of the results is presented in the Table. Overall, standard CT angiography and timing-invariant CT angiography provided similar high diagnostic accuracy for occlusion detection in acute ischemic stroke with a sensitivity of 96% (95% CI, 90%–100%) and a specificity of 100% (95% CI, 99%–100%) for standard CTA and a sensitivity of 98% (95% CI, 94%–100%) and a specificity of 100% (95% CI, 100%–100%) for timing-invariant CTA. For proximal large-vessel occlusions, defined as occlusions of the ICA, basilar artery, and M1, the sensitivity and specificity were 100% (95% CI, 100%–100%) for both techniques. For distal middle cerebral artery occlusions (M2+), standard CTA provided a sensitivity of 75% (95% CI, 45%–100%) and a specificity of 100% (95% CI, 100%–100%) compared with a sensitivity and specificity of 100% (95% CI, 100%–100%) for timing-invariant CTA (not significant). An example of a M2 occlusion that was detected on both techniques is shown in Fig 1.
Diagnostic performance of standard CTA and timing-invariant CTA for assessment of artery occlusion in the various territoriesa
| Territory | Occlusions | Standard CTA |
Timing-Invariant CTA |
||
|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | ||
| Overall | 47 | 96% (90–100) | 100% (99–100) | 98% (94–100) | 100% (100–100) |
| Internal carotid artery | 9 | 100% (100–100) | 100% (100–100) | 100% (100–100) | 100% (100–100) |
| Anterior cerebral artery | 2 | 100% (100–100) | 99% (98–100) | 100% (100–100) | 99% (98–100) |
| Middle cerebral artery | 33 | 94% (86–100) | 100% (100–100) | 100% (100–100) | 99% (98–100) |
| Segment 1 (M1) | 25 | 100% (100–100) | 100% (100–100) | 100% (100–100) | 100% (100–100) |
| Segment 2+ (M2+) | 8 | 75% (45–100) | 100% (100–100) | 100% (100–100) | 100% (100–100) |
| Basilar artery | 1 | 100% (100–100) | 100% (100–100) | 100% (100–100) | 100% (100–100) |
| Posterior cerebral artery | 2 | 100% (100–100) | 99% (98–100) | 50% (0–100) | 100% (100–100) |
Data in parentheses are 95% confidence intervals.
Fig 1.
Standard CT angiography (A) and timing-invariant CT angiography (B) images in a patient with a left-sided middle cerebral artery occlusion in the M2 segment. The occlusion was detected on both CT angiography techniques.
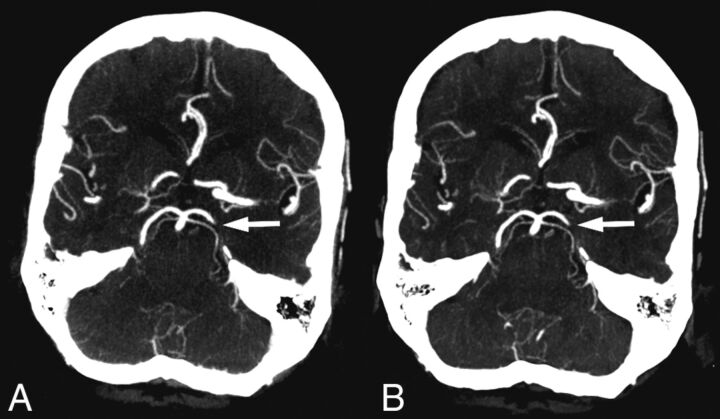
For the posterior cerebral artery, 1 of 2 occlusions was missed on timing-invariant CTA, while both were diagnosed on standard CTA. Overall, only this 1 occlusion was missed on timing-invariant CTA (missed by 3 observers on timing-invariant CTA but also by 2 observers on standard CTA), shown in Fig 2.
Fig 2.
Standard CT angiography (A) and timing-invariant CT angiography (B) images in a patient with a left-sided posterior cerebral artery occlusion. This was the only occlusion missed on timing-invariant CTA and was considered a result of observer variation because it was scored as a false-negative finding by 3 observers on timing-invariant CTA and by 2 observers on standard CTA.
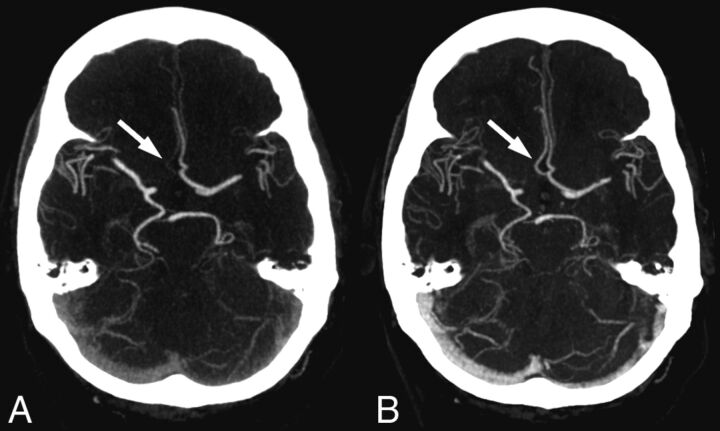
In 8 patients, differences were found between the observer scores on standard CTA and timing-invariant CTA, and these cases were presented to the expert panel. In all cases, the expert panel found that the standard CTA and timing-invariant CTA images provided the same diagnosis. In 4 cases, however, standard CTA showed faint enhancement in an artery segment, whereas timing-invariant CTA showed high enhancement. Figure 3 shows an example of these differences in a patient with a hypoplastic A1 segment that was scored as a false-positive finding on standard CTA as a combined A1 and A2 occlusion, resulting from only faint A2 enhancement, while on timing-invariant CTA, the segment was scored as a true-negative finding because it showed clear A2 enhancement. In the remaining 4 cases, the differences in observer scores between standard CTA and timing-invariant CTA could only be explained by observer variation because there was mostly 1 observer difference and the images were virtually identical (Figs 1 and 2).
Fig 3.
Images show the effect of delayed contrast material arrival in a patient with right-sided hemiparesis. A, On standard CT angiography, the right-sided anterior cerebral artery was considered occluded in both segments A1 and A2. B, On timing-invariant CTA, the right-sided anterior cerebral artery was considered hypoplastic in segment A1 and patent in segment A2. Standard CTA shows only faint enhancement in the right-sided A2 segment (arrow) because the bulk of contrast material arrived after the standard CTA acquisition. Timing-invariant CT shows strong A2 enhancement (arrow) because it is delay-insensitive and displays maximal contrast enhancement with time.
Overall interrater agreement was good for both timing-invariant CTA (mean κ value = 0.76; range observers, 0.69–0.83; mean agreement = 97%; range observers, 96%–98%), and standard CTA (mean κ value = 0.75; range observers, 0.63–0.85; mean agreement = 97%; range observers, 96%–98%).
Discussion
In acute stroke, CT angiography is performed to identify proximal large-vessel occlusions suitable for endovascular treatment. In this study, we found that CTA derived from CT perfusion data can successfully detect such occlusions; this feature makes it unnecessary to perform a separate CTA acquisition of the brain. Timing-invariant CTA integrates information across the whole time sequence of the CT perfusion acquisition and is, therefore, less susceptible to delays in regional enhancement, which may hamper standard CTA.5 Our results imply that standard CTA for the evaluation of intracranial occlusions can be omitted if CT perfusion imaging has been performed. The advantage of such an approach lies in improvement of patient safety: Because 1 scan can be performed instead of 2 separate ones, total scanning time, contrast material usage, and radiation dose will be reduced.5 The exact benefits will depend on the scan protocol, but the approach will typically allow a combined CTA and CT perfusion imaging protocol that can be performed within 60 to 90 seconds, reduces the radiation dose by 0.5–1.0 mSv, and requires only 40–50 mL of intravenous contrast material.4,5,10,11 Within this time, tPA can be prepared for administration; therefore, advanced imaging need not delay thrombolytic therapy.4
In our study, we found a few differences in occlusion detection between standard CTA and timing-invariant CTA. We analyzed these cases and found that most of these differences could be explained only by observer variation because the images were virtually identical. In some cases however, we found that segments that seemed occluded on standard CTA actually showed contrast enhancement on timing-invariant CTA. This difference implies that these segments cannot be occluded because they fill with contrast material, albeit at a later point in time than that used for acquiring the standard CTA. Our results imply that standard CTA may not be able to differentiate delayed enhancement of a vascular segment from vessel occlusion. Standard CTA is delay-sensitive because it is performed at 1 moment in time: Vessels will not be visible if contrast material has not yet arrived at the time of acquisition. Timing-invariant CTA, on the other hand, is delay-insensitive because it displays the maximum contrast during the total time of the CT perfusion acquisition, typically some 60 seconds. Timing-invariant CTA is, therefore, less susceptible to suboptimal contrast enhancement resulting from variations in cardiac output or delayed arrival due to vascular pathology.5,9,12,13 A strength of the suggested approach is that CT perfusion images and 4D-CTA images are simultaneously available for side-by-side comparison. This combined information can resolve discrepancies and helps differentiate hypoplastic vessel segments, vessel stenosis, and occlusions with good collateralization.5,9,12,13
Our study has limitations. First, our scanner could not cover the whole brain during CT perfusion but only a 5- to 6-cm range around the circle of Willis. We could, therefore, evaluate the main cerebral arteries that are relevant for endovascular treatment but not the distal pericallosal and cortical branches. In this comparison study, we focused on the question of whether occlusions can be reliably detected on the CTA images that are derived from the CT perfusion data. To make sure that the results were not biased by the greater coverage of standard CTA, we cropped the CTA data so that identical volumes could be evaluated and compared. Most manufactures now have scanners that provide whole-brain coverage during CT perfusion imaging. With this equipment, no additional brain CTA is necessary if timing-invariant CTA is calculated from the CT perfusion acquisition. For assessing the extracranial portions of the carotid artery, cervical CTA, MR angiography, or sonography may be performed.14–16 Cervical imaging remains important for assessing the extracranial extent of ICA occlusion for neurointerventional planning or detecting carotid plaques as a source of emboli. Second, timing-invariant CTA is currently not commercially available but can be easily implemented in commercial or research software, and 1 major vendor has announced the implementation on its CT workstation.5
Conclusions
Timing-invariant CTA derived from CT perfusion data provides diagnostic performance similar to that of standard CTA for the detection of artery occlusions in acute stroke.
Footnotes
Disclosures: Ewoud J. Smit—UNRELATED: Payment for Lectures (including service on Speakers Bureaus): Toshiba Medical Systems. Frederick J.A. Meijer—UNRELATED: Payment for Lectures (including service on Speakers Bureaus): Toshiba Medical Systems (Speakers Bureau). Jan W. Dankbaar—RELATED: Grant: Dutch Heart Foundation (2012T061). Birgitta Velthuis—UNRELATED: Grants/Grants Pending: Netherlands Heart Foundation (grant number 2008 T034) Dutch Acute Stroke Trial*; Payment for Lectures (including service on Speakers Bureaus): Philips Healthcare.* Mathias Prokop—RELATED: Grant: Philips Healthcare*; UNRELATED: Grants/Grants Pending: Philips Healthcare,* Toshiba Medical Systems*; Payment for Lectures (including service on Speakers Bureaus): Bracco, Toshiba Medical Systems, Bayer-Schering, CME Science; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Toshiba Medical Systems, Philips Healthcare, European School of Radiology, CME Science. *Money paid to the institution.
REFERENCES
- 1. Shuaib A, Butcher K, Mohammad AA, et al. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol 2011;10:909–21 [DOI] [PubMed] [Google Scholar]
- 2. Schellinger PD, Bryan RN, Caplan LR, et al. ; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Evidence-based guideline: the role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke—report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2010;75:177–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wintermark M, Rowley HA, Lev MH. Acute stroke triage to intravenous thrombolysis and other therapies with advanced CT or MR imaging: pro CT. Radiology 2009;251:619–926 [DOI] [PubMed] [Google Scholar]
- 4. Lev MH. Acute stroke imaging: what is sufficient for triage to endovascular therapies? AJNR Am J Neuroradiol 2012;33:790–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smit EJ, Vonken E-J, van der Schaaf IC, et al. Timing-invariant reconstruction for deriving high-quality CT angiographic data from cerebral CT perfusion data. Radiology 2012;263:216–25 [DOI] [PubMed] [Google Scholar]
- 6. Yang CY, Chen YF, Lee CW, et al. Multiphase CT angiography versus single-phase CT angiography: comparison of image quality and radiation dose. AJNR Am J Neuroradiol 2008;29:1288–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Willems PW, Brouwer PA, Barfett JJ, et al. Detection and classification of cranial dural arteriovenous fistulas using 4D-CT angiography: initial experience. AJNR Am J Neuroradiol 2010;32:49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frölich AM, Psychogios MN, Klotz E, et al. Angiographic reconstructions from whole-brain perfusion CT for the detection of large vessel occlusion in acute stroke. Stroke 2012;43:97–102 [DOI] [PubMed] [Google Scholar]
- 9. Smit EJ, Vonken EJ, van Seeters T, et al. Timing-invariant imaging of collateral vessels in acute ischemic stroke. Stroke 2013;44:2194–99 [DOI] [PubMed] [Google Scholar]
- 10. Balemans CE, Reichert LJM, van Schelven BI, et al. Epidemiology of contrast material-induced nephropathy in the era of hydration. Radiology 2012;263:706–13 [DOI] [PubMed] [Google Scholar]
- 11. Huda W, Ogden KM, Khorasani MR. Converting dose-length product to effective dose at CT. Radiology 2008;248:995–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 2010;256:32–61 [DOI] [PubMed] [Google Scholar]
- 13. Frölich AM, Wolff SL, Psychogios MN, et al. Time-resolved assessment of collateral flow using 4D CT angiography in large-vessel occlusion stroke. Eur Radiol 2014;24:390–96 [DOI] [PubMed] [Google Scholar]
- 14. Chappell FM, Wardlaw JM, Young GR, et al. Carotid artery stenosis: accuracy of noninvasive tests—individual patient data meta-analysis. Radiology 2009;251:493–502 [DOI] [PubMed] [Google Scholar]
- 15. El-Saden SM, Grant EG, Hathout GM, et al. Imaging of the internal carotid artery: the dilemma of total versus near total occlusion. Radiology 2001;221:301–08 [DOI] [PubMed] [Google Scholar]
- 16. Corti R, Fuster V. Imaging of atherosclerosis: magnetic resonance imaging. Eur Heart J 2011;32:1709–19b [DOI] [PubMed] [Google Scholar]