Abstract
Schizophrenia is a biologically complex disorder with multiple regional deficits in cortical brain morphology. In addition, interindividual heterogeneity of cortical morphological metrics is larger in patients with schizophrenia when compared to healthy controls. Exploiting interindividual differences in the severity of cortical morphological deficits in patients instead of focusing on group averages may aid in detecting biologically informed homogeneous subgroups. The person-based similarity index (PBSI) of brain morphology indexes an individual’s morphometric similarity across numerous cortical regions amongst a sample of healthy subjects. We extended the PBSI such that it indexes the morphometric similarity of an independent individual (eg, a patient) with respect to healthy control subjects. By employing a normative modeling approach on longitudinal data, we determined an individual’s degree of morphometric dissimilarity to the norm. We calculated the PBSI for sulcal width (PBSI-SW) in patients with schizophrenia and healthy control subjects (164 patients and 164 healthy controls; 656 magnetic resonance imaging scans) and associated it with cognitive performance and cortical sulcation index. A subgroup of patients with markedly deviant PBSI-SW showed extreme deficits in cognitive performance and cortical sulcation. Progressive reduction of PBSI-SW in the schizophrenia group relative to healthy controls was driven by these deviating individuals. By explicitly leveraging interindividual differences in the severity of PBSI-SW deficits, neuroimaging-driven subgrouping of patients is feasible. As such, our results pave the way for future applications of morphometric similarity indices for subtyping of clinical populations.
Keywords: MRI, adolescence, brain development, cortex
Introduction
Neurobiological research and biomarker discovery efforts in the field of psychiatry have been substantially hampered by insufficient biological validity of current diagnostic categories, stalling the development of precision medicine in psychiatry.1 Given the large body of evidence for clinical, etiological, and biological heterogeneity within psychotic disorders, extensive research is being conducted to identify more biologically homogeneous subgroups based on clinical, neuroimaging, neurophysiological, molecular, or biochemical variables, which might improve knowledge of the underlying pathophysiology and guide stratified treatments.2–6
Schizophrenia is consistently associated with gray and white matter deficits that vary in severity, and some of these abnormalities are progressive over time.7–9 However, the clinical utility of brain imaging for guiding diagnosis and treatment is challenged by the large heterogeneity in location and severity of brain deficits among patients with schizophrenia.10–12 The degree of interindividual heterogeneity in brain deficits is not evenly distributed across the cortex in schizophrenia.11 Large interindividual heterogeneity in cortical deficits affecting a particular brain region in schizophrenia may be the result of separable neurobiological underpinnings of brain anatomy in that region across individuals, thus pointing to different biological subgroups within the disorder. In contrast, regional brain deficits with low interindividual variability may point to mechanisms shared by a significant proportion of patients with schizophrenia, thus supporting their involvement in its general pathophysiology.11–13 Collectively, these findings underline the importance of focusing on interindividual differences in severity in addition to assessing mean differences.14
Novel morphometric approaches assessing the similarity of cortical morphology across regions, instead of focusing on single regions, may thus be well suited for assessing the widespread, variable cortical deficits in schizophrenia.15,16 A recent cross-sectional study showed that, in 3 independent samples of adults with schizophrenia, average global and regional morphometric similarity was reduced, and this reduction was associated with schizophrenia-related genes.15 However, it is unclear if reductions in morphometric similarity in schizophrenia are progressive over time. In addition, reduced morphometric similarity was based on mean differences, which, in combination with widespread enlarged dispersion of cortex morphology in schizophrenia, suggests that results may not be equally applicable to all patients. The recently developed person-based similarity index (PBSI) combines the concepts of morphometric similarity and interindividual heterogeneity. By calculating an individual’s morphometric similarity to the other individuals in a group across cortical regions,16 it may be more sensitive to distributed disease-associated cortical atypicalities compared to standard univariate measures.
The next step is to identify patients whose brain morphology is markedly dissimilar to that of a normative group. In a normative modeling approach, the deviation from the norm value of some quantity is examined. Usually, the quantity in question is a univariate measure, such as the volume of a certain brain region. How a number of such univariate normative measures should be combined for stratification of individuals is still a topic of debate.17 Here, we chose to apply normative modeling to a multivariate summary measure, PBSI. We extended the PBSI for patients such that it indexes the degree of similarity between the morphological profile of an individual patient to those of healthy control subjects.
Sulcal morphological parameters have been recently developed as complementary to volume and cortical thickness measurements. Compared to these traditional measurements, sulcal parameters, such as sulcal width (SW), are sensitive to shrinking in adjacent gyri, reductions in subcortical white matter, changes in sulcal cytoarchitecture, and less biased by age-related decreases in magnetic resonance imaging (MRI) gray–white contrast.18–27 Reductions in sulcal cytoarchitecture, cortical thickness, and subcortical white matter have consistently been implicated in the pathology of schizophrenia pointing to SW as an optimal metric for this disease.7,8,27
The PBSI for SW (PBSI-SW) has dispersion, as is expected from previous publications, reflecting more heterogeneous brain morphology in individuals with schizophrenia.11,12 Normative modeling leverages this dispersion, allowing for statistical thresholding of the dissimilarity and facilitates the identification of participants who are markedly dissimilar.
We leverage a large longitudinal lifespan sample of participants with schizophrenia and healthy participants to investigate whether the lifespan PBSI-SW trajectories differ between patients and healthy subjects and whether such differences are driven by individuals with extremely deviating PBSI-SW. In addition, we investigate whether extreme PBSI-SW deviance is associated with deficits in cognition and—in line with the neurodevelopmental hypothesis of schizophrenia—the sulcation index, a marker of perinatal neurodevelopmental perturbations, which is associated with schizophrenia.28
Methods
Sample and Neuroimaging
From a large longitudinal sample of patients with schizophrenia and healthy participants aged 16–70 years (at baseline), we included individuals who had T1-weighted MRI scan acquisitions at 2 time points. Detailed information regarding diagnostic criteria and clinical assessments of the Utrecht Schizophrenia project and the Genetic Risk and Outcome of Psychosis consortium are described in 29–31. Details on the inclusion and exclusion criteria, clinical and cognitive measurements, MRI acquisition, quality assessment and processing to obtain total brain volume, regional SW, and the sulcation index can be found in supplementary material and supplementary figures 1 and 2. The final data set consisted of 656 scans from 164 healthy participants and 164 patients. Demographic, cognitive, clinical, and imaging information sample is provided in table 1. The institutional review board at the University Medical Center Utrecht reviewed the study protocols and provided ethical approval. All participants provided written informed consent.
Table 1.
Demographics, cognitive, clinical, and imaging characteristics of patients with schizophrenia and healthy controls
| Schizophrenia patients | Healthy controls | ||||
|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | P | |
| Number of subjects | 164 | 164 | |||
| Sociodemographics | |||||
| Sex, n (%), males | 126 (77) | 126 (77) | |||
| Age, y: mean (SD) | 29.52 (8.95) | 33.60 (9.21) | 31.04 (11.65) | 35.05 (11.88) | |
| Education, y: mean (SD) | 11.94 (2.68) | 12.11 (2.85) | 13.61 (2.65) | 13.66 (2.63) | |
| Scanner (I/II) | 81/83 | 55/109 | ** | ||
| Cognitive and clinical variables | |||||
| Estimated-scale IQ total: mean (SD) | 97.62 (16.56) | 102.97 (20.47) | 112.59 (15.78) | 115.46 (16.11) | *** |
| Age of onset, y: mean (SD)a | 21.63 (5.70) | ||||
| Duration of illness, y: mean, (SD)b | 6.77 (7.61) | 11.23 (8.27) | |||
| PANSS score, total: mean (SD) | 62.78 (18.78) | 50.24 (14.30) | |||
| Total positive symptoms | 14.83 (5.78) | 12.36 (4.63) | |||
| Total negative symptoms | 16.17 (6.01) | 12.09 (5.66) | |||
| Total general symptoms | 30.88 (11.04) | 24.99 (7.05) | |||
| Antipsychoticsc | |||||
| Without medication, n (%) | 7 (4.14) | 7 (4.16) | |||
| Exclusively typical, n (%) | 23 (13.6) | 12 (7.14) | |||
| Exclusively atypical, n (%) | 89 (52.66) | 92 (54.76) | |||
| Both, n (%) | 4 (2.36) | 2 (1.19) | |||
| Cumulative dose exposure (mg): mean (SD) | 275837.8 (397345.9) | 945754.9 (1285851) |
|||
| Missing, n (%) | 103 (61.54) | 102 (60.71) | |||
| Imaging | |||||
| Total brain volume | 1181.27(112.70) | 1171.50 (115.42) | 1218.15 (111.20) | 1213.57 (110.68) | ** |
Note: The total amount of scans is 912.
IQ, intelligence quotient; PANSS, Positive and Negative Symptom Scale.
aAge of onset is the age at which the first positive symptom occurs.
bDuration of illness is calculated based on the date of the appearance of first positive symptoms of schizophrenia until the date of scan.
cAntipsychotic treatment data is not available for all the patients included (82 patients had medication information). Cumulative doses were calculated per time of scan and given in chlorpromazine equivalents using standard conversion factors and estimated by the daily doses of each of the antipsychotics used by the patient. Significance calculated by chi-square or Welch t tests when appropriate. Significant diagnostic differences are for one or both time points.
*P < .05, **P < .01, ***P < .001.
PBSI-SW
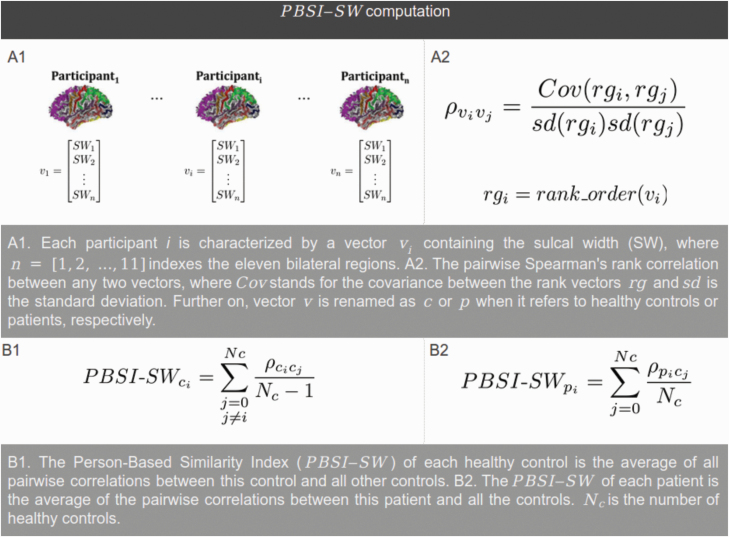
The PBSI-SW calculation was based on 16. At each time point and for each individual, we computed a PBSI-SW value using a 3-step procedure (see figure 1). First, we created the SW profile of each participant by concatenating her/his SW for 11 bilateral sulcal regions. Second, for each of the Nc controls, we calculated the Spearman’s rank correlation coefficients between the participant’s SW profile and the profiles of the other Nc − 1 controls. For each patient, we calculated the interindividual Spearman’s rank correlation coefficient between the participant’s SW profile and the profiles of all Nc controls. Third, for each control, a PBSI-SW was calculated as the average of the Nc − 1 correlation coefficients. For each patient, a PBSI-SW was calculated as the average of the Nc correlation coefficients. Higher PBSI-SW (with a maximum of 1) denotes greater similarity between an individual’s SW profile and those of the (other) controls. The R script used for the PBSI-SW calculation is available from GitHub. There was no change of variability of PBSI-SW across the age span (see supplementary material).
Fig. 1.
Pipeline for computing a person-based similarity index for sulcal width (PBSI-SW) for healthy controls (based on 16) and patients. See online version for color figure.
Statistical Analyses
All analyses were performed in R (https://cran.rstudio.com/).
Longitudinal Trajectory of PBSI-SW
To investigate the longitudinal trajectories of PBSI-SW over the age range, generalized additive mixed models (GAMMs) were used.32 GAMM models were implemented to examine age, diagnosis (ie, patient vs control), and an age × diagnosis interaction while controlling for scanner, total brain volume, and the random effect of the individual. We first fitted models, including a sex and age × sex term, but these terms were not significant. We, therefore, excluded these terms from the model. Including scanner as a random effect did not change the results. To better understand the age × diagnosis interaction, GAMM estimates for age were also implemented and visualized for patients and controls separately.
Cross-Sectional Comparisons
To compare PBSI-SW between groups at baseline and follow-up separately, PBSI-SW values were residualized for age, scanner, and total brain volume at each time point and within each diagnostic group using the following formula:
where PBSI-SW[t]D yields for the PBSI-SW at the time point t and D represents the 2 diagnostic groups. Next, after adding back group mean values for each metric, we used the Welch t test to assess differences between the diagnostic groups at baseline and follow-up.
Normative Modeling
First, we created a normative reference for PBSI-SW by calculating the average of the residualized PBSI-SW values for the healthy control subjects (C) at time point t (baseline or follow-up). Then, individual deviance of a participant’s residualized PBSI-SW value at time point t with respect to the normative (ie, control) group was calculated:
This PBSI-SW-Z value is the distance between a participant’s PBSI-SW and the normative reference, reflecting how deviant the PBSI-SW value of a given individual is compared to the normative value at a certain time point, independent of the participant’s age, scanner, and total brain volume.
For comparison, we also calculated Z-values using the method described in 33. The Pearson correlation coefficient between them was 0.94 (see supplementary figure 4). Then, we applied a threshold, |PBSI-SW-Z| >2 (as in 34), to identify individuals whose morphometric profile is markedly dissimilar (at any time point) to the morphometric profiles of the normative group: the outliers. Density plots of PBSI-SW-Z are shown in supplementary figure 5.
To assess the impact of deviance on the PBSI-SW lifespan trajectories, we recalculated GAMM models without healthy control and patient outliers. To investigate whether the group of patients with deviant PBSI-SW shared differential clinical, cognitive, and morphological characteristics relative to the rest of the patients, we compared age at baseline, sex, Positive and Negative Symptom Scale (PANSS) total scores, and positive, negative, and general subscores at baseline, illness duration, estimated intelligence quotient (IQ) at baseline, and sulcation index at baseline and follow-up between the outlier and nonoutlier patient groups. Finally, we also assessed whether image quality differed in patients at the extremes of the distributions.
Regional Contribution to PBSI-SW
A leave-one-out method was used to determine differences in regional contribution to PBSI-SW (see supplementary figure 13).
Results
Longitudinal Trajectory of PBSI-SW
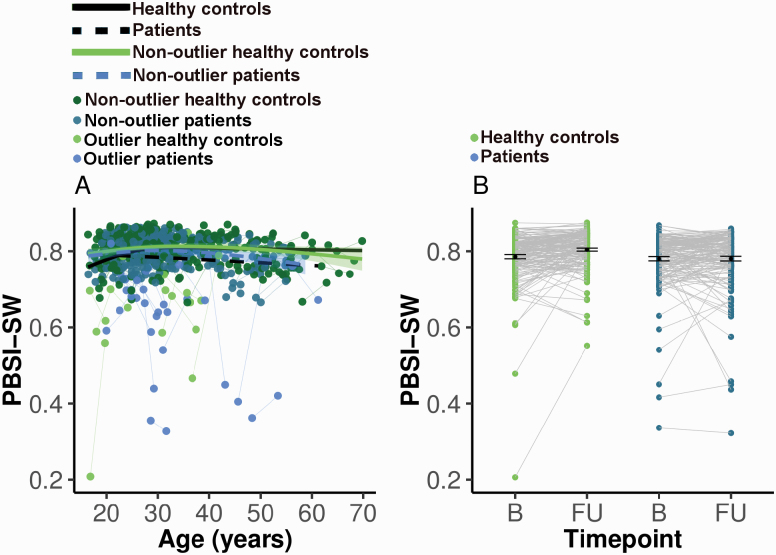
The PBSI-SW trajectories were significantly different between groups (age-by-diagnosis interaction: F = 14.57, P < .001; see table 2 and figure 2A). Patients displayed a close to linear reduction with age. Controls showed an increase of PBSI-SW until 30 years approximately, after which PBSI-SW remained stable. Cross-sectional comparisons of PBSI-SW between patients and controls at baseline and at follow-up demonstrated a significant difference with small to intermediate effect size at follow-up (mean PBSI-SW for controls = 0.80, for patients = 0.78, t = 3.23, degrees of freedom [df] = 278.16, P = .001, mean difference = 0.02, 95% CI = 0.01, 0.04, Cohen’s d = 0.36; see figure 2B).
Table 2.
Generalized additive model estimates for age, diagnosis, scanner, total brain volume, and age × diagnosis for person-based similarity index for sulcal width
(PBSI-SW) before and after outlier removal
| Intercept | Estimate | SE | T | P |
|---|---|---|---|---|
| Diagnosis | −1.302e-02 | 7.032e-03 | −1.851 | 0.0649 |
| Total brain volume | 6.446e-05 | 3.100e-05 | 2.079 | 0.0382 |
| Scanner | 7.521e-03 | 7.407e-03 | 1.015 | 0.3105 |
| Slope | Edf | Ref.df | F | P |
| s(age) | 2.204 | 3 | 11.864 | 0.07815 |
| s(age):patients | 1.042 | 3 | 14.570 | 0.00975 |
| Outliers removed | ||||
| Intercept | Estimate | SE | T | P |
| Diagnosis | −7.168e-03 | 3.941e-03 | −1.819 | 0.069694 |
| Total brain volume | 5.944e-05 | 1.781e-05 | 3.338 | 0.000919 |
| Scanner | 1.031e-03 | 4.172e-03 | 0.247 | 0.804988 |
| Slope | Edf | Ref.df | F | P |
| s(age) | 2.5759 | 3 | 24.974 | 0.000234 |
| s(age):patients | 0.5552 | 3 | 1.217 | 0.139211 |
Smooth function (edf), as well as degrees of freedom (Ref.df), and F-statistic and associated significance (***P < .001; **P < .01; *P < .05).
Fig. 2.
(A) Generalized additive mixed models (GAMMs) for person-based similarity index for sulcal width (PBSI-SW). Four fits over the spaghetti plot are shown: (1) healthy controls, (2) patients, (3) nonoutlier healthy controls, (4) nonoutlier patients. Outliers were participants with |PBSI-SW-Z| >2, ie, markedly deviating from the normative morphometric value at any time point. GAMMs included total brain volume and scanner as covariates. The age × diagnosis interaction was no longer significant after removal of the outliers. (B) PBSI-SW values for healthy controls and patients at baseline and follow-up. PBSI-SW was residualized for age, scanner, and total brain volume. B, baseline; FU, follow-up. See online version for color figure.
PBSI-SW Deviance (PBSI-SW-Z)
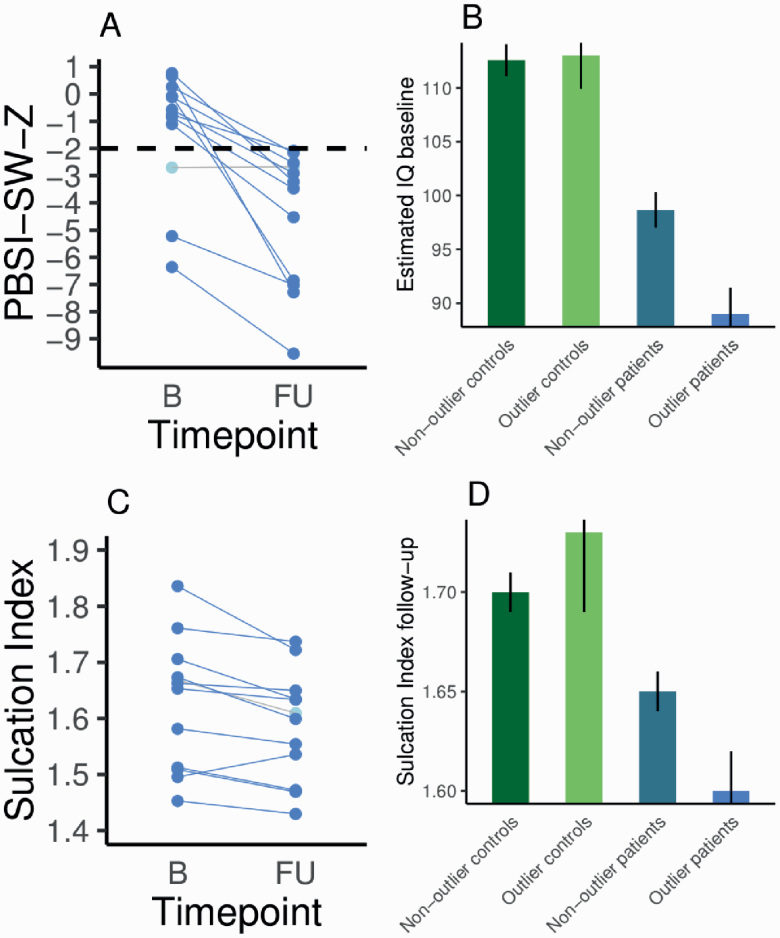
As expected, more patients than controls had significant deviance at any of the time points: 10 controls had PBSI-SW-Z <−2 and 19 patients (χ 2 = 3.06, df = 1, P = .04). Of these 19 patients, 16 patients showed a decrease in PBSI-SW-Z between baseline and follow-up (see figure 3A), which is, assuming equal proportions of increase and decrease, a disproportionally high number (χ 2 = 8.89, df = 1, P < .01). In nonoutlier patients, the effect was opposite; 57 out of 145 participants showed a decrease (χ 2 = 6.63, df = 1, P = .01). We recalculated lifespan PBSI-SW trajectories excluding outliers, and the age × diagnosis interaction was no longer significant (see table 2 and figure 2A)
Fig. 3.
(A) Person-based similarity index for sulcal width Z-values (PBSI-SW-Z) for 13 outlier patients (6 outlier patients did not have estimated intelligence quotient (IQ) at baseline available) with PBSI-SW-Z < −2 at any time point. All but 1 patient had a more negative PBSI-SW-Z value at follow-up as compared to baseline. (B) Average and SE for estimated IQ at baseline for controls and patients, nonoutlier patients, and outlier patients. (C) Sulcation index at baseline and follow-up for outlier patients. (D) Average sulcation index and SE at follow-up for controls and patients, nonoutlier patients, and outlier patients. See online version for color figure.
The PBSI-SW-Z <−2 outlier group of 19 patients did not differ from the other patients in terms of age, sex, scanner, baseline total PANSS scores, positive, negative, and general PANSS subscores, illness duration, or image quality metrics (see supplementary figure 6), but they did differ in estimated IQ at baseline (mean [SE] estimated IQ nonoutliers = 98.66 [1.64], outliers = 89.00 [2.14], t = −3.32, df = 21.06, P < 0.01, mean difference = −9.66, 95% CI = −16.46, −3.79, Cohen’s d = −0.62; see figure 3B). In order to deal with potential problems related to unbalanced and small sample sizes, we repeated the comparison of estimated IQ between the outlier patient group and the nonoutlier group using nonparametric permutation testing (1000 permutations, F = 4.145, P < .05).
To assess whether the change of PBSI-SW over time was related to image quality, Z-scores for the symmetrized annual percent change of PBSI-SW were calculated. Outliers did not have extreme values for image quality metrics (see supplementary material and supplementary figures 7–9).
Patients had a lower sulcation index compared to healthy control subjects at follow-up (mean [SE] sulcation index healthy control subjects = 1.68 (0.01), patients = 1.64 [0.01], t = −3.44, df = 321.79, P < .01, mean difference = −0.04, 95% CI = −0.06, −0.02, Cohen’s d = −0.38). The outlier patient group had a lower sulcation index compared to the nonoutlier patient group (mean [SE] sulcation index nonoutliers: 1.65 [0.01], outliers: 1.60 [0.02], t = −2.40, df = 13.25, P = .03, mean difference = −0.05, 95% CI = −0.13, −0.01, Cohen’s d = −0.76; see figures 3C and 3D below). For patients, estimated IQ at baseline and sulcation index at baseline and follow-up were not significantly associated with PBSI-SW (all P-values >.05; see supplementary figure 10). In addition, PBSI-SW (as a continuous measure) did not correlate with medication use and change in PANSS scores (see supplementary figure 11).
In controls, the PBSI-SW-Z <−2 outlier group did not differ from the nonoutlier healthy control group in estimated IQ at baseline (mean [SE] estimated IQ nonoutliers = 112.57 [1.49], outliers = 113.00 [3.08], t = 0.13, df = 10.63, P = .90, mean difference = 0.43, 95% CI = −7.12, 7.99, d = 0.03) and sulcation index at baseline (mean [SE] sulcation index nonoutliers = 1.68 [0.01], outliers = 1.71 [0.05], t = 0.72, df = 9.52, P = .49, mean difference = 0.03, 95% CI = −0.08, 0.15, d = 0.34) or follow-up (mean [SE] sulcation index nonoutliers = 1.70 [0.01], outliers: 1.73 [0.04], t = 0.59, df = 9.65, P = .57, mean difference = 0.03, 95% CI = −0.07, 0.13, d = 0.25).
To compare the results of PBSI-SW with another commonly used metric, cortical thickness (CT), PBSI-CT-Z was calculated (see supplementary material and supplementary figure 12). Outlier and nonoutlier patients, based on PBSI-CT-Z, did not differ in estimated IQ at baseline (mean [SE] estimated IQ nonoutliers = 97.65 [16.89], outliers: 97.3 [12.99], t = −0.08, df = 11.93, P = .94, mean difference = −0.35, 95% CI = −9.96, 9.26, Cohen’s d = −0.02).
Regional Contribution to PBSI
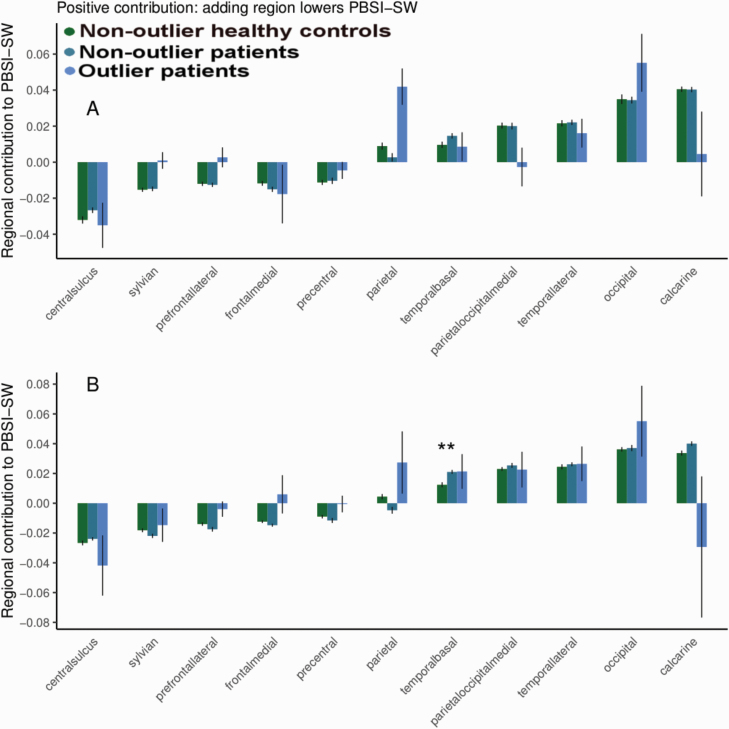
The outlier patient group did not differ from the other groups in contribution value for any of the regions (see figure 4, supplementary material, and supplementary figure 13).
Fig. 4.
Average and SE of each region’s contribution value to the person-based similarity index for sulcal width (PBSI-SW) for healthy controls, nonoutlier, and outlier patients (PBSI-SW-Z <−2 at any time point) at baseline (A) and at follow-up (B). Regions are ranked by contribution values for healthy controls at baseline. **P < .01 between controls and nonoutlier patients after false discovery rate correction (baseline and follow-up, controls vs nonoutlier patients, controls vs outlier patients, and nonoutlier patients vs outlier patients). See online version for color figure.
Discussion
This study is the first to use the PBSI, a recently developed metric that quantifies variation in brain structural profiles across the cortex at the level of the individual. We extended the PBSI such that it quantifies the similarity between the SW profile of an individual patient with schizophrenia to that of healthy control subjects. This approach allowed us to index for each patient the deviance of PBSI-SW with respect to a reference group. Our main finding is that significant deviance of PBSI-SW was present in a small group of patients only. These patients had more severe deficits in estimated IQ and a lower sulcation index at follow-up when compared to nondeviating patients and controls. On average, schizophrenia was associated with progressive reduction of PBSI-SW over the lifespan when compared to controls, but this diagnostic effect was primarily driven by the relatively small subgroup of participants who deviated markedly in PBSI-SW.
Schizophrenia is characterized by complex and heterogeneous neurobiological and genetic underpinnings. Brain structural deficits are spread out over the cortex and there is great variability in the pattern of regional deficits among patients.7,12 This makes global measures, such as the PBSI-SW, suitable metrics as they summarize deviations in multiple regions instead of focusing on a single region.16 Using the adapted PBSI approach, we were able to translate the heterogeneity of the pattern of SW deficits present in patients with schizophrenia into variation in a single number, PBSI-SW. However, in contrast to the traditional case-control approach, this variation could be used as a measure of individual deviance and, therefore, facilitate detection of biologically more homogeneous subgroups with schizophrenia.14
Marked deviance of PBSI-SW was sparse, limited to only a small subset of patients. This finding is in line with recent reports using normative modeling, showing that deviance (with respect to a control group) for cortical thickness was present only in small subsets of patients with schizophrenia, bipolar disorder, or autism spectrum disorders.33–35 Furthermore, in patients with bipolar disorder, greater deviance was associated with worse performance on tasks of processing speed and executive functioning but not with age. However, deviance was not used to classify patients into subgroups.33 The current study extends these findings by demonstrating that, while PBSI-SW did not correlate significantly with cognitive performance and global sulcation in the whole group, patients with markedly deviating PBSI-SW had lower cognitive performance and decreased global sulcation compared to nondeviating patients. The clinical importance of PBSI-SW deviance was further underlined by our finding that the effect of diagnosis on change of PBSI-SW over time was driven by the same small subset of deviating patients. This finding undermines the notion of the “average patient” who is the focus of the traditional case-control design and its accompanying first-order statistics, the group means. Normative modeling does not rely on (structural brain) abnormalities shared by all individuals with schizophrenia but rather concentrates on the individual differences. As such, normative modeling is consistent with the notion of multiple pathological pathways that can lead to heterogeneous symptom presentations, appraising the complex neurobiology of psychiatric disorders.17
Leveraging the heterogeneity among patients with schizophrenia, bipolar disorder, or schizo-affective disorder, patients with 1 of these 3 psychotic disorders have been regrouped into 3 biotypes using brain electrophysiological and neuropsychological measurements; these subgroups were validated by assessing the patients’ structural brain deficits.2 The biotype that clustered on low cognitive control performance also had the severest structural brain deficits compared to the other biotypes but diagnostic categories were spread out over the 3 biotypes. These findings together with ours demonstrate large interindividual differences in severity, type, and location of structural brain deficits in schizophrenia and strongly suggest that criteria from diagnostic manuals do not adhere to neurobiology. As such, proposed alternative approaches, such as the Research Domain Criteria, may be important for harmonizing clinical characterization and neurobiological underpinnings.1
Abnormally increased SW and decreased sulcation index have been associated with schizophrenia, bipolar disorder, and senescence.18,21,28,36 Schizophrenia has been associated with aberrant early life neurodevelopmental processes. Indeed, exposure to adverse environmental factors during fetal life may increase the risk of developing psychotic disorders.37 Sulcal morphology is strongly linked to early life neurodevelopmental processes responsible for changing the cortical surface from lissencephalic to its archetypical folded appearance.19,38 The sulcation index may be used to retrospectively assess potential impairments in these processes. However, the sulcation index also reduces during adolescence as a consequence of cortical thinning and white matter growth.20 Synaptic pruning, trophic glial, and vascular changes and/or cell shrinkage in combination with genetics may be underlying decreases in sulcation 19,39,40; some of these processes may be particularly pronounced in schizophrenia.41,42
In order to evaluate our results using PBSI-SW, we used cortical thickness to calculate PBSI-CT. PBSI-CT did not show progression over time in patients, nor did PBSI-CT outliers differ in estimated IQ from PBSI-CT nonoutliers. This may indicate increased sensitivity of SW, as compared to cortical thickness, to schizophrenia-associated atrophic changes over time and advocates the inclusion of sulcal morphological measurements in multivariate morphological metrics.
Although we did not find differences between outliers and nonoutliers on imaging quality metrics, we cannot rule out subtle effects (eg, more severely ill patients moving slightly more during scan acquisition and taking more medication) on the results. Future studies focusing on this question are warranted. IQ was estimated from the performances on 4 subtests of the Wechsler Adult Intelligence Scale III; this procedure may show some limitations in particular populations relative to using full-scale IQ scores.43 However, it has shown good validity as a measure of general cognitive ability, thus supporting the association between marked deviance and poorer cognitive performance.44 Although we assessed a large sample of patients with schizophrenia, the small proportion of patients with considerable deviance in PBSI-SW led to small sample sizes for the clinical and cognitive characterization. If applied to larger (multicenter) samples, our methodological approach could enable the identification of larger groups with marked deviance to further characterize this phenotype using additional clinical and cognitive measures. Moreover, although the sample was well characterized, with diagnostic, clinical, and cognitive assessments conducted by experienced professionals, we did not have information about development or premorbid adjustment. Adding such variables, and extending the range of age at onset with patients with adolescent-onset or even childhood-onset schizophrenia, could aid in characterizing the subgroup with marked deviance, especially considering its association with deficits in sulcation index as a measure of developmental impairments.
Supplementary Material
Acknowledgments
The authors thank Zimbo Boudewijns, Joyce van Baaren, and Diego Muñoz Beltrán for technical assistance. C.M.D.-C. has received honoraria from Sanofi-Aventis and Abbvie. C.A. has been a consultant to or has received honoraria or grants from Acadia, Angelini, Gedeon Richter, Janssen-Cilag, Lundbeck, Otsuka, Roche, Sage, Servier, Shire, Schering-Plough, Sumitomo Dainippon Pharma, Sunovion, and Takeda. W.C. has received unrestricted research grants from or served as an independent symposium speaker or consultant for Eli Lilly, Bristol-Myers Squibb, Lundbeck, Sanofi-Aventis, Janssen-Cilag, AstraZeneca, and Schering-Plough. The other authors report no financial relationships with commercial interests.
Funding
This work was supported by the Spanish Ministry of Science, Innovation and Universities, Instituto de Salud Carlos III (PI16/02012, PI17/01249, PI17/00997, and PI19/01024), cofinanced by European Regional Development Fund funds from the European Commission, “A way of making Europe,” and Centro de Investigación Biomédica en Red de Salud Mental, Regional Government (B2017/BMD-3740 AGES-CM-2 and JR19/00024; C.M.D.-C.).
References
- 1. Insel T, Cuthbert B, Garvey M, et al. . Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. [DOI] [PubMed] [Google Scholar]
- 2. Clementz BA, Sweeney JA, Hamm JP, et al. . Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173(4):373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ivleva EI, Clementz BA, Dutcher AM, et al. . Brain structure biomarkers in the psychosis biotypes: findings from the bipolar-schizophrenia network for intermediate phenotypes. Biol Psychiatry. 2017;82(1):26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schnack HG. Improving individual predictions: machine learning approaches for detecting and attacking heterogeneity in schizophrenia (and other psychiatric diseases). Schizophr Res. 214: 34– 42. doi: 10.1016/j.schres.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 5. Reininghaus U, Böhnke JR, Chavez-Baldini U, et al. . Transdiagnostic dimensions of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). World Psychiatry. 2019;18(1):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martinuzzi E, Barbosa S, Daoudlarian D, et al. . Stratification and prediction of remission in first-episode psychosis patients: the OPTiMiSE cohort study. Transl Psychiatry. 2019;9(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Erp TGM, Walton E, Hibar DP, et al. ; Karolinska Schizophrenia Project . Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biol Psychiatry. 2018;84(9):644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kelly S, Jahanshad N, Zalesky A, et al. . Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 2018;23(5):1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arango C, Kahn R. Progressive brain changes in schizophrenia. Schizophr Bull. 2008;34(2):310–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weinberg D, Lenroot R, Jacomb I, et al. . Cognitive subtypes of schizophrenia characterized by differential brain volumetric reductions and cognitive decline. JAMA Psychiatry. 2016;73(12):1251–1259. [DOI] [PubMed] [Google Scholar]
- 11. Brugger SP, Howes OD. Heterogeneity and homogeneity of regional brain structure in schizophrenia: a meta-analysis. JAMA Psychiatry. 2017;74(11):1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alnæs D, Kaufmann T, van der Meer D, et al. ; Karolinska Schizophrenia Project Consortium . Brain heterogeneity in schizophrenia and its association with polygenic risk. JAMA Psychiatry. 2019;76(7):739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gopal S, Miller RL, Michael A, et al. . Spatial variance in resting fMRI networks of schizophrenia patients: an independent vector analysis. Schizophr Bull. 2016;42(1): 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marquand AF, Rezek I, Buitelaar J, Beckmann CF. Understanding heterogeneity in clinical cohorts using normative models: beyond case-control studies. Biol Psychiatry. 2016;80(7):552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morgan SE, Seidlitz J, Whitaker KJ, et al. . Cortical patterning of abnormal morphometric similarity in psychosis is associated with brain expression of schizophrenia-related genes. Proc Natl Acad Sci USA. 2019;116(19):9604–9609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doucet GE, Moser DA, Rodrigue A, Bassett DS, Glahn DC, Frangou S. Person-Based brain morphometric similarity is heritable and correlates with biological features. Cereb Cortex. 2019;29(2):852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marquand AF, Kia SM, Zabihi M, Wolfers T, Buitelaar JK, Beckmann CF. Conceptualizing mental disorders as deviations from normative functioning. Mol Psychiatry. 2019;24(10):1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Janssen J, Alemán-Gómez Y, Schnack H, et al. . Cortical morphology of adolescents with bipolar disorder and with schizophrenia. Schizophr Res. 2014;158(1–3):91–99. [DOI] [PubMed] [Google Scholar]
- 19. Pizzagalli F, Auzias G, Yang Q, et al. ‘ The reliability and heritability of cortical folds and their genetic correlations across hemispheres’, bioRxiv, doi: 10.1101/795591, January 1, 2019, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed]
- 20. Alemán-Gómez Y, Janssen J, Schnack H, et al. . The human cerebral cortex flattens during adolescence. J Neurosci. 2013;33(38):15004–15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu T, Sachdev PS, Lipnicki DM, et al. . Limited relationships between two-year changes in sulcal morphology and other common neuroimaging indices in the elderly. Neuroimage. 2013;83:12–17. [DOI] [PubMed] [Google Scholar]
- 22. Im K, Lee J-M, Seo SW, Hyung Kim S, Kim SI, Na DL. Sulcal morphology changes and their relationship with cortical thickness and gyral white matter volume in mild cognitive impairment and Alzheimer’s disease. NeuroImage. 2008;43(1):103–113. [DOI] [PubMed] [Google Scholar]
- 23. Fish AM, Cachia A, Fischer C, et al. . Influences of brain size, sex, and sex chromosome complement on the architecture of human cortical folding. Cereb Cortex. 2017;27(12):5557–5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kochunov P, Thompson PM, Coyle TR, et al. . Relationship among neuroimaging indices of cerebral health during normal aging. Hum Brain Mapp. 2008;29(1):36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kochunov P, Mangin JF, Coyle T, et al. . Age-related morphology trends of cortical sulci. Hum Brain Mapp. 2005;26(3):210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lemaitre H, Goldman AL, Sambataro F, et al. . Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol Aging. 2012;33(3):617.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wagstyl K, Ronan L, Whitaker KJ, et al. . Multiple markers of cortical morphology reveal evidence of supragranular thinning in schizophrenia. Transl Psychiatry. 2016;6:e780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cachia A, Amad A, Brunelin J, et al. . Deviations in cortex sulcation associated with visual hallucinations in schizophrenia. Mol Psychiatry. 2015;20(9):1101–1107. [DOI] [PubMed] [Google Scholar]
- 29. Hulshoff Pol HE, Schnack HG, Mandl RC, et al. . Focal gray matter density changes in schizophrenia. Arch Gen Psychiatry. 2001;58(12):1118–1125. [DOI] [PubMed] [Google Scholar]
- 30. Korver N, Quee PJ, Boos HB, Simons CJ, de Haan L; GROUP investigators . Genetic Risk and Outcome of Psychosis (GROUP), a multi-site longitudinal cohort study focused on gene-environment interaction: objectives, sample characteristics, recruitment and assessment methods. Int J Methods Psychiatr Res. 2012;21(3):205–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kubota M, van Haren NE, Haijma SV, et al. . Association of IQ changes and progressive brain changes in patients with schizophrenia. JAMA Psychiatry. 2015;72(8): 803–812. [DOI] [PubMed] [Google Scholar]
- 32. Wood SN Generalized Additive Models: An Introduction with R. Boca Raton, Florida, USA: Chapman Hall/CRC; 2006. [Google Scholar]
- 33. Wolfers T, Doan NT, Kaufmann T, et al. . Mapping the heterogeneous phenotype of schizophrenia and bipolar disorder using normative models. JAMA Psychiatry. 2018;75(11):1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bethlehem RAI, Seidlitz J, Romero-Garcia R, Trakoshis S, Dumas G, Lombardo MV. A normative modelling approach reveals age-atypical cortical thickness in a subgroup of males with autism spectrum disorder. Commun Biol. 2020; 3(1): 486. doi: 10.1038/s42003-020-01212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zabihi M, Oldehinkel M, Wolfers T, et al. . Dissecting the heterogeneous cortical anatomy of autism spectrum disorder using normative models. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(6):567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kochunov P, Mangin JF, Coyle T, et al. . Age-related morphology trends of cortical sulci. Hum Brain Mapp. 2005;26(3):210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rapoport J, Giedd J, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17(12):1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Llinares-Benadero C, Borrell V. Deconstructing cortical folding: genetic, cellular and mechanical determinants. Nat Rev Neurosci. 2019;20(3):161–176. [DOI] [PubMed] [Google Scholar]
- 39. Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167–178. [DOI] [PubMed] [Google Scholar]
- 40. Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997;88(3):287–298. [DOI] [PubMed] [Google Scholar]
- 41. Boksa P. Abnormal synaptic pruning in schizophrenia: urban myth or reality? J Psychiatry Neurosci. 2012;37(2):75–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10(1):40–68. [DOI] [PubMed] [Google Scholar]
- 43. Merchán-Naranjo J, Mayoral M, Rapado-Castro M, et al. . Estimation of the intelligence quotient using Wechsler Intelligence Scales in children and adolescents with Asperger syndrome. J Autism Dev Disord. 2012;42(1):116–122. [DOI] [PubMed] [Google Scholar]
- 44. Blyler CR, Gold JM, Iannone VN, Buchanan RW. Short form of the WAIS-III for use with patients with schizophrenia. Schizophr Res. 2000;46(2–3):209–215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.