Abstract
Cost-effectiveness studies of early intervention services (EIS) for psychosis have not included extension beyond the first 2 years. We sought to evaluate the cost-effectiveness of a 3-year extension of EIS compared to regular care (RC) from the public health care payer’s perspective. Following 2 years of EIS in a university setting in Montreal, Canada, patients were randomized to a 3-year extension of EIS (n = 110) or RC (n = 110). Months of total symptom remission served as the main outcome measure. Resource use and cost data for publicly covered health care services were derived mostly from administrative systems. The incremental cost-effectiveness ratio (ICER) and cost-effectiveness acceptability curve were produced. Relative cost-effectiveness was estimated for those with duration of untreated psychosis (DUP) of 12 weeks or less vs longer. Extended early intervention had higher costs for psychiatrist and nonphysician interventions, but total costs were not significantly different. The ICER was $1627 per month in total remission. For the intervention to have an 80% chance of being cost-effective, the decision-maker needs to be willing to pay $5942 per month of total symptom remission. DUP ≤ 12 weeks was associated with a reduction in costs of $12 276 even if no value is placed on additional months in total remission. Extending EIS for psychosis for people, such as those included in this study, may be cost-effective if the decision-maker is willing to pay a high price for additional months of total symptom remission, though one commensurate with currently funded interventions. Cost-effectiveness was much greater for people with DUP ≤12 weeks.
Keywords: first-episode psychosis, cost-effectiveness analysis, duration of untreated psychosis, randomized controlled trial
Introduction
Early intervention services (EIS) for treating first-episode psychosis (FEP) comprise a package of evidence-based interventions, adapted to the needs of FEP patients and delivered either through a case management1,2 or a coordinated care model.3,4 Recent meta-analyses have provided strong evidence for the superior effectiveness of EIS compared to routine care on clinical and functional outcomes at 1 and 2 years.5 Cost-effectiveness of EIS delivered over the first 2 years has also been reported.6–9 Longer duration of untreated psychosis (DUP), a known predictor of clinical and functional outcome, may undermine the benefits of EIS.10 One previous study has reported that a DUP of less than 74 weeks was associated with much greater cost-effectiveness.9
In response to reports that benefits of EIS may not be sustained once patients are transferred to regular care (RC),10 several randomized controlled trials (RCTs) have investigated the benefits of extending EIS beyond the first 2 years.11–13 There have been no reports of the cost-effectiveness of such an extension of EIS. In one of these RCTs, we reported the clinical effectiveness of a 3-year extension of an EIS, following 2 years of EIS, in comparison to 3 years of RC following 2 initial years of EIS.11 Here, we report results on the cost-effectiveness of this RCT extension of EIS and the association between lower DUP and costs, as well as cost-effectiveness. As the trial was of 3-year duration, we also investigate the differences in the evolution of costs between the experimental and control conditions.
Methods
Setting, Design, and Participants
The original study design and efficacy outcomes have been reported previously.11,14 Briefly, patients with an FEP who had received EIS for 2 years within the McGill University network of EIS in Montreal, Canada, were randomized to either continue to receive EIS (extended early intervention [EEI]) or transferred to RC for an additional 3 years. Data span the period from July 2008 to May 2016.
The EEI service included modified assertive case management, lowest effective dose of a second-generation antipsychotic medication, family intervention booster sessions, cognitive-behavioral therapy when indicated, and crisis intervention. Patients allocated to RC were transferred from the EIS either to primary care or to standard psychiatric care, depending on their course over the first 2 years. Primary care in Québec includes local community health and social service centers (CLSCs) and family physicians with variable support from psychiatric services. Standard psychiatric care is provided through hospital-based outpatient services by psychiatrists often with nursing or other professional support. All types of primary and specialist care are available free of charge to all Quebec residents.
All patients (18–35 y old) with a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition diagnosis of an FEP (nonaffective or affective) who had received 2 years of EIS, irrespective of their clinical remission and comorbid substance abuse status, were invited to participate.11,14 For random allocation to the intervention or control arm of the trial, a computerized urn randomization protocol15 was used, adjusting for known prognostic characteristics (gender and substance abuse).
Economic Evaluation
The perspective adopted for this economic evaluation was that of Québec’s Ministry of Health and Social Services (MSSS). Costs and effects were discounted at 1.5%, consistent with the recommendation of the Canadian Agency for Drugs and Technologies in Health.16
Measures
Cumulative time in symptom remission (positive, negative, and total, ie, remission from both), selected a priori as the primary outcome measure for the trial,11 was measured using the Scale for Assessment of Positive Symptoms17 and the Scale for Assessment of Negative Symptoms18 at randomization and every 3 months over the following 3 years.14 For the cost-effectiveness analysis, we used total symptoms as our primary measure of effectiveness. Because of the different ways that positive and negative symptoms contribute to patient well-being and because they tend to respond differently to different treatments (ie, especially medications for the first and psychosocial interventions for the second), we also report results using them as secondary measures of effectiveness.
Resource use and cost data were obtained from the MSSS public administrative databases on all consented patients, following approval by the government’s data access commission. These data, linked via an individual’s health insurance number, describe hospitalizations and physician services for all patients. They also describe filled prescriptions for patients with public coverage (90%). These data were extracted from 12 months before randomization to 36 months after. Data concerning visits with nonphysician health care professionals (eg, social workers and case managers) were collected from case records of the EIS for the EEI arm, as well as hospital clinics and CLSCs where study participants had received care. Nonphysician service data were not found for 35 patients.
Administrative data included fees for physician services and the costs of prescription pharmaceuticals. Costs associated with hospitalizations in Québec are normally estimated using the resource intensity weights derived from the All Patient Refined Diagnosis-Related Groups (APR-DRG) classification system as adjusted by the provincial government.19 However, these data were often (87% of the time) not provided, especially in the case of psychiatric hospitalizations. For these, hospitalization costs were estimated using a per diem cost based on previous work in Montreal.20 Unit costs for nonphysician interventions were also taken from the same study.20 Costs were adjusted for inflation to 2018 dollars from the year they were reported using the consumer price index.21
Data Analysis
Data were analyzed by year from the date of randomization to account for consecutive enrollment. Costs from government databases were categorized as follows: physician services, medications, and hospitalizations, separated as psychiatric or somatic, based on International Statistical Classification of Diseases and Related Health Disorders (ICD-10) diagnostic codes, physician billing codes, and drug identification numbers. This process was verified by the study lead investigator (A.M.). Nonphysician visits were all classified as having a mental-health-related purpose. Data were analyzed on an intention-to-treat basis. Patients who died or were incarcerated during the extension trial were included in the analysis up until the point of their death or incarceration.
Linear regression using ordinary least squares was applied to estimate between-group differences for total, positive, and negative symptoms remission (months) and nonphysician services. Differences between groups in accrued costs and resource use were analyzed longitudinally using generalized estimating equations22 and cross-sectionally using generalized linear models (GLM)23 adjusting for baseline costs.24 Given the right-skewed nature of cost data, GLMs using different variance and link functions were compared using the Akaike information criterion23; the gamma distribution and identity link function best fit the data.23 When frequencies were low and resource use could be modeled as counts, with variance greater than the mean (eg, visits), a negative binomial model was estimated.25
The incremental cost-effectiveness ratio (ICER) was calculated as the difference in mean costs between the 2 groups (intervention minus control) divided by the difference in the mean number of months in remission. In order to describe uncertainty in the ICER, 5000 bootstrap replicates were computed for each outcome and plotted on a cost-effectiveness plane.26 These estimates were then used to generate cost-effectiveness acceptability curves,26 which show the probability that the intervention is cost-effective according to the decision-maker’s willingness to pay for an additional month in (total, positive, or negative) symptom remission. We carried out a 1-way sensitivity analysis of each ICER on the discount rate, using 0% and 3%, and also evaluated the impact of using per diem costing instead of the APR-DRG method on the 36 patients for whose hospitalizations resource intensity weights were available.
Given prior evidence of greater effectiveness of EEI among patients with shorter DUP,27 we used net-benefit regression to assess to what extent cost-effectiveness differs according to whether DUP is ≤12 or >12 weeks.28 This cutoff, based on a joint recommendation from the World Health Organization and International Early Psychosis Association,29 and supported by data from our RCT study,27 is the median DUP for our sample. We computed each individual’s net monetary benefit during the 3-year follow-up for different values of the decision-maker’s willingness to pay ($0–$2500) for an additional month of remission. If λ denotes this willingness to pay, ri the individual’s number of months in remission, and ci the total cost they have incurred, then each individual’s net monetary benefit is: λ ∙ ri – ci. Net monetary benefit, computed using several representative values of λ, was regressed on group assignment, DUP >12 weeks, and several covariates. Finally, the interaction between treatment and DUP was added to the model and interpreted as the degree to which cost-effectiveness depends on whether DUP is short or long. All analyses were performed using STATA 15.0 (StataCorp). The study was approved by the institutional ethics board of McGill University and all patients signed informed consent. The trial was registered (ISRCTN11889976).
Results
Participants
Of a total of 220 consenting participants, 110 were randomized to continue to receive EEI and the remaining 110 transferred to RC. Details of recruitment are provided in earlier publications11,14 and reproduced in supplementary figure 1. There were no meaningful demographic or clinical differences between the groups at the time of randomization (table 1).
Table 1.
Baseline demographic and clinical characteristics
| Total (n = 220) | EEI (n = 110) | Regular care (n = 110) | |
|---|---|---|---|
| Age at onset of first-episode psychosis, y, mean (SD) | 22.4 (4.4) | 21.9 (4.1) | 22.9 ± 4.7 |
| Male, n (%) | 151 (68.6%) | 75 (68.2%) | 76 (69.1%) |
| Single, n (%) | 200 (90.9%) | 103 (93.6%) | 97 (88.2%) |
| High school education or less, n (%) | 103 (46.8%) | 53 (48.2%) | 50 (45.4%) |
| Duration of untreated psychosis, wk, mean (SD) | 49.3 (123.6; median: 11.6) | 52.4 (148.8; median: 8.3) | 46.3 (92.7; median: 12.7) |
| Low (≤12 wk) duration of untreated psychosis, n (%)a | 73 (47.1%) | 38 (22.5%) | 35 (24.5%) |
| Primary diagnosis of schizophrenia spectrum, n (%) | 143 (65.0%) | 74 (67.3%) | 69 (62.7%) |
| Secondary diagnosis of substance abuse/ dependence, n (%) | 105 (47.7%) | 52 (47.3%) | 53 (48.2%) |
| Antipsychotic dose in chlorpromazine equivalents, mg, mean (SD) | 314.6 (332.6) | 299.9 (350.1) | 329.7 (342.9) |
| SAPS total score, mean (SD) | 6.5 (9.7; n = 216) | 7.1 (10.4; n = 107) | 6.0 (8.9; n = 109) |
| SANS total score, mean (SD) | 13.8 (11.6; n = 204) | 13.6 (10.4; n = 103) | 14.0 (12.8; n = 101) |
| Positive symptom remission, n (%) | 161 (73.2%) | 81 (73.6%) | 80 (72.7%) |
| Negative symptom remission, n (%) | 107 (48.6%) | 53 (48.2%) | 54 (49.1%) |
| Total symptom remission, n (%) | 92 (41.8%) | 45 (40.9%) | 47 (42.7%) |
Note: EEI, extended early intervention; SAPS, Scale for Assessment of Positive Symptoms; SANS, Scale for Assessment of Negative Symptoms.
aPercentages calculated out of 155 for which duration of untreated psychosis status was available.
Clinical (Primary) Outcome
As previously reported, consistent with our hypothesis,11 during the 36-month trial, patients who received EEI services spent more time in positive, negative, and total symptoms remission than the RC group: 59.3%, 47.1%, and 42.6% compared to 40.7%, 38.2%, and 36.4%, respectively.
Resource Use
Cumulative resource use was similar between the 2 groups during the 3-year extension trial (supplementary table 1), except that patients in the EEI group received 2.3 times more (in absolute terms, 30 more) nonphysician interventions than the RC group. Additionally, the EEI group had 25.2% more visits with psychiatrists (15.5 more) and filled 7.6% more days’ worth of psychiatric medication prescriptions (114.8 d more) than the RC group. There were no other meaningful differences with regards to the use of other measured resources, including general practitioner visits, hospital admissions, or days of hospitalization between the 2 groups.
Costs
Approximately 97% of the predicted total cost was attributable to mental-health-related care in both groups. The predicted mean cumulative cost over 3 years of a participant was comparable for the 2 groups (table 2). While the cost of mental health care for the EEI group, reflecting the higher number of nonphysician and psychiatrist interventions, was somewhat larger than that for the RC group, the latter had marginally higher costs related to hospitalizations. The predicted mean cumulative cost of participants’ somatic health care use was similar between the groups.
Table 2.
Cumulative predicted costs during the 3-year trial, by type of cost and by experimental group, in 2018 Canadian dollars
| Control group | Intervention group | Adjusted differencea | |
|---|---|---|---|
| Costs | Mean (95% CI) | Mean (95% CI) | Coefficient (95% CI) |
| Mental health costs | |||
| Hospitalizations | 13 348 (7516 to 19 180) | 12 342 (6628 to 18 056) | −1006 (−9172 to 7160) |
| Medications | 5432 (4373 to 6491) | 6712 (5674 to 7750) | 1280 (−203 to 2763) |
| Physician services | 4468 (3227 to 5709) | 4294 (3077 to 5510) | −174 (−1912 to 1564) |
| Total mental health costs | 27 222 (19 285 to 35 158) | 31 233 (23 457 to 39 009) | 4011 (−7101 to 15 123) |
| Somatic health costs | |||
| Hospitalizations | 128 (20 to 236) | 71 (−35 to 177) | −58 (−209 to 94) |
| Medications | 302 (163 to 441) | 199 (63 to 335) | −103 (−298 to 92) |
| Physician services | 528 (145 to 911) | 847 (472 to 1222) | 319 (−217 to 856) |
| Total somatic health costs | 979 (522 to 1437) | 1097 (649 to 1545) | 118 (−523 to 758) |
| Nonphysician visits | 3777 (2248 to 5307) | 8073 (6575 to 9572) | 4296 (2155 to 6437) |
| Total costs | 28 199 (20 089 to 36 309) | 32 332 (24 386 to 40 278) | 4133 (−7222 to 15 488) |
aEstimated difference adjusting for differences at baseline.
While at the end of year 1, the EEI group cost an average of $2725 more than the RC group, the costs began to converge over time to a difference of $−322 at the end of year 3 in favor of EEI (supplementary table 2). This evolution is primarily the result of both nonphysician visits and psychiatric hospitalizations tending to go down more in the EEI than in the RC group. Of less consequence to the overall cost, psychiatric medication expenditures in the EEI group increased on average to the end of year 3 by $798 per participant compared to a decrease of $48 for the RC group.
Cost-Effectiveness
Table 3 shows the difference in average costs and in average months in remission, as well as the ICERs. The intervention cost the MSSS $1627 per additional month in total symptom remission. The ICERs are lower for positive ($614) and negative ($1247) symptoms.
Table 3.
Incremental cost-effectiveness ratios (ICERs), differences in costs, and differences in months in remission, by type of symptom, with bootstrapped CIs
| Value | 95% CI | ||
|---|---|---|---|
| Additional month in total remission | |||
| ICER, $/month | $1627 | Undefined | $40 682 |
| Cost difference, $ | $4112 | $7790 | $15 078 |
| Difference in number of months | 2.5 | −0.9 | 5.8 |
| Additional month in positive remission | |||
| ICER, $/month | $614 | Cost-saving | $2751 |
| Cost difference, $ | $4112 | $7059 | $15 122 |
| Difference in number of months | 6.70 | 3.60 | 9.63 |
| Additional month in negative remission | |||
| ICER, $/month | $1247 | Undefined | $16 776 |
| Cost difference, $ | $4112 | $7349 | $14 988 |
| Difference in number of months | 3.3 | −0.2 | 6.6 |
Reducing the discount rate to 0 had a larger impact than increasing it by 3%. Doing so decreased the ICER for total symptoms by 9.4% (supplementary table 3). Our sensitivity analysis for method of hospitalization costing revealed no significant effect.
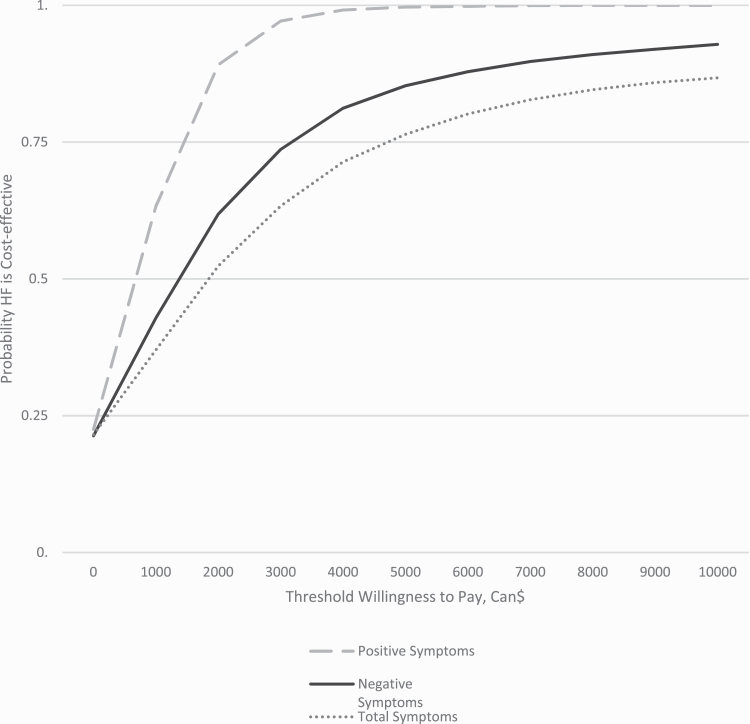
The cost-effectiveness acceptability curves (figure 1) show that if the decision-maker is willing to pay about $5942 per additional month free of any symptoms, the probability that the intervention is cost-effective reaches 0.8. The cost-effectiveness planes with 5000 bootstrapped replicates of the ICER are shown in supplementary figure 2.
Fig. 1.
Probability that extended early intervention services compared to regular care is cost-effective as a function of the decision-maker’s willingness to pay for an additional month of total, positive, and negative symptom remission.
DUP and Cost-Effectiveness of EEI
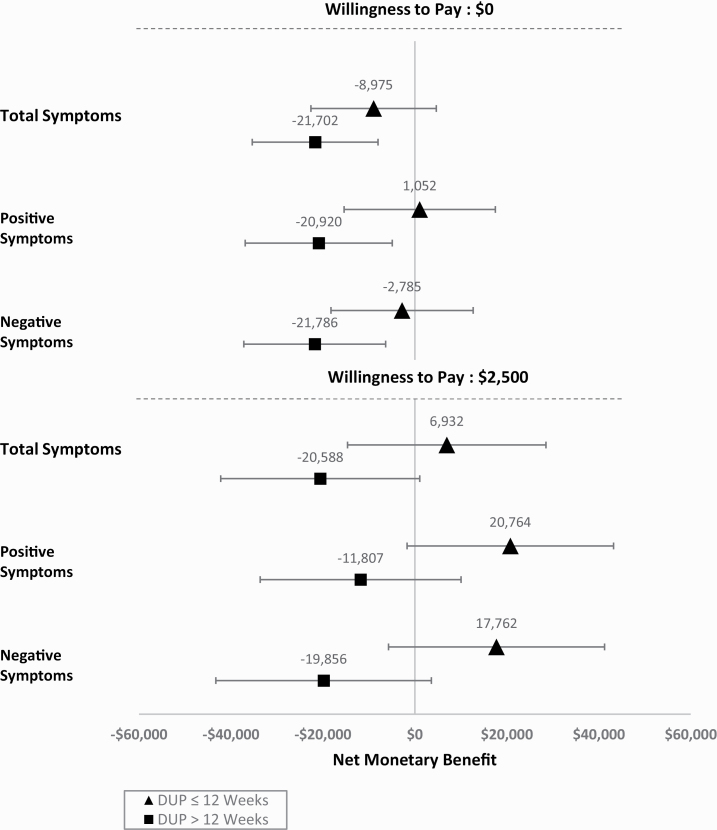
Averaging between the intervention and control groups, if a decision-maker is unwilling to pay anything for total symptom remission (represented by λ = 0), the adjusted net benefit of a patient with a short DUP (≤12 wk) is initially positive (net cost $8541). The contribution of a shorter DUP to net benefit continues to rise with the increase in decision-maker’s willingness to pay, reflecting the fact that shorter DUP is associated with greater months in remission (supplementary table 4). Figure 2 shows that even at a high willingness to pay of $2500 per month of symptom remission, the net monetary benefit is positive for individuals with DUP of 12 weeks or less, but negative for others.
Fig. 2.
Association between average duration of untreated psychosis and effect of extended early intervention services on the net monetary benefit for different values of willingness to pay (0$ and $2500) for a month of remission by type of symptom.
Supplementary table 5 shows that if a decision-maker is not willing to pay anything for an additional month in total symptom remission, all else equal having a shorter DUP is associated with a higher net benefit of the intervention (ie, reduces its net cost) by $12 276. Because the intervention is associated with more months in remission for people with shorter DUP, as the decision-maker’s willingness to pay for an additional month in remission increases, a shorter DUP is associated with a progressively greater net benefit from the intervention. At a willingness to pay of $2500 per month in remission, a shorter DUP is associated with a 2.2 times greater net benefit for total symptoms than when the decision-maker is not willing to pay anything.
Discussion
We had previously reported that an extension of EIS from 2 to 5 years vs transferring patients to RC after 2 years of EIS resulted in more months in remission from positive, negative, and total symptoms.11 In this cost-effectiveness analysis, the cost of an additional month of total symptom remission (the ICER) was $1627, while the respective amounts for positive and negative symptom remission were smaller, $614 and $1247. The lower cost for positive symptom remission reflects the greater effectiveness of the intervention at reducing positive symptoms, which mainly respond to medications.
The greater intensity of interventions required in EEI was associated with a nonsignificant increase in costs, especially those related to nonphysician, as well as psychiatric visits (mostly related to psychosocial interventions and case management) and higher cost of medications. In contrast, hospitalizations were more costly (in both absolute and relative terms) in the RC group.
The data show a temporal pattern suggesting that cost-effectiveness was increasing over time: in particular, hospitalization costs for the EEI group decreased after the first year compared to those for the RC group. This may be because a proportion of patients at the time of randomization showed continued instability (were not in complete remission) and received fairly intensive interventions in the EEI group for the first 1–2 years of the trial. Such intensity likely decreased over time as patients maintained longer periods of remission. RC would not have been equipped to provide such an intensive intervention. The increase in psychiatric medication costs for the EEI compared to the RC group, while not a very large part of overall costs, may be related to the longer period of actual exposure to treatment in EEI compared to RC (mean 133.2 vs 101.7 wk, respectively).11 A higher intensity of psychiatric and psychosocial interventions, as alluded to above, often facilitates patients’ continued use of medications to prevent relapse.
The cost-effectiveness acceptability curve indicates that to be 80% sure that the intervention is cost-effective in terms of total symptom remission, the decision-maker needs to be willing to pay nearly $6000 per month for total remission.
We did not initially plan to assess effectiveness using quality-adjusted life years (QALYs) due to their acknowledged limitations, especially in mental health.30 QALYs are, however, useful for comparing the cost-effectiveness of different interventions. Our data do not allow a precise conversion of the ICER into a cost per QALY. A previous report using the Positive and Negative Symptom Scale, however, suggests that a transition from moderate to mild symptoms is associated with an increase in utility of about 0.13 and from severe to mild symptoms with an increase ranging from 0.21 to 0.32.31 If we then assume conservatively that a transition from moderate or severe symptoms to total remission (meaning absence of any symptoms) is associated with an increase in utility of 0.18, then the ICER translates into a cost per QALY of $108 467. This situates the intervention in a somewhat gray zone: conventionally, interventions costing between $50 000 and $100 000 are considered cost-effective, but the cost-effectiveness of medications currently in use, as well as the estimated value of a statistical life, suggest that a more realistic threshold must lie well beyond that range.32 Thus, our results suggest that extending early intervention from 2 to 5 years, for a population similar to the one that entered our study, yields benefits at an acceptable cost.
As could have been expected, EIS emerged as more cost-effective in terms of remission from positive than negative symptoms—the intervention was more effective at increasing months of remission from the former. Evidence suggests that the rating of a state depends on its overall severity, with the predominance of negative vs positive symptoms making little difference.31 The intervention may, thus, have been more likely to meet a given cost-effectiveness threshold, had QALYs been used, for individuals for whom positive symptoms were dominant. Should further investigation confirm this, it would highlight the need for cost-effective ways of addressing negative symptoms.
This study is the first to investigate the cost-effectiveness of EIS beyond the first 2 years within an RCT design in which 1 group received additional EIS for 3 years, while the control group received RC after the first 2 years of EIS. In the cost-effectiveness study of the related OPUS trial,33 the authors reported outcomes for 5 years from the start of EIS for FEP patients, who had originally been randomized to EIS or RC for the first 2 years. For the subsequent 3 years, all patients received RC. Unlike in our study, point estimates of costs showed lower costs (but not to a statistically significant extent) in the EIS group, and no meaningful difference in the outcome measure (Global Assessment of Functioning Scale). Overall, the results showed a high probability of cost-effectiveness for EIS. This was partly due to lower use and costs of supported housing in the EIS group—a type of cost not included in our analysis.
A key finding of our study is that the cost-effectiveness of EEI varies according to DUP. Our results suggest that, for patients with DUP of 12 weeks or less, the treatment in EEI is associated with lower costs, as well as increased benefits, compared with those for patients with DUP longer than 12 weeks. This benefit increases significantly with the decision-maker’s willingness to pay. The intervention does not, in fact, appear cost-effective for people with DUP greater than 12 weeks for any reasonable value a decision-maker might attribute to an additional month of symptom remission. The RAISE study similarly found that a shorter DUP (<74 wk) was associated with greater cost-effectiveness.9 More research is needed to determine whether reducing DUP would increase the cost-effectiveness of EEI. Evidence of a significant positive long-term (10 y) impact of experimentally reducing DUP in a cohort of FEP patients8 suggests that this may turn out to be the case.
Another possible avenue for increasing the cost-effectiveness of EEI might be to provide the extended EIS selectively to patients with unstable clinical outcomes (not remitted or not able to remain in remission) over the first 2 years. Among the patients randomized to RC, those whose clinical outcomes were better during the first 2 years were transferred to primary care (family physicians and community primary care clinics), whereas those whose outcomes had been worse were transferred to secondary care (hospital-based psychiatric clinics). Previously published11 clinical outcome results reveal that the first group had much better subsequent outcomes. Evidently, they were doing well enough 2 years after the start of EIS that they needed less specialized care after that. Thus, if the trial had targeted only the subgroup with worse clinical outcomes during the first 2 years of EIS, the cost-effectiveness of EEI may have been greater.
Our study has significant strengths. It was carried out within a single integrated early intervention program of 3 individual clinics that followed identical treatment protocols. Furthermore, the majority (n = 178, 81%) of patients were recruited from the lead clinical program (PEPP-Montréal),11 which further reduced the variability of interventions provided within the EEI. Finally, reliance on administrative data means that we had access to accurate resource use data on all patients (90% for medications).
Some limitations of the analysis may also be noted. The perspective of the health care payer does not consider the impacts of the intervention on productivity, justice-related costs, or private costs. Results from a societal perspective could have been different. Furthermore, as is inevitable in a study of this nature, the results are contingent on how EIS was implemented, the nature of RC with which it was compared, and the characteristics of study participants.
In conclusion, our findings indicate that, if all patients are provided 3 years of additional EIS beyond the first 2 years, for this extended EIS to have a high probability of being cost-effective, the decision-maker has to be willing to pay a high price per month for symptom remission. Cost-effectiveness appears, however, within the range of cost-effectiveness for other currently funded medications or interventions. The intervention emerged as much more cost-effective for people with DUP less than 12 weeks, suggesting that increased effort to reduce delay in initial treatment of FEP might further increase the cost-effectiveness of extended EIS. Finally extended EIS may be more cost-effective if offered to only patients with unstable outcome at 2 years, while those with good outcome may be treated equally well in less expensive primary care.
Supplementary Material
Acknowledgments
We would like to thank Nicole Pawliuk, Marie-Christine Rondeau, and Aldanie Rho for assistance in data collection and management and participating clients in this study. The authors E.L., S.I., N.S., N.C., E.J., and S.A. have no conflict of interest. A.M. served as a research consultant to and gave lectures at conferences supported by Lundbeck and Otsuka and was on an advisory board meeting for the same two companies for which he received honoraria. H.C.M. reports grants from Acadia, Amgen, and SyneuRx international; grants, honoraria, and nonfinancial support from Janssen, Lundbeck, and Otsuka; honoraria and nonfinancial support from Pfizer; honoraria from Sunovion, Purdue, HLS therapeutics, Mylan, and Shire within the last 3 years unrelated to the submitted work. M.G. reports personal fees from Purple Squirrel Economics Canada outside the submitted work. R.J. served as a speaker and member of advisory board committees for Pfizer, Janssen, BMS, Sunovian, Myelin, Otsuka, Lundbeck, Shire, and Perdue. He also received grants from Janssen, BMS, Otsuka, Lundbeck, Astra Zeneca, and HLS within the last 3 years unrelated to the submitted work.
Funding
This research was funded by the Canadian Institutes of Health Research (grant #MCT 94189; P.I.: A.M.). The funding source had no further role in study design, data collection and analysis, writing of the report, and in the decision to submit the report for publication. A.M. was supported by the Canada Research Chairs program. S.I. received salary awards from the Fonds de recherche du Québec - Santé and Canadian Institutes of Health Research.
References
- 1. Malla A, Norman R, McLean T, Scholten D, Townsend L. A Canadian programme for early intervention in non-affective psychotic disorders. Aust N Z J Psychiatry. 2003;37(4):407–413. [DOI] [PubMed] [Google Scholar]
- 2. McGorry PD, Edwards J, Mihalopoulos C, Harrigan SM, Jackson HJ. EPPIC: an evolving system of early detection and optimal management. Schizophr Bull. 1996;22(2):305–326. [DOI] [PubMed] [Google Scholar]
- 3. Dixon LB, Goldman HH, Bennett ME, et al. Implementing coordinated specialty care for early psychosis: the RAISE Connection Program. Psychiatr Serv. 2015;66(7):691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-Year Outcomes From the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mihalopoulos C, Harris M, Henry L, Harrigan S, McGorry P. Is early intervention in psychosis cost-effective over the long term? Schizophr Bull.. 2009;35(5):909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCrone P, Craig TK, Power P, Garety PA. Cost-effectiveness of an early intervention service for people with psychosis. Br J Psychiatry. 2010;196(5):377–382. [DOI] [PubMed] [Google Scholar]
- 8. Hegelstad WtV, Larsen TK, Auestad B, et al. Long-term follow-up of the TIPS early detection in psychosis study: effects on 10-year outcome. Am J Psychiatry. 2012;169(4):374–380. [DOI] [PubMed] [Google Scholar]
- 9. Rosenheck R, Leslie D, Sint K, et al. Cost-effectiveness of comprehensive, integrated care for first episode psychosis in the NIMH RAISE Early Treatment Program. Schizophr Bull. 2016;42(4):896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bertelsen M, Jeppesen P, Petersen L, et al. Five-year follow-up of a randomized multicenter trial of intensive early intervention vs standard treatment for patients with a first episode of psychotic illness: the OPUS trial. Arch Gen Psychiatry. 2008;65(7):762–771. [DOI] [PubMed] [Google Scholar]
- 11. Malla A, Joober R, Iyer S, et al. Comparing three-year extension of early intervention service to regular care following two years of early intervention service in first-episode psychosis: a randomized single blind clinical trial. World Psychiatry. 2017;16(3):278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang WC, Chan GH, Jim OT, et al. Optimal duration of an early intervention programme for first-episode psychosis: randomised controlled trial. Br J Psychiatry. 2015;206(6):492–500. [DOI] [PubMed] [Google Scholar]
- 13. Albert N, Melau M, Jensen H, et al. Five years of specialised early intervention versus two years of specialised early intervention followed by three years of standard treatment for patients with a first episode psychosis: randomised, superiority, parallel group trial in Denmark (OPUS II). BMJ. 2017;356:i6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lutgens D, Iyer S, Joober R, et al. A five-year randomized parallel and blinded clinical trial of an extended specialized early intervention vs. regular care in the early phase of psychotic disorders: study protocol. BMC Psychiatry. 2015;15:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 1994;12:70–75. [DOI] [PubMed] [Google Scholar]
- 16. Canadian Agency for Drugs Technologies in Health. Guidelines for the Economic Evaluation of Health Technologies: Canada. 4th ed. Ottawa, Canada: CADTH; 2017. [Google Scholar]
- 17. Andreasen N Scale for the Assessment of Positive Symptoms (SAPS). Iowa City. University of Iowa; 1984. [Google Scholar]
- 18. Andreasen N The Scale for the Assessment of Negative Symptoms (SANS) Iowa City. University of Iowa; 1983. [Google Scholar]
- 19. Ministère de la Santé et Services Sociaux Québec. Cadre Normatif du Système MED-ÉCHO. Québec, Canada: Ministère de la Santé et Services Sociaux Québec; 2019. https://publications.msss.gouv.qc.ca/msss/document-000170/. Accessed January 31, 2019. [Google Scholar]
- 20. Latimer EA, Rabouin D, Cao Z, et al. ; At Home/Chez Soi Investigators . Costs of services for homeless people with mental illness in 5 Canadian cities: a large prospective follow-up study. CMAJ Open. 2017;5(3):E576–E585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Statistics Canada. Consumer Price Index: annual review, 2018. Ottawa, Canada: Statistics Canada; 2018. https://www150.statcan.gc.ca/n1/daily-quotidien/190118/dq190118c-eng.htm. Accessed November 7, 2018. [Google Scholar]
- 22. Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 23. Barber J, Thompson S. Multiple regression of cost data: use of generalised linear models. J Health Serv Res Policy. 2004;9(4):197–204. [DOI] [PubMed] [Google Scholar]
- 24. van Asselt AD, van Mastrigt GA, Dirksen CD, Arntz A, Severens JL, Kessels AG. How to deal with cost differences at baseline. PharmacoEcon. 2009;27(6):519–528. [DOI] [PubMed] [Google Scholar]
- 25. Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. 2nd ed. Oxford, UK: Oxford University Press; 2015. [Google Scholar]
- 26. Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. 4th ed. Oxford, UK: Oxford University Press; 2015. [Google Scholar]
- 27. Dama M, Shah J, Norman R, et al. Short duration of untreated psychosis enhances negative symptom remission in extended early intervention service for psychosis. Acta Psychiatr Scand. 2019;140(1):65–76. [DOI] [PubMed] [Google Scholar]
- 28. Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ. 2002;11(5):415–430. [DOI] [PubMed] [Google Scholar]
- 29. Bertolote J, McGorry P. Early intervention and recovery for young people with early psychosis: consensus statement. Br J Psychiatry Suppl. 2005;48:s116–s119. [DOI] [PubMed] [Google Scholar]
- 30. Knapp M, Mangalore R. The trouble with QALYs. Epidemiol Psychiatr Sci. 2007;16(4):289–293. [DOI] [PubMed] [Google Scholar]
- 31. Lenert LA, Sturley AP, Rapaport MH, Chavez S, Mohr PE, Rupnow M. Public preferences for health states with schizophrenia and a mapping function to estimate utilities from positive and negative symptom scale scores. Schizophr Res. 2004;71(1):155–165. [DOI] [PubMed] [Google Scholar]
- 32. Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med. 2003;163(14):1637–1641. [DOI] [PubMed] [Google Scholar]
- 33. Hastrup LH, Kronborg C, Bertelsen M, et al. Cost-effectiveness of early intervention in first-episode psychosis: economic evaluation of a randomised controlled trial (the OPUS study). Br J Psychiatry. 2013;202(1):35–41. [DOI] [PubMed] [Google Scholar]
- 34. Malla A, McGorry P. Early intervention in psychosis in young people: a population and public health perspective. Am J Public Health. 2019;109(S3):S181–S184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.