Abstract
Studies on the long-term development and early predictors of treatment-resistant schizophrenia (TRS) and clozapine-resistant TRS (CR-TRS) in patients with first-episode schizophrenia-spectrum disorders (FES) are limited and have not considered the impact of early intervention services (EIS). This study aimed to explore the development of TRS and CR-TRS among patients with FES over 12 years of follow-up. Of the 1234 patients with FES, 15% developed TRS. A total of 450 patients with schizophrenia or schizoaffective disorder were included in a nested case-control study (157 TRS and 293 non-TRS). Younger age of onset, poorer premorbid social adjustment during adulthood, longer duration of first episode, a greater number of relapses, and a higher antipsychotic dose in the first 24 months were associated with earlier TRS. CR-TRS patients, constituting 25% of TRS patients, had a poorer premorbid social adjustment in late adolescence and longer delay before clozapine initiation compared with non-CR-TRS. CR-TRS had poorer clinical and functional outcomes at 12-year follow-up. However, TRS patients on clozapine had a lower mortality rate compared with non-TRS patients. EIS did not have a significant impact on the development of TRS, but patients in the EIS group had a shorter delay of clozapine initiation. Results suggested that neurodevelopmental factors, early clinical characteristics, and requirement for higher antipsychotic dose may be associated with TRS development, highlighting multiple pathways leading to this form of illness. Specific interventions including relapse prevention and early initiation of clozapine during the early course of illness may reduce the rate of TRS and improve patient outcomes.
Keywords: treatment resistant schizophrenia, clozapine-resistant schizophrenia, long-term outcomes, clinical predictors, early intervention service
Introduction
Schizophrenia affects 1% of the population and is the eighth leading cause of disability-associated life years lost.1 Studies suggest that about 20%–30% of patients do not respond to standard antipsychotic medication and are considered to have treatment-resistant schizophrenia (TRS).2–4 Patients with TRS have poorer functional outcomes, including higher rates of unemployment and worse quality of life,5,6 and were found to have 3- to 11-fold higher direct healthcare costs than the schizophrenia population as a whole, mostly attributable to higher rates of hospitalization.6,7 Understanding the mechanisms of TRS development and identifying patients who are likely to have TRS onset early in the course of illness may be important to expedite targeted interventions to those at higher risk. Thus, understanding the early predictors of TRS would be crucial.
A recent systematic review8 found 12 studies reporting predictors of TRS; however, the length of follow-up was less than 5 years or unreported in half of these. Since TRS may develop over as long as a decade,3,9 a shorter duration of follow-up may limit the likelihood of capturing those who develop TRS later in the course of the illness. Most of the predictors examined were basic demographics; the only consistent predictor was a younger age of onset.8 Among the 6 studies with longer than 10 years of follow-up, only 2 followed cohorts of first-episode psychosis patients. Systematic explorations of clinical features of illness and early treatment outcomes as predictors were limited. One of the possible reasons for inconsistency in identifying predictors, and the wide range of TRS rates in different studies is the variation in the definition of TRS. Although the framework defining TRS always includes “insufficient response” and “adequate antipsychotic treatments,” definitions of these features vary among studies.10 Consensus agreement on the definition of TRS for research was obtained only recently.11
Clozapine is one of the most effective antipsychotic drugs for TRS.12 However, due to its side-effect profile, clozapine is only recommended for patients with TRS and as third-line treatment in most developed countries and regions, including Hong Kong.13,14 Despite the established efficacy of clozapine, about 30%–70% of patients with TRS4,15 have a poor or no response to clozapine and are considered as clozapine-resistant schizophrenia (CR-TRS). In clinical practice, studies suggest that only 30% of TRS patients receive clozapine treatment,16 and the long delay in clozapine initiation is common.17 Understanding factors contributing to the poor clozapine response and the origins of CR-TRS may facilitate our understanding of the illness and intervention decisions. A systematic review of 96 studies on biological predictors of clozapine response failed to find any consistent predictors.18 A more recent meta-analysis19 found that younger age, fewer negative symptoms at onset, and paranoid schizophrenia subtypes were associated with better response to clozapine. However, to our knowledge, there are no studies reporting clinical predictors of CR-TRS from the first episode.
Worldwide, early intervention services (EIS) for psychosis has been established with an aim to improve the long-term outcomes of patients by providing intensive and comprehensive intervention during the first 2–3 years after the first onset of psychosis. Although the benefits of EIS on short-term clinical and functional outcomes are convincing,20 long-term outcomes are less consistent.21 Some described improvements in longitudinal functioning22,23 and less mortality related to suicide,24 without an impact on long-term symptom improvement. None explored the impact of EIS on rates of TRS. The EIS in Hong Kong was implemented as a publicly available, regionwide service in 2001, providing 2-year phase-specific intervention for patients aged 15–25 years with the first-episode psychosis.25
The current study aimed to explore the pattern of development of TRS and CR-TRS among patients with schizophrenia-spectrum disorder over 12 years or more following their first onset of illness. Demographic and early clinical characteristic predictors of the development of TRS and CR-TRS as well as the impact of the EIS on rates of and time of developing TRS were specifically explored. Clinical and functional outcomes of TRS and CR-TRS were studied. The findings could help to identify risk factors for TRS and CR-TRS, contribute to understanding mechanisms, and inform the planning of targeted care pathways to improve long-term outcomes of patients with first-episode schizophrenia-spectrum disorders (FES).
Methods
Research Sample and Setting
As the Hong Kong EIS was implemented regionwide, a historical control study design was adopted (supplementary methods provide detail on the service). A total of 617 patients with a diagnosis of FES consecutively enrolled in the EIS for the first time between July 1, 2001 and June 30, 2003 were identified from the centralized hospital database (Clinical Management System [CMS]). The same number of patients who received the standard care service (SCS) between July 1, 1998 and June 30, 2001 in Hong Kong was identified from the same CMS system and matched individually by sex, diagnosis, and age with the EIS sample. Patients with substance-induced psychosis, organic conditions, or intellectual disability and those who received prior psychiatric treatment for more than 1 month were excluded from the study. Details of the matching and basic demographic information were reported previously.24 Using this cohort of 1234 FES patients, a nested case-control study design was adopted to determine the predictors for TRS and CR-TRS.
Research Procedure
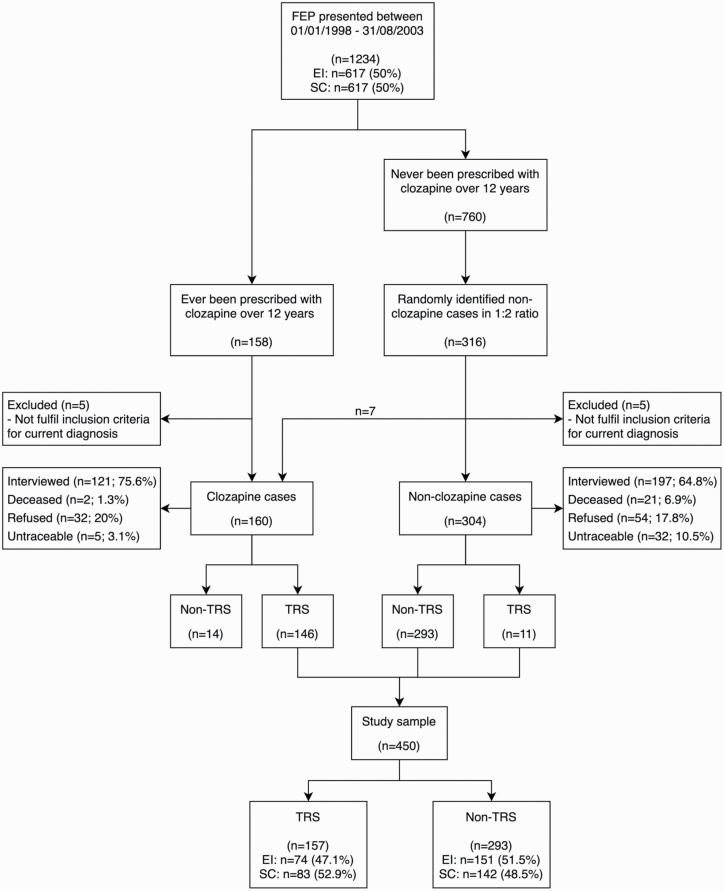
A 4-phase approach was adopted for identifying TRS patients. In the first phase, clozapine prescription was considered as the initial proxy indicator of treatment resistance. Using the CMS, a detailed screening of medication history of all patients was conducted from the first contact with the service through June 2015, and 158 patients (12.8%) ever prescribed clozapine (the clozapine group) were identified. A non-clozapine comparison group was then formed by random selection from the remaining patients using a 1:2 ratio, considering that patients in this group may be treatment resistant without having been prescribed clozapine. A total of 316 patients were identified as non-clozapine patients. The total sample for the nested case-control study was 474.
In the second phase, all written clinical notes of the sample (N = 474) were screened for diagnosis at the time of study and any evidence of clozapine prescription for over 12 years. Five of the 158 patients treated with clozapine and 5 of the non-clozapine patients were subsequently excluded as they did not fulfill the inclusion criteria of diagnosis for the study. The clinical note screening identified 7 patients from the comparison group prescribed with clozapine, and these were reassigned to the clozapine group. Therefore, the total sample was 464, with 160 clozapine patients and 304 non-clozapine patients (figure 1).
Fig. 1.
Flowchart of the screening and interview process. FEP, first-episode psychosis; EI, early intervention; SC, standard care; TRS, treatment-resistant schizophrenia.
In the third phase, a detailed case note review using a standardized data entry form was conducted for all participants from the first contact with service until June 2015. Information obtained includes demographics, baseline and longitudinal clinical variables, and antipsychotics prescribed. The baseline clinical variables include baseline diagnosis, duration of untreated psychosis (DUP), positive and negative symptom severity in the first month after presentation using the Clinical Global Impressions-Schizophrenia (CGI-SCH) scale,26 and duration of the first episode (defined by the start of the treatment to the time point of CGI-SCH positive symptom severity score of <=2 and being discharged from hospital if admission was required). DUP is the period (in days) from the first emergence of psychotic symptoms until the use of effective psychiatric treatment as determined by clinicians. Clinical variables from the first year and initial 24 months following presentation include the duration of hospitalization and number of relapses. Relapse was operationally defined as an increase of CGI-SCH positive scores, from 1 to 3 or from 4–6 to 5–7, followed with hospitalization or adjustment of antipsychotic medication.27 Dosage and duration of antipsychotics prescribed for the entire duration were documented, and chlorpromazine equivalent dose28 and daily defined dose (DDD)29 were calculated. The clozapine prescription pattern includes clinician-documented reasons for clozapine prescription, clozapine start and end date (if any), reasons for termination if any, and maximum dose of clozapine.
Finally, all patients were approached for a face-to-face interview. Information including symptom severity measured with the Positive and Negative Syndrome Scale for Schizophrenia (PANSS),30 functioning measured with the Social and Occupational Functioning Assessment Scale (SOFAS),31 and premorbid functioning rated with the Premorbid Adjustment Scale.32 Mortality information was obtained from the CMS and coroner’s court reports. Informed consent was obtained from all eligible patients. The study had institutional ethics approval from all 7 hospital clusters in Hong Kong.
Definition of TRS and CR-TRS
The definition of TRS and CR-TRS was based on the principles of a consensus report.11 TRS status was operationalized as having clozapine prescribed for clinically determined TRS or schizoaffective disorder and having persistent moderate positive psychotic symptoms (CGI-SCH positive score >= 4) after at least 2 trials of antipsychotics with chlorpromazine equivalent dose of >=600 mg per day for >=6 weeks. For those without a clozapine prescription, TRS status was determined upon scoring >=4 on any PANSS positive symptom item for >=12 weeks with a moderate functioning impairment (SOFAS < 60) and a history of at least 2 trials of antipsychotics above a chlorpromazine equivalent dose of >=600 mg per day for >=6 weeks. The time point when these criteria were fulfilled was considered as the onset of TRS. Delay of clozapine initiation was the time difference between the TRS onset and clozapine initiation. CR-TRS status was determined when patients on clozapine scored 4 or greater on the CGI-SCH scale in spite of sufficient clozapine dosage (350 mg) for >=6 weeks and having any PANSS positive symptom item scored >= 4 for >=12 weeks at interview. Though the CMS provided records of medications that have been picked up by patients, reliable adherence information was not able to be obtained retrospectively based on clinical records systematically; therefore, adherence was not taken into consideration in the definition.
Statistical Analysis
The Kolmogorov-Smirnov test was used to test for normality of data. To compare univariate differences in baseline demographic, clinical and premorbid adjustment variables between TRS and non-TRS groups, Mann-Whitney U, and chi-square tests were performed. As patients became TRS at different time points, Cox proportional hazard regression analysis was used to identify the predictors of TRS. The time function was calculated as the number of months from the FES onset to the onset of TRS. Variables that were significantly different between TRS and non-TRS groups were included as the independent variable in Cox proportional hazard regression analyses. Hazard ratios with 95% confidence intervals were calculated. Kaplan-Meier (KM) plots with log-rank analysis were used to explore the difference in time to TRS between EIS and SCS groups. Repeated measures ANOVA was conducted to explore the differences in DDD between TRS and non-TRS over the first 2 years.
Mann-Whitney U and chi-square tests were used to assess univariate differences in baseline demographic, clinical, and premorbid adjustment variables between CR-TRS and non-clozapine-resistant TRS (nCR-TRS) groups. The effects of clozapine prescription time on the status of CR-TRS and nCR-TRS were explored using KM plots with log-rank analysis, with time function calculated as the number of months from the determination of TRS to the date of clozapine prescription, described as the delay of clozapine prescription in this study.
Clinical outcomes assessed by face-to-face interviews were compared between TRS and non-TRS using Mann-Whitney U tests, and between CR-TRS, nCR-TRS, and non-TRS using Kruskal-Wallis tests with Bonferroni-corrected post hoc pairwise comparisons. Logistic regression analysis was performed to explore the effects of age of onset, gender, clozapine use, service cohorts, and number of relapses on mortality. All statistical analyses were performed with SPSS version 25. Details of inter-rater reliability are reported in the supplementary method section.
Results
The final analysis sample included 450 patients (figure 1). Fourteen (8.75%) clozapine-prescribed patients were found to be non-TRS (supplementary table 1) and were excluded from the main analysis. Among the 450 patients, 157 were TRS (34.9%) (including 11 TRS without clozapine, 3.6%). For the total FES sample of 1234 patients, adding the percentage of TRS patients prescribed clozapine (N = 146, 11.8%) plus the estimate of TRS not treated with clozapine (3.6%) yields an overall estimate of TRS to be approximately 15%. Among the total FES sample, 74 TRS patients were from the EIS group (12%) and 83 were from the SCS group (13.5%).
Development and Predictors of TRS
About 10% of TRS patients were treatment resistant from the illness onset. By the end of the third episode of illness (second relapse), TRS was present in 61% of patients who eventually developed this form of illness (supplementary figure 1).
The overall premorbid social functioning was similar in the 2 groups of patients but was lower in adulthood (proximal to illness onset) in the TRS group (table 1). The TRS group had a lower age of onset by approximately 1 year. History of substance use disorder did not differ and no difference in DUP between groups was noted.
Table 1.
Basic Demographics and Premorbid Functioning Compared Between Treatment-Resistant (TRS) and Non-treatment-Resistant (non-TRS) Schizophrenia Groups
| TRS (n = 157) | non-TRS (n = 293) | U/χ2 | P-value | |
|---|---|---|---|---|
| Basic demographics | ||||
| Current age (SD) | 36.28 (3.78) | 37.27 (3.66) | 19256.0 | .004 |
| Age of onset (SD) | 19.37 (3.31) | 20.51 (3.46) | 18631.0 | .001 |
| Years of education (SD) | 10.18 (2.27) | 10.62 (2.32) | 21109.5 | .158 |
| Gender (male) (%) | 83 (52.9%) | 159 (54.3%) | 0.081 | .776 |
| Substance use disorder (SUD) history | 2.385 | .122 | ||
| No SUD (%) | 139 (88.5%) | 272 (92.8%) | ||
| Had SUD (%) | 18 (11.5%) | 21 (7.2%) | ||
| Log DUP (d) (SD) | 1.89 (0.7) | 2.00 (0.77) | 21034.0 | .135 |
| Baseline and year 1 clinical variables | ||||
| M1 positive symptoms (SD) | 4.54 (1.03) | 4.39 (0.89) | 20229.0 | .031 |
| M1 negative symptoms (SD) | 2.94 (1.29) | 2.80 (1.23) | 21272.5 | .211 |
| EP1 duration (d), mean/median | 117.43 (158.3) | 87.22 (105.35) | 20343.5 | .043 |
| Y1 hospitalization (d) (SD) | 90.31 (137.8) | 62.3 (97.47) | 19843.5 | .016 |
| Y1 number of relapses (SD) | 0.38 (0.62) | 0.18 (0.45) | 19305.0 | <.001 |
| Y1 DDD (SD) | 0.85 (0.63) | 0.61 (0.5) | 17075.0 | <.001 |
| Combined years 1 and 2 clinical variables | ||||
| 24-month hospitalization (d) (SD) | 118.66 (144.38) | 78.08 (111.11) | 16280.0 | <.001 |
| 24-month number of relapses (SD) | 0.92 (0.93) | 0.49 (0.7) | 15187.0 | <.001 |
| 24-month DDD (SD) | 0.9 (0.57) | 0.59 (0.47) | 14816.0 | <.001 |
| Service cohort | 0.792 | .373 | ||
| Early intervention (%) | 74 (47.1%) | 151 (51.5%) | ||
| Standard care (%) | 83 (52.9%) | 142 (48.5%) | ||
| Premorbid Adjustment Scale | Interviewed (n = 112) | Interviewed (n = 170) | ||
| PAS academic Child | 0.42 (0.19) | 0.37 (0.21) | 8394.5 | .124 |
| PAS academic Early adolescent | 0.45 (0.19) | 0.43 (0.2) | 8297.0 | .446 |
| PAS academic Late adolescent | 0.46 (0.17) | 0.46 (0.2) | 4777.5 | .737 |
| PAS academic total | 0.45 (0.16) | 0.42 (0.18) | 8970.5 | .459 |
| PAS social Child | 0.29 (0.23) | 0.27 (0.26) | 8488.5 | .214 |
| PAS social Early adolescent | 0.31 (0.23) | 0.29 (0.27) | 8415.5 | .164 |
| PAS social Late adolescent | 0.35 (0.24) | 0.32 (0.26) | 7920.0 | .198 |
| PAS social Adult | 0.55 (0.25) | 0.47 (0.25) | 7550.0 | .010 |
| PAS social total | 0.42 (0.22) | 0.38 (0.24) | 8236.5 | .066 |
| PAS overall | 0.41 (0.16) | 0.37 (0.18) | 8536.0 | .142 |
Note: U, Mann-Whitney-U; χ 2, Chi-square; SD, standard deviation; DUP, duration of untreated psychosis; M1, month 1; EP1, episode 1; Y1, year 1; DDD, defined daily dose; PAS, Premorbid Adjustment Scale.
aThe bold value are all significant P-values.
The TRS patients had more severe positive symptoms during the first month of illness after presentation (table 1). The duration of the first episode was longer by about 1 month. Over the first year, although only 16 patients (10% of TRS) were defined as TRS by the end of their first episode (supplementary figure 1), the mean DDD of antipsychotic medication was higher in TRS patients (table 1). Repeated measures ANOVA found that TRS patients had a steady increase of DDD, while non-TRS had a stable DDD: the interaction of time and group was significant [F(1.602, 672.945) = 14.891, P < .001] (supplementary figure 2). The TRS patients had more relapses and longer hospitalization over the first year of treatment. Number of relapses, duration of hospitalization, and DDD over the first 2 years were also significantly higher in the TRS group. However, no difference in the number of TRS between EIS and SCS was found (table 1).
Cox regression analysis found that poor premorbid adjustment in early adulthood was associated with greater risk for TRS, as was a younger age of onset of illness (table 2). Longer duration of the first episode, a greater number of relapses, and a higher dose of antipsychotic medication over the first 2 years were also found to be significant in the model. Survival analysis of the 450 patients showed that the proportion of TRS and time from the illness onset to becoming TRS were similar for patients treated in the EIS and SCS (log-rank χ2 = 0.526, P = .468) (supplementary figure 3).
Table 2.
Cox Regression of Predictors of Treatment Resistance (n = 272)
| Estimate | SE | P-value | HR (95% CI) | |
|---|---|---|---|---|
| PAS social Adult | 1.068 | 0.373 | .004 | 2.910 (1.400–6.049) |
| Age of onset | −0.084 | 0.030 | .004 | 0.919 (0.867–0.974) |
| M1 positive symptoms | 0.102 | 0.131 | .435 | 1.108 (0.857–1.432) |
| EP1 duration (d) | 0.002 | 0.001 | .020 | 1.002 (1.000–1.003) |
| 24-month hospitalization (d) | −0.00046 | 0.001 | .605 | 1.000 (0.998–1.001) |
| 24-month number of relapses | 0.546 | 0.120 | <.001 | 1.726 (1.365–2.183) |
| 24-month DDD | 0.704 | 0.174 | <.001 | 2.021 (1.437–2.842) |
Note: SE, standard error; HR, hazard ratio; 95% CI, 95% confidence interval; PAS, premorbid adjustment scale; M1, month 1; EP1, episode 1; DDD, defined daily dose.The bold value are all significant P-values.
Development and Predictors of CR-TRS
Among the 157 TRS patients, 146 were ever prescribed with clozapine. Of these patients, 36 (24.7%) did not respond sufficiently and were described as CR-TRS; 91 patients were considered as nCR-TRS; 19 were excluded from subsequent analysis due to early clozapine termination as a result of intolerance (n = 14), or insufficient clozapine dosage (<350 mg) despite persistent positive symptoms (n = 5). The 11 TRS patients who were not prescribed with clozapine were also excluded from the following analysis.
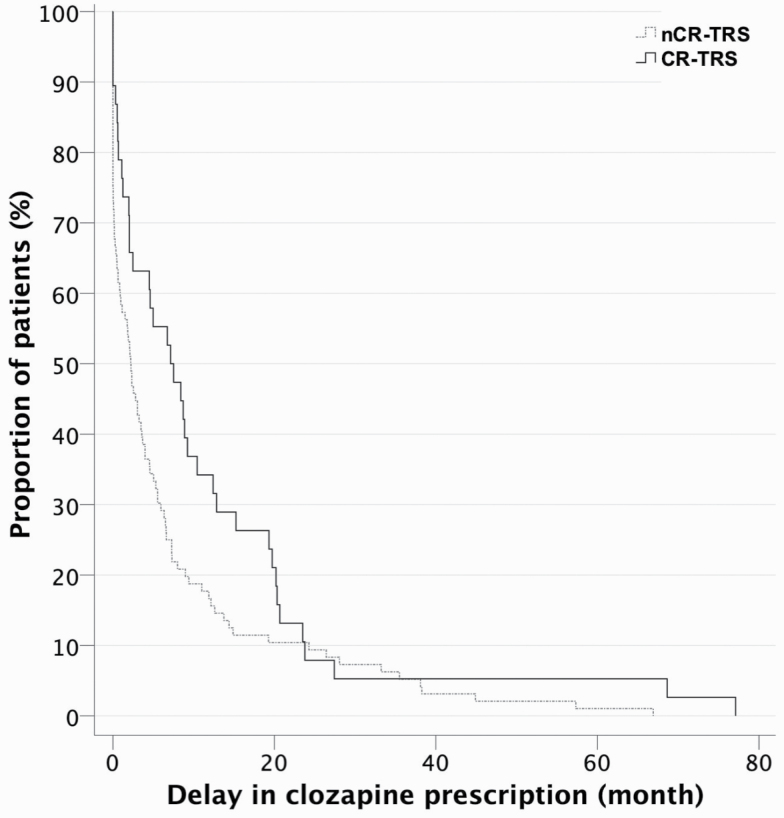
The CR-TRS group showed more impairment in late adolescent social adjustment compared with the nCR-TRS group (table 3). The clinical characteristics over the first 2 years of illness were similar between these 2 TRS subgroups (supplementary table 2). KM survival analysis (figure 2) showed that the delay of clozapine prescription was longer in the CR-TRS group than in the nCR-TRS group (log-rank χ2 = 3.871, P = .049), with an estimated mean time from TRS status to clozapine prescription of 11.82 months (95% CI = 6.23–17.40) for the CR-TRS group vs 7.23 months (95% CI = 4.60–9.85) for the nCR-TRS group. Patients in the SCS group had significantly greater delay of clozapine initiation (M = 8.60, SD = 11.45) compared with those in the EIS group (M = 8.05, SD = 15.85; U = 2159.0, P =.049). However, only a trend significance was seen (P = .091) with more CR-TRS patients in the SCS group than that in the EIS group.
Table 3.
Basic demographics, Early Clinical Characteristics, and Premorbid Functioning Compared Between Clozapine-Resistant Schizophrenia (CR-TRS) and Non-clozapine-Resistant Schizophrenia (nCR-TRS) Groups
| CR-TRS (n = 36) | nCR-TRS (n = 91) | U/χ2 | P-value | |
|---|---|---|---|---|
| Basic demographics | ||||
| Current age (SD) | 36.53 (3.53) | 35.65 (3.69) | 1411.5 | .224 |
| Age of onset (SD) | 19.67 (3.22) | 18.87 (3.24) | 1445.0 | .299 |
| Gender (male) (%) | 19 (52.8%) | 49 (53.8%) | 0.012 | .913 |
| Smoking | 0.238 | .626 | ||
| Nonsmoker (%) | 26 (72.2%) | 61 (67.8%) | ||
| Smoker & ex-smoker (%) | 10 (27.8%) | 29 (32.2%) | ||
| Substance use disorder (SUD) history | 0.830 | .362 | ||
| No SUD (%) | 33 (91.7%) | 78 (85.7%) | ||
| Had SUD (%) | 3 (8.3%) | 13 (14.3%) | ||
| Log DUP (d) (SD) | 1.96 (0.61) | 1.82 (0.71) | 1487.0 | .419 |
| Diagnosisa | 0.038 | 1.000 | ||
| ATPD & Psychosis NOS (%) | 1 (2.8%) | 2 (2.2%) | ||
| Schizophrenia & schizoaffective disorder (%) | 35 (97.2%) | 89 (97.8%) | ||
| Premorbid Adjustment Scale | ||||
| PAS academic Child | 0.44 (0.2) | 0.41 (0.19) | 2.235 | .327 |
| PAS academic Early adolescent | 0.47 (0.2) | 0.44 (0.19) | 1.013 | .602 |
| PAS academic Late adolescent | 0.5 (0.17) | 0.43 (0.18) | 1.725 | .422 |
| PAS academic total | 0.47 (0.17) | 0.43 (0.15) | 0.829 | .661 |
| PAS social Child | 0.33 (0.24) | 0.27 (0.24) | 2.551 | .279 |
| PAS social Early adolescent | 0.36 (0.23) | 0.28 (0.22) | 4.147 | .126 |
| PAS social Late adolescent | 0.45 (0.26) | 0.3 (0.23) | 7.712 | .021 |
| PAS social Adult | 0.58 (0.27) | 0.54 (0.27) | 6.101 | .047 |
| PAS social total | 0.48 (0.23) | 0.39 (0.23) | 5.317 | .070 |
| PAS overall | 0.45 (0.16) | 0.38 (0.16) | 4.575 | .102 |
| Baseline and clinical variables | ||||
| M1 positive symptoms (SD) | 4.71 (0.86) | 4.52 (1.07) | 1450.5 | .407 |
| M1 negative symptoms (SD) | 2.83 (1.36) | 2.81 (1.22) | 1575.0 | .921 |
| EP1 duration (d), mean/media | 127.58/56.0 | 114.23/76.0 | 1417.5 | .238 |
| 24-month hospitalization (d) (SD) | 110.86 (104.77) | 125.22 (154.60) | 1564.0 | .952 |
| 24-month number of relapses (SD) | 1.00 (0.84) | 0.93 (0.97) | 1461.0 | .505 |
| 24-month DDD (SD) | 1.02 (0.67) | 0.88 (0.53) | 1482.0 | .404 |
| Service cohort | 2.860 | .091 | ||
| Early intervention (%) | 13 (36.1%) | 48 (52.7%) | ||
| Standard care (%) | 23 (63.9%) | 43 (57.3%) | ||
| Delay in clozapine prescription (mo) | 11.82 (17.10) | 7.23 (12.78) | 1191.0 | .016 |
Note: U, Mann-Whitney U; χ 2, Chi-square; SD, standard deviation; DUP, duration of untreated psychosis; ATPD, acute and transient psychotic disorder; Psychosis NOS, psychotic disorders not otherwise specified; PAS, Premorbid Adjustment Scale; M1, month 1; EP1, episode 1; DDD, defined daily dose.
The bold value are all significant P-values.
aFisher’s exact test.
Fig. 2.
Kaplan-Meier survival plot for delay in clozapine prescription in non-clozapine-resistant (nCR-TRS) and clozapine-resistant (CR-TRS) patients.
Outcomes of TRS and Non-TRS 12 Years After Illness Onset
The interviewed and non-interviewed groups were compared (supplementary table 3) with no significant difference in basic demographics. The face-to-face interview found that CR-TRS had poorer clinical and functional outcomes compared with non-TRS and nCR-TRS; only negative symptoms were found to be significantly different between CR-TRS and non-TRS (supplementary table 4). Notably, 34.6% of non-TRS patients (N = 66) were considered clinically remitted based on operational criteria,33 a similar proportion as in the nCR-TRS sample (N = 35, 38.5%). A total of 22 patients were deceased with 21 due to suicide. Logistic regression analysis suggested a significantly lower mortality of the group prescribed clozapine (P = .014) after controlling for gender, age of onset, intervention group, and number of relapses in the first 24 months (supplementary table 5).
Discussion
Among the whole FES sample of this study (N = 1234), 13% (N = 160) were prescribed clozapine. A total of 450 patients with FES were included in the nested case-control study with 157 as TRS and 293 were non-TRS. Patients with younger age of onset, poorer premorbid social adjustment during adulthood, longer duration of first-episode, a greater number of relapses, and a higher level of DDD of antipsychotic medication in the first 24 months had an increased risk of developing TRS earlier. Among the TRS patients prescribed clozapine, 25% were clozapine resistant (CR-TRS). The CR-TRS patients had a poorer premorbid social adjustment in late adolescence and longer delay of clozapine initiation compared with nCR-TRS. CR-TRS had poorer clinical and functional outcomes at 12-year follow-up. Significantly more non-TRS patients died from suicide compared with patients prescribed with clozapine.
The rate of TRS in the whole FES sample over 12 years is estimated to be 15%. Because of the design of EIS service, the current study is limited to patients with age of first service contact between 15 and 25 years old. Despite the younger age and limited age range, the TRS rate of the current study is relatively lower compared with the previous studies that had samples of wider age ranges and mostly adult population.34–36 Inasmuch as comorbid substance use is suggested as a possible predictor of treatment resistance,37 the much lower substance use rate (8.6%) of the current sample compared with most of the previous studies (30%)34,35 could be a contributing factor. The variation of TRS definition in previous studies may be another factor. Some of the previous studies used clozapine prescription as a proxy indicator38; others used lack of treatment response despite adequate antipsychotic treatment based on clinical records.36 The current study followed the principle of the consensus TRS definition11 while also considering the naturalistic clinical use of clozapine. Notably, the clozapine prescription rate reported here (13%, N = 160) is similar to the previous population-based cohort study of Danish national registry data (13.2%).34 The use of new criteria and low rate of substance use might also explain the relatively lower rate of CR-TRS (25%) compared with previous reports of 30%–40%.15,39
To our knowledge, this is the first study exploring the development of TRS and CR-TRS in the same FES sample with follow-up beyond 10 years. Even within the limited range of age in our sample, younger age of onset of illness was found as a risk factor for TRS, consistent with a recent systematic review.8 Premorbid adjustment, particularly in the social domain during adulthood, was a predictor of TRS with a trend level of significance for the overall premorbid social adjustment; no effect was observed for academic domains. This echoed previous studies suggesting an association between poor premorbid social functioning and TRS,37,40,41 with social domains of premorbid adjustment showing a stronger relationship with clinical outcomes, while academic domains more clearly associated with cognitive function.42,43 Compared with nCR-TRS patients, CR-TRS patients had poorer premorbid social adjustment during late adolescence. Overall, younger age of onset and poorer premorbid social functioning supported the role of neurodevelopmental risk factors for TRS. Premorbid social impairment might be more related to the development of the clozapine-resistant type of TRS.
Clinical features of illness and provision of treatment in the early years after onset also play important roles. Longer duration of the first episode and a higher level of positive symptoms in the first month were risk factors for subsequent TRS, corroborating findings from a previous study.34 Higher antipsychotic dosage in the first 2 years was associated with TRS. We also found that TRS patients had a continuous increase in DDD of antipsychotic medications over the first 2 years, compared with a stable DDD for non-TRS patients. This may suggest the development of tolerance to the antipsychotic medication in the TRS patients, possibly implicating dopamine supersensitivity in the development of TRS.44,45 However, another possible explanation could be that these patients are less responsive to dopaminergic antagonists and have an illness with different neurochemical pathophysiology. Furthermore, TRS patients had more relapses compared with the non-TRS patients and over 60% of TRS development occurred within the first 2 episodes of relapse. This highlights the importance of relapse prevention and management of the first few relapses.
CR-TRS had poorer clinical and functional outcomes compared with non-TRS and nCR-TRS patients. The clinical remission rates of non-TRS and nCR-TRS were similar. This suggested that these young TRS patients who are not resistant to clozapine had compatible long-term clinical outcomes as non-TRS patients. Delay in clozapine prescription was a modifiable difference between CR-TRS and nCR-TRS; CR-TRS patients had about 5 months more delay than nCR-TRS patients. This supports previous literature39,46 indicating that delay in clozapine prescription decreases the likelihood of response to clozapine, highlighting the importance of early prescription of clozapine for TRS patients and the implications for longer-term outcomes of TRS. Similar to previous studies,47,48 this study also demonstrated lower all-cause mortality for TRS patients prescribed with clozapine. This further emphasizes the potential benefit of clozapine for patients with schizophrenia.
EIS was found to have no measurable impact on the development of TRS but patients in the EIS group had a shorter delay of clozapine prescription. A subgroup of patients may continue to do poorly even with the intensive care provided by EIS.22 Identifying this subgroup of patients early in the course of illness and developing targeted interventions are a clinical priority. The findings of the current study highlight that patients with early age of onset, poor premorbid social functioning, more severe symptomatology, and poor response to antipsychotic treatment during the first episode may be more likely to develop TRS. Furthermore, relapses in the first 2 years were also significantly associated with TRS. Developing a specific relapse prevention and management program together with strategies to reduce the delay of clozapine prescription within EIS may be important for preventing the development of TRS and improving outcomes.
Strengths of the study were the long follow-up of FES patients, use of an updated consensus definition of TRS and CR-TRS, and comparison of EIS and SCS groups. However, the inclusion criteria of the study and the age limit of the EIS restricted the generalizability of the study results. The attrition rate of the follow-up assessment might also introduce selection bias though the basic demographics and clinical variables between the interviewed and non-interviewed groups were not significant. The historical control design may have introduced a cohort effect leading to the bias of results. Patients’ medication adherence was not measured systematically nor were clozapine plasma levels obtained. This may have limited the reliability of the rates of TRS and CR-TRS estimated in the study. As the baseline and early clinical variables were obtained from clinical records, the reliability of this information depends on the quality of the records. The small sample size of the study, particularly the CR-TRS sample, might limit the power of the study to identify risk factors.
Conclusions
The study found that 13% of FES patients followed up over 12 years were prescribed with clozapine and 15% were estimated to be TRS. The nested case-control study results suggest that neurodevelopmental factors, early clinical characteristics, and requirement for higher antipsychotic dose may all contribute to the TRS development, highlighting the heterogeneity and multiple pathways leading to this form of illness. Although the development of TRS was similar in the EIS group compared with the SCS group, delay in clozapine prescription, an important risk factor for the development of clozapine resistance, was shorter in the EIS group. This emphasized the need to establish specific interventions, including relapse prevention and an early clozapine prescription program during the first 1 to 2 years of illness, to potentially reduce the TRS rate and improve the outcomes of patients with TRS.
Supplementary Material
Acknowledgments
We are grateful to the clinical staff of all 7 psychiatric units in Hong Kong for supporting patient recruitment. We also thank Ms Stephanie Wing Yan Chan, Sharon Kao, Ruby Lau, and Olivia Wai Tung Li for supporting patient recruitment and data analysis. E.Y.H.C is the Chairperson of the steering committee of the EASY service of the Hospital Authority of Hong Kong. S.K.W.C., and W.C.C. are the conveners of the EASY service of the Hospital Authority of Hong Kong. There are no other conflict of interest reported.
Funding
The research is supported by the Health and Medical Research Fund, Hong Kong, grant [11121721 to S.K.W.C.]. W.G.H. was supported by the Jack Bell Chair in Schizophrenia.
References
- 1. Rössler W, Salize HJ, van Os J, Riecher-Rössler A. Size of burden of schizophrenia and psychotic disorders. Eur Neuropsychopharmacol. 2005;15(4):399–409. [DOI] [PubMed] [Google Scholar]
- 2. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789–796. [DOI] [PubMed] [Google Scholar]
- 3. Meltzer HY. Treatment-resistant schizophrenia–the role of clozapine. Curr Med Res Opin. 1997;14(1):1–20. [DOI] [PubMed] [Google Scholar]
- 4. Conley RR, Kelly DL. Management of treatment resistance in schizophrenia. Biol Psychiatry. 2001;50(11):898–911. [DOI] [PubMed] [Google Scholar]
- 5. Iasevoli F, Giordano S, Balletta R, et al. Treatment resistant schizophrenia is associated with the worst community functioning among severely-ill highly-disabling psychiatric conditions and is the most relevant predictor of poorer achievements in functional milestones. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:34–48. [DOI] [PubMed] [Google Scholar]
- 6. Meltzer HY, Cola P, Way L, et al. Cost effectiveness of clozapine in neuroleptic-resistant schizophrenia. Am J Psychiatry. 1993;150(11):1630–1638. [DOI] [PubMed] [Google Scholar]
- 7. Kennedy JL, Altar CA, Taylor DL, Degtiar I, Hornberger JC. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int Clin Psychopharmacol. 2014;29(2):63–76. [DOI] [PubMed] [Google Scholar]
- 8. Smart SE, Kępińska AP, Murray RM, MacCabe JH. Predictors of treatment resistant schizophrenia: a systematic review of prospective observational studies. Psychol Med. 2019:1–10. doi: 10.1017/S0033291719002083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wiersma D, Nienhuis FJ, Slooff CJ, Giel R. Natural course of schizophrenic disorders: a 15-year followup of a Dutch incidence cohort. Schizophr Bull. 1998;24(1):75–85. [DOI] [PubMed] [Google Scholar]
- 10. Suzuki T, Remington G, Mulsant BH, et al. Defining treatment-resistant schizophrenia and response to antipsychotics: a review and recommendation. Psychiatry Res. 2012;197(1-2):1–6. [DOI] [PubMed] [Google Scholar]
- 11. Howes OD, McCutcheon R, Agid O, et al. Treatment-resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017;174(3):216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry. 2017;74(7):686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Möller HJ; WFSBP Task Force on Treatment Guidelines for Schizophrenia . World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: long-term treatment of schizophrenia. World J Biol Psychiatry. 2006;7(1):5–40. [DOI] [PubMed] [Google Scholar]
- 14. Warnez S, Alessi-Severini S. Clozapine: a review of clinical practice guidelines and prescribing trends. BMC Psychiatry. 2014;14:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(1):772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farooq S, Taylor M. Clozapine: dangerous orphan or neglected friend? Br J Psychiatry. 2011;198(4):247–249. [DOI] [PubMed] [Google Scholar]
- 17. Doyle R, Behan C, OʼKeeffe D, et al. Clozapine use in a cohort of first-episode psychosis. J Clin Psychopharmacol. 2017;37(5):512–517. [DOI] [PubMed] [Google Scholar]
- 18. Samanaite R, Gillespie A, Sendt KV, McQueen G, MacCabe JH, Egerton A. Biological predictors of clozapine response: a systematic review. Front Psychiatry. 2018;9:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okhuijsen-Pfeifer C, Sterk AY, Horn IM, Terstappen J, Kahn RS, Luykx JJ. Demographic and clinical features as predictors of clozapine response in patients with schizophrenia spectrum disorders: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2020;111:246–252. [DOI] [PubMed] [Google Scholar]
- 20. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chan SKW, Chan HYV, Devlin J, et al. A systematic review of long-term outcomes of patients with psychosis who received early intervention services. Int Rev Psychiatry. 2019;31(5-6):425–440. [DOI] [PubMed] [Google Scholar]
- 22. Chan SKW, Pang HH, Yan KK, et al. Ten-year employment patterns of patients with first-episode schizophrenia-spectrum disorders: comparison of early intervention and standard care services. Br J Psychiatry. 2020;217(3):491–497. [DOI] [PubMed] [Google Scholar]
- 23. Chan SK, So HC, Hui CL, et al. 10-Year outcome study of an early intervention program for psychosis compared with standard care service. Psychol Med. 2015;45(6):1181–1193. [DOI] [PubMed] [Google Scholar]
- 24. Chan SKW, Chan SWY, Pang HH, et al. Association of an early intervention service for psychosis with suicide rate among patients with first-episode schizophrenia-spectrum disorders. JAMA Psychiatry. 2018;75(5):458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang JY, Wong GH, Hui CL, et al. Early intervention for psychosis in Hong Kong–the EASY programme. Early Interv Psychiatry. 2010;4(3):214–219. [DOI] [PubMed] [Google Scholar]
- 26. Haro JM, Kamath SA, Ochoa S, et al. The Clinical Global Impression–Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophrenia. Acta Psychiatr Scand. 2003;107:16–23. [DOI] [PubMed] [Google Scholar]
- 27. Haro JM, Novick D, Bertsch J, Karagianis J, Dossenbach M, Jones PB. Cross-national clinical and functional remission rates: Worldwide Schizophrenia Outpatient Health Outcomes (W-SOHO) study. Br J Psychiatry. 2011;199(3):194–201. [DOI] [PubMed] [Google Scholar]
- 28. Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167(6):686–693. [DOI] [PubMed] [Google Scholar]
- 29. Nosè M, Tansella M, Thornicroft G, et al. Is the defined daily dose system a reliable tool for standardizing antipsychotic dosages? Int Clin Psychopharmacol. 2008;23(5):287–290. [DOI] [PubMed] [Google Scholar]
- 30. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 31. Diagnostic and Statistical Manual of Mental Disorders (4th ed., text revision) (DSM-IV-TR). 2000. Washington DC: American Psychiatric Association. [Google Scholar]
- 32. Cannon-Spoor HE, Potkin SG, Wyatt RJ. Measurement of premorbid adjustment in chronic schizophrenia. Schizophr Bull. 1982;8(3):470–484. [DOI] [PubMed] [Google Scholar]
- 33. Andreasen NC, Carpenter WT Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162(3):441–449. [DOI] [PubMed] [Google Scholar]
- 34. Wimberley T, Støvring H, Sørensen HJ, Horsdal HT, MacCabe JH, Gasse C. Predictors of treatment resistance in patients with schizophrenia: a population-based cohort study. Lancet Psychiatry. 2016;3(4):358–366. [DOI] [PubMed] [Google Scholar]
- 35. Lally J, Ajnakina O, Di Forti M, et al. Two distinct patterns of treatment resistance: clinical predictors of treatment resistance in first-episode schizophrenia spectrum psychoses. Psychol Med. 2016;46(15):3231–3240. [DOI] [PubMed] [Google Scholar]
- 36. Demjaha A, Lappin JM, Stahl D, et al. Antipsychotic treatment resistance in first-episode psychosis: prevalence, subtypes and predictors. Psychol Med. 2017;47(11):1981–1989. [DOI] [PubMed] [Google Scholar]
- 37. Bozzatello P, Bellino S, Rocca P. Predictive factors of treatment resistance in first episode of psychosis: a systematic review. Front Psychiatry. 2019;10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim JS, Park CM, Choi JA, et al. The association between season of birth, age at onset, and clozapine use in schizophrenia. Acta Psychiatr Scand. 2017;136(5):445–454. [DOI] [PubMed] [Google Scholar]
- 39. Shah P, Iwata Y, Brown EE, et al. Clozapine response trajectories and predictors of non-response in treatment-resistant schizophrenia: a chart review study. Eur Arch Psychiatry Clin Neurosci. 2020;270(1):11–22. [DOI] [PubMed] [Google Scholar]
- 40. Legge SE, Dennison CA, Pardiñas AF, et al. Clinical indicators of treatment-resistant psychosis. Br J Psychiatry. 2020;216(5):259–266. [DOI] [PubMed] [Google Scholar]
- 41. Caspi A, Reichenberg A, Weiser M, et al. Premorbid behavioral and intellectual functioning in schizophrenia patients with poor response to treatment with antipsychotic drugs. Schizophr Res. 2007;94(1-3):45–49. [DOI] [PubMed] [Google Scholar]
- 42. Monte RC, Goulding SM, Compton MT. Premorbid functioning of patients with first-episode nonaffective psychosis: a comparison of deterioration in academic and social performance, and clinical correlates of Premorbid Adjustment Scale scores. Schizophr Res. 2008;104(1-3):206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barajas A, Usall J, Baños I, et al. ; GENIPE group . Three-factor model of premorbid adjustment in a sample with chronic schizophrenia and first-episode psychosis. Schizophr Res. 2013;151(1-3):252–258. [DOI] [PubMed] [Google Scholar]
- 44. Yamanaka H, Kanahara N, Suzuki T, et al. Impact of dopamine supersensitivity psychosis in treatment-resistant schizophrenia: an analysis of multi-factors predicting long-term prognosis. Schizophr Res. 2016;170(2-3):252–258. [DOI] [PubMed] [Google Scholar]
- 45. Sheitman BB, Lieberman JA. The natural history and pathophysiology of treatment resistant schizophrenia. J Psychiatr Res. 1998;32(3-4):143–150. [DOI] [PubMed] [Google Scholar]
- 46. Shah P, Iwata Y, Plitman E, et al. The impact of delay in clozapine initiation on treatment outcomes in patients with treatment-resistant schizophrenia: a systematic review. Psychiatry Res. 2018;268:114–122. [DOI] [PubMed] [Google Scholar]
- 47. Cho J, Hayes RD, Jewell A, et al. Clozapine and all-cause mortality in treatment-resistant schizophrenia: a historical cohort study. Acta Psychiatr Scand. 2018;61:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vermeulen JM, van Rooijen G, van de Kerkhof MPJ, Sutterland AL, Correll CU, de Haan L. Clozapine and long-term mortality risk in patients with schizophrenia: a systematic review and meta-analysis of studies lasting 1.1-12.5 years. Schizophr Bull. 2019;45(2):315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.