Abstract
The antisaccade task is considered a test of cognitive control because it creates a conflict between the strong bottom-up signal produced by the cue and the top-down goal of shifting gaze to the opposite side of the display. Antisaccade deficits in schizophrenia are thought to reflect impaired top-down inhibition of the prepotent bottom-up response to the cue. However, the cue is also a highly task-relevant stimulus that must be covertly attended to determine where to shift gaze. We tested the hypothesis that difficulty in overcoming the attentional relevance of the cue, rather than its bottom-up salience, is key in producing impaired performance in people with schizophrenia (PSZ). We implemented 3 versions of the antisaccade task in which we varied the bottom-up salience of the cue while holding its attentional relevance constant. We found that difficulty in performing a given antisaccade task—relative to a prosaccade version using the same stimuli—was largely independent of the cue’s bottom-up salience. The magnitude of impairment in PSZ relative to control subjects was also independent of bottom-up salience. The greatest impairment was observed in a version where the cue lacked bottom-up salience advantage over other locations. These results indicate that the antisaccade deficit in PSZ does not reflect an impairment in overcoming bottom-up salience of the cue, but PSZ are instead impaired at overcoming its attentional relevance. This deficit may still indicate an underlying inhibitory control impairment but could also reflect a hyperfocusing of attentional resources on the cue.
Keywords: inhibitory control, schizophrenia, antisaccade, attention/hyperfocusing
Introduction
Schizophrenia is associated with disturbances in cognitive control, which manifest in clinical symptoms, such as distractibility, loosening of associations, and disorganized behavior. These disturbances are thought to be mediated by prefrontal cortical dysfunction1,2 and posited to underlie deficits of attention, working memory (WM), and behavioral inhibition.3,4
The antisaccade task has been a gold standard test of cognitive control because it places top-down goals in conflict with strong bottom-up inputs that automatically activate oculomotor control structures in the cortex and midbrain.5,6 In a typical antisaccade task, viewers fixate a central location, a cue is flashed on one side of fixation, and the goal is to move the eyes toward the mirror-image location. The sudden onset of the cue automatically activates saccade neurons corresponding to the cue location, and top-down control is needed to suppress these neurons and activate the neurons that code the mirror-image location.5 It is well-documented that people with schizophrenia (PSZ) incorrectly fixate the cue instead of the mirror-image location more frequently than healthy control subjects (HCS), whereas PSZ have little or no deficit in prosaccade tasks.7–13Indeed, to our knowledge, there has never been a failure to replicate the finding of higher antisaccade error rates in PSZ than in matched controls.
Successful antisaccade performance requires suppressing the automatic activation of a prosaccade to the cue. Lesions of dorsolateral prefrontal cortex (dlPFC) appear to impair the suppression process,14–17leading to an increase in erroneous saccades to the cue and difficulty making corrective saccades.14 Impaired top-down inhibition of the bottom-up response to the cue18–20 is widely assumed to be implicated in the schizophrenia antisaccade deficit.
However, the cue is not just a salient bottom-up stimulus, but it is also a highly task-relevant stimulus that must be covertly attended so that the subject knows where to shift gaze. Consequently, performing an antisaccade requires suppressing an eye movement toward a task-relevant, covertly attended stimulus, not just a physically salient stimulus. Given close links between covert and overt attention,21 the act of shifting covert attention to the cue presumably increases the likelihood that it will attract a shift of gaze. Making an eye movement toward the mirror-image location, therefore, requires suppressing this attentionally boosted signal, not just suppressing a purely bottom-up sensory signal. Indeed, research with healthy populations has found that antisaccade performance becomes progressively more impaired (relative to prosaccade performance) as the attentional demands of the cue increase.22,23
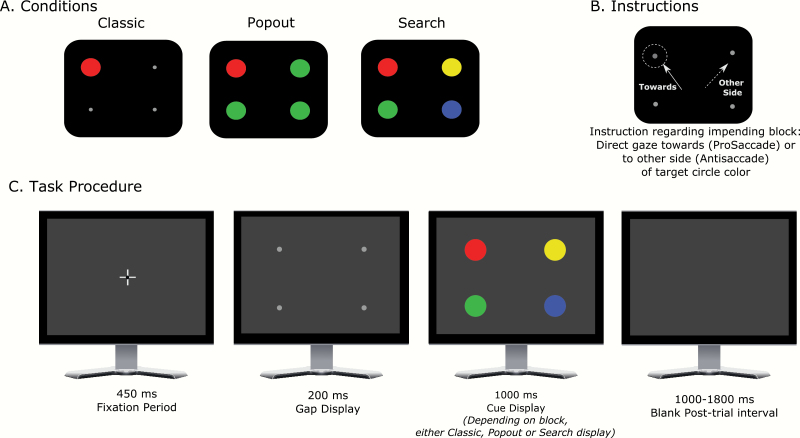
To better understand antisaccade deficits in schizophrenia, we developed new variants of the antisaccade paradigm that manipulate bottom-up salience of the cue while holding constant its task relevance. Here, we define bottom-up salience as the physical property of the stimulus that makes it stand out, whereas attentional relevance is determined by the task instructions. As illustrated in figure 1, we altered the traditional design by using 4 potential cue locations (one in each visual quadrant) rather than 2 cue locations on the horizontal midline. In the condition approximating the traditional antisaccade task (the classic condition), a single cue stimulus was presented at one of the 4 potential locations, and participants were instructed to saccade to the horizontal mirror-image location. In this condition, the cue not only had strong bottom-up salience but also had attentional relevance in terms of guiding the eye movement direction. We expected higher error rates in PSZ than HCS, resembling the broader literature.
Fig. 1.
Task conditions and procedure. (A) In one condition, a single cue stimulus was presented at one of the 4 locations indicated. In another “pop-out” condition, colored disks appeared in each of the 4 locations, with 3 disks of one color and 1 disk of another color (either red or green and vice-versa)—in this example, red is the cue. In the “search” condition, all 4 locations had an equiluminant colored disk, but only one color was relevant—here, the red disk is the cue. For half of the participants within each group, the cue disk was red, and for the other half, it was green. The cue location was selected randomly on each trial. (B) At the beginning of each block, participants viewed an instruction screen indicating the condition (classic, pop-out, or search) and whether they should look toward the cue (pro-saccade) or to the mirror-image location (anti-saccade). (C) Each trial began with the appearance of the fixation point. Once the participant maintained gaze on the fixation point for 450 ms, it disappeared and a gap display consisting of location markers at each of the 4 locations appeared. After 200 ms, the gap display was replaced by the cue display (consisting of one cue and 0 or 3 distractors depending on the condition: in this example, the search condition is displayed). This was presented for 1000 ms, during which participants were required to shift their gaze toward or away from the cue location, followed by a blank post-trial interval of 1000–1800 ms.
The other 2 conditions used the same cue but included distractors at the other 3 locations. The cue’s salience is a function of the combination of properties of the cue and other items in the display,24 so adding distractors effectively manipulated salience of the cue. Moreover, this approach does not require changing the cue’s physical properties and, therefore, avoids confounding salience of the cue with its other properties.
In the pop-out condition, all distractors shared a single, dissimilar color from the cue color, causing the cue to “pop out” from the display. As in the classic condition, participants were instructed to make an eye movement to the horizontal mirror-image location. Color pop-outs automatically produce an attentional priority signal25 but do not capture attention as strongly as the sudden-onset cue in the classic antisaccade task.26 Thus, in this condition, the cue has a moderate bottom-up salience and a strong attentional relevance.
In the search condition (figure 1C), each of the 3 distractors was a different color, and the task was again to make an eye movement to the horizontal mirror-image location. Here, the cue not only had the same bottom-up salience as the distractors but also had strong attentional relevance. Note that classic, pop-out, and search conditions mirror the most widely used manipulations of salience in basic cognitive science literature (ie, onsets, popouts/singletons, and non-popout search; see reviews 27,28). These conditions may also differ in factors other than salience, but these differences would be present for the pro- and anti-saccade versions of the conditions.
Because the cue had the same attentional relevance in all 3 conditions but varied in salience, this design facilitates contrasting 2 competing hypotheses about the origins of the SZ antisaccade deficit. If the deficit is caused by an inability to overcome the bottom-up salience of the cue, then PSZ should exhibit minimal impairment (relative to HCS) in the search condition, modest impairment in the pop-out condition, and a large impairment in the classic condition. However, if the deficit is a result of failure to suppress the relevance-driven activation of the cue (or by an overcommitment of covert attention to the cue), then the deficit should be as large (and perhaps even larger) in the search than in the classic and pop-out conditions.
Performing tasks such as these involves 2 phases, a search phase in which the cue is located and a response phase in which the saccade is programmed and executed. The classic, popout, and search conditions differed in the difficulty of the search phase but were identical in terms of the response phase. That is, both versions of a given condition required finding the cue within physically identical displays, and subjects needed to either program a saccade toward or away from this location. Given that some (but not all) prior research has shown that visual search processes are impaired in PSZ,29–33 one might expect that PSZ would be slower than HCS to find the cue and this would be exaggerated in the more difficult conditions. However, the difficulty of finding the cue should be identical in the pro- and anti-saccade versions of a given condition. Thus, we can factor out the differences in difficulty across conditions by examining the difference in performance between the pro- and anti-saccade versions of a given condition.
Methods
Participants
We used eye-tracking methods to test 43 outpatients from the Maryland Psychiatric Research Center and other outpatient clinics meeting criteria for schizophrenia or schizoaffective disorder (PSZ) and 34 nonpsychiatric control subjects (HCS). Consensus diagnosis was established using the Structured Clinical Interview for DSM-IV.34Table 1 summarizes participant characteristics.
Table 1.
Participant Characteristics
| HCS (N = 34) |
PSZ (N = 43) |
Statistic | P-value | |
|---|---|---|---|---|
| Age | 38.32 (10.48) | 36.63 (9.64) | t = 0.74 | .46 |
| Gender (M | F) | 20 | 14 | 27 | 16 | φ = 0.13 | .72 |
| Race (African American | Caucasian | Other) | 14 | 15 | 5 | 20 | 20 | 3 | φ = 1.24 | .54 |
| Participant education | 16.21 (2.28) | 13.47 (2.35) | t = 5.14 | <.001 |
| Maternal education | 14.24 (3.53) | 14.67 (3.03) | t = −0.57 | .57 |
| Paternal education | 14.03 (4.63) | 14.26 (3.91) | t = −0.23 | .82 |
| Neurocognitive test resultsa | ||||
| WASI-II IQ | 112.24 (10.34) | 95.03 (14.61) | t = 5.69 | <.001 |
| WRAT 4 | 111.62 (13.76) | 99.05 (14.77) | t = 3.82 | <.001 |
| WTAR | 113.65 (10.77) | 100.93 (17.03) | t = 3.79 | <.001 |
| MD processing speed | 55.88 (7.66) | 41.31 (12.27) | t = 5.11 | <.001 |
| MD attention vigilance | 52.17 (8.37) | 44.91 (11.4) | t = 2.63 | .011 |
| MD working memory | 56.08 (9.78) | 41.34 (11.54) | t = 5.04 | <.001 |
| MD verbal learning | 50.21 (8.78) | 37.91 (8.72) | t = 5.21 | <.001 |
| MD visual learning | 49.08 (8.3) | 37.19 (11.99) | t = 4.16 | <.001 |
| MD reasoning | 53 (9.28) | 43.63 (9.07) | t = 3.79 | <.001 |
| MD social cognition | 50.25 (10.9) | 40.5 (10.16) | t = 3.45 | .001 |
| MCT overall | 53.38 (7.31) | 35.34 (12.37) | t = 6.35 | <.001 |
| Visual WM capacity (K) from change localization task | 2.94 (0.27) | 2.43 (0.53) | t = 4.34 | <.001 |
| Overall d′ from 12-AX-CPT task | 3.64 (0.58) | 2.69 (0.68) | t = 5.45 | <.001 |
| Antipsychotic medication | ||||
| Antipsychotic medication (atypical | typical) | 34 | 9 | |||
| Total CPZ equiv | 572.95 (330.63) | |||
| Other psychotropic medicationb | ||||
| Mood stabilizers + anxiolytics + antidepressants | 3 | |||
| Antidepressants + anxiolytics | 6 | |||
| Mood stabilizers + antidepressants | 4 | |||
| Anxiolytics | 8 | |||
| Mood stabilizers | 1 | |||
| Antidepressants | 9 | |||
| Clinical ratings | ||||
| BPRS total | 33.38 (8.75) | |||
| SANS total | 22.38 (9.93) |
Note: WASI, Wechsler Abbreviated Scale of Intelligence; WRAT, Wide Range Achievement Test; WTAR, Wechsler Test of Adult Reading; MD, MCCB (MATRICS Consensus Cognitive Battery) Cognitive Domain; MCT, MCCB Composite Total; WM, Working Memory; d′, D-prime; CPZ, Chlorpromazine equivalent; BPRS, Brief Psychiatric Rating Scale; SANS, Scale for the Assessment of Negative Symptoms.
aMeasures of these variables were available in 32 out of 43 PSZ and 24 out of 34 HCS.
bOut of 43 PSZ, 31 were also receiving other psychotropic medications(in addition to antipsychotics) as indicated.
Stimuli and Procedure
The task is illustrated in figure 1 (details on methods are provided in supplementary material).
Analysis
Data processing and analyses details are provided in supplementary material. For each condition (Classic/Popout/Search) and direction (Pro/Anti), we derived the directional error rate and saccade latency as the main outcome measures. Table 2 contains ANOVA results.
Table 2.
Statistics
| A.ERROR RATES | F | p | η²p |
|---|---|---|---|
| (I)PROSACCADE ERROR RATES | |||
| Condition | 36.46 | < .001GG | .33 |
| Condition X Group | 0.27 | .72GG | .004 |
| Group | 1.85 | .18 | .02 |
| (II)ANTISACCADE ERROR RATES | |||
| Condition | 169.32 | < .001 GG | .69 |
| Condition X Group | 7.21 | .004 GG | .09 |
| Group | 53.92 | < .001 | .42 |
| (III)3-WAY ANOVA FOR ERROR RATES | |||
| Direction | 1006.75 | < .001 | .93 |
| Direction X Group | 58.32 | < .001 | .44 |
| Condition | 155.59 | < .001 GG | .68 |
| Condition X Group | 2.93 | .07 GG | .038 |
| Direction X Condition | 15.29 | < .001 GG | .17 |
| Direction X Condition X Group | 3.38 | .047 GG | .04 |
| Group | 45.76 | < .001 | .38 |
| B.SACCADIC LATENCIES | F | p | η²p |
| (I)PROSACCADE LATENCIES | |||
| Condition | 168.10 | < .001 | .70 |
| Condition X Group | 0.42 | .66 | .006 |
| Group | 24.61 | < .001 | .25 |
| (II)ANTISACCADE LATENCIES | |||
| Condition | 225.16 | < .001 GG | .75 |
| Condition X Group | 3.51 | .038 GG | .05 |
| Group | 21.05 | < .001 | .22 |
| (III)3-WAY ANOVA FOR LATENCIES | |||
| Direction | 316.13 | < .001 | .81 |
| Direction X Group | 5.81 | .02 | .07 |
| Condition | 406.04 | < .001 GG | .84 |
| Condition X Group | 1.121 | .33 GG | .015 |
| Direction X Condition | 17.78 | < .001 GG | .19 |
| Direction X Condition X Group | 3.37 | .04 GG | .04 |
| Group | 27.27 | < .001 | .27 |
| (IV)ANTISACCADE LATENCIES FOR ERROR TRIALS | |||
| Condition | 118.17 | < .001 GG | .61 |
| Condition X Group | 1.41 | .25 GG | .019 |
| Group | 0.42 | .52 | .006 |
GG The Greenhouse-Geisser correction was applied in ANOVAs to adjust for lack of sphericity, and the reported p-values reflect this correction.
Results
Error Rates
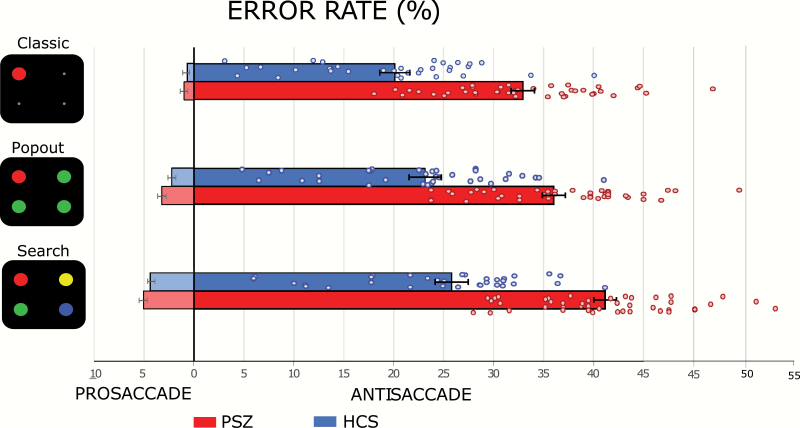
Figure 2 displays error rates
Fig. 2.
Error rates for each condition. The mean percent of errors for prosaccade trials are displayed on the left side and those for antisaccade trials are presented on the right side. Error bars indicate the standard error of the mean.
Prosaccade Error Rates
Prosaccade error rates were lowest in the classic condition, higher in the popout condition, and highest in the search condition (significant main effect of Condition, table 2A[I]), indicating that the cue was most salient in the classic condition and least salient in the search condition. Prosaccade error rates were slightly higher for PSZ than for HCS but the main effect of Group and Condition × Group interaction were not significant. Thus, variations in search difficulty across conditions had similar effects on PSZ and HCS.
Antisaccade Error Rates
Antisaccade error rates were lowest in the classic condition, higher in the pop-out condition, and highest in the search condition (significant main effect of Condition, table 2A[II]). Antisaccade error rates were much higher in PSZ than in HCS, as confirmed by a significant main effect of Group. The difference in antisaccade errors between groups was most pronounced in the search condition, leading to a significant Condition by Group interaction. The proportion of PSZ exhibiting high error rates was relatively similar across the 3 antisaccade conditions (figure 2).
To decompose this interaction, we calculated difference scores between the classic and popout conditions and between the classic and search conditions for each participant. We used t-tests (not assuming equal variances) to compare these difference scores. We found that the mean antisaccade error rate increased from the classic condition to the popout condition by approximately the same amount in PSZ (3.10 ± 1.73) and HCS (3.03 ± 2.62), with no significant difference between groups (t = 0.13, P = .90, Cohen’s d = 0.03). In contrast, the increase from the classic condition to the search condition was significantly greater in PSZ (8.24 ± 4.29) than in HCS (5.71 ± 3.89; t = 2.68, P = .009, Cohen’s d = 0.62). These results provide no evidence that the antisaccade impairment in schizophrenia is driven by the physical salience of the cue. Indeed, impairment was largest in the condition in which the cue had no bottom-up advantage over the other locations.
The pattern of results across the 3 antisaccade conditions was clearly different from the pattern across the 3 prosaccade conditions, with a large impairment in PSZ relative to HCS for the antisaccade tasks but not for the prosaccade tasks. This was confirmed by a 3-way ANOVA with factors of Group, Condition, and Direction (table 2A[III]). The larger patient impairment in the antisaccade variants led to a significant Group × Direction interaction. The fact that antisaccade impairment was particularly strong for the search condition led to a significant Group × Condition × Direction interaction.
Corrective Saccades
To determine whether greater antisaccade error rate in PSZ might reflect a failure of task comprehension, we examined corrective saccades after antisaccade errors. Majority of erroneous antisaccades were corrected in both groups (HCS: 89.2 ± 20.9%; PSZ: 88.0 ± 19.0%), with no significant main effect of Condition (F1,75 = 1.61, P = .20), Group (F1,75 = 0.17, P = .68), or Condition × Group interaction (F1,75 = 0.22, P = .80). Thus, both groups understood and were motivated to perform the tasks.
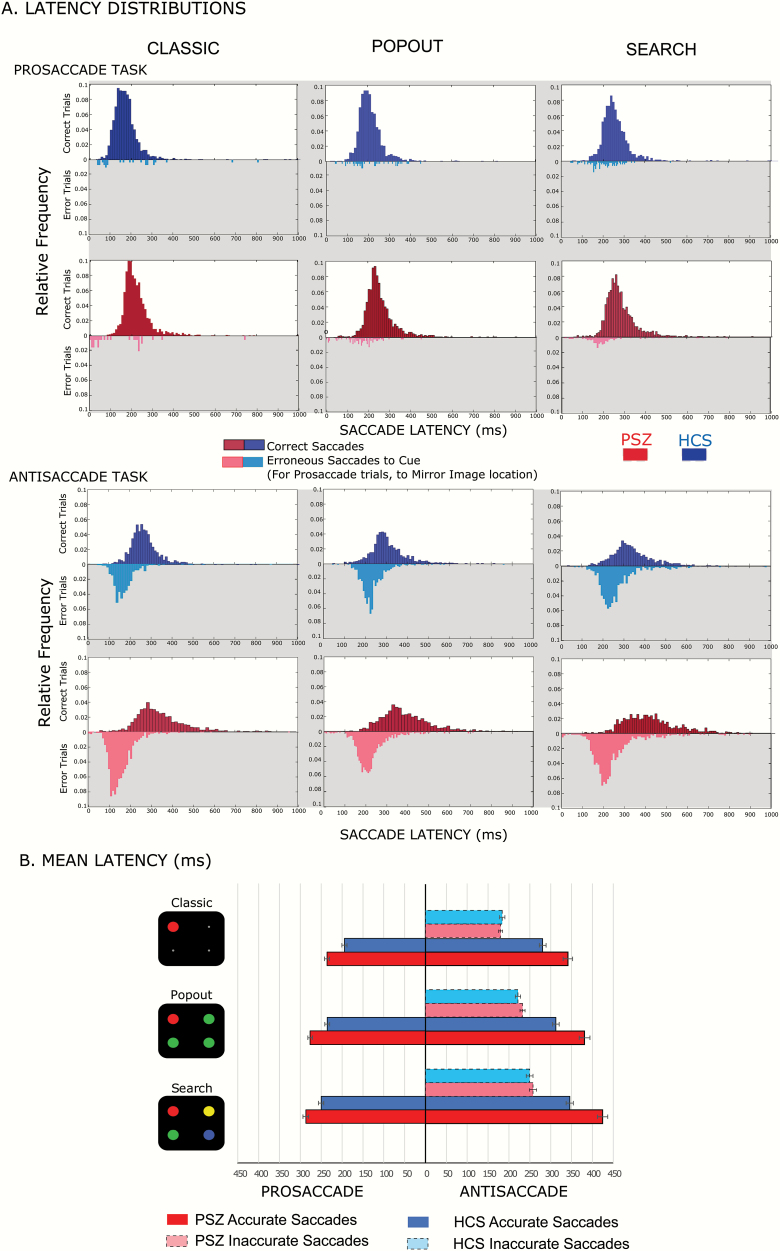
Saccade Latencies
Figure 3A displays saccade latency distributions for correct and error responses in pro-saccade and anti-saccade versions of the 3 conditions. Latencies were clearly shorter in the prosaccade versions in both groups.
Fig. 3.
(A) Latency distributions. Histograms represent relative frequency distributions of saccade latencies prosaccades (top panel) and antisaccades (bottom panel). Correct latencies are represented by darker colored histograms in the upper half of each plot, while erroneous latencies are plotted as vertically flipped histograms in lighter shading. Blue histograms represent data for HCS and red for PSZ. (B) Mean latencies for the 6 task combinations. The mean latencies for correct prosaccade trials are displayed on the left side and those for correct and incorrect antisaccade trials are presented on the right side of the figure. Bars displayed in lighter shading are mean latencies for erroneous saccades made to the cue instead of the mirror location.
Figure 3B shows mean saccade latencies on correct trials for both pro- and antisaccade versions across conditions, along with mean saccade latencies on error trials for the antisaccade versions; prosaccade errors were too rare to provide meaningful latency data. Prosaccade latencies were fastest in the classic condition, slower in the pop-out condition, and slowest for the search condition (table 2B[I], main effect of Condition), additional evidence that our manipulation of cue saliency was effective. Prosaccade latencies were slower in PSZ than in HCS (significant main effect of Group), but PSZ and HCS were similarly impacted by condition (nonsignificant Group × Condition interaction). Thus, variations in search difficulty across the 3 conditions had similar effects on PSZ and HCS in the prosaccade versions of the conditions.
Analogous analyses were performed for correct antisaccade trials (table 2B[II]). These antisaccade latencies were slower in PSZ than in HCS (main effect of Group) and were fastest in the classic condition, slower in the pop-out condition, and slowest for the search condition (significant main effect of Condition). The slowing in PSZ relative to HCS was most pronounced in the search condition (significant Group × Condition interaction).
In a single ANOVA with factors of Group, Condition, and Direction (table 2B[III]), we found that saccade latencies on correct trials were slower in the antisaccade than in prosaccade versions of the tasks (main effect of Direction), that the magnitude of this slowing varied across the classic, popout, and search tasks (significant Condition × Direction effect), and that this slowing was more prominent in PSZ than in HCS (significant Group × Direction interaction). The slowing for PSZ relative to HCS in antisaccade versions was exaggerated in the search condition, leading to a significant Group × Condition × Direction interaction.
To decompose this interaction, we obtained difference scores (for correct antisaccade latencies) between the classic and popout conditions and between the classic and search conditions. PSZ and HCS were slowed approximately equally in the popout condition relative to the classic condition (Popout-Classic difference scores: HCS: 31.37 ± 23.20; PSZ: 40.05 ± 33.18, Welch’s t(73.99) = 01.35, P = .18, Cohen’s d = 0.30). However, PSZ were significantly more slowed than HCS in the search condition compared with the classic condition (Search-Classic latency difference scores, HCS: 64.73 ± 29.80; PSZ: 83.19 ± 38.96, Welch’s t(74.93) = 2.36, P = .03, Cohen’s d = 0.52). Thus, in PSZ, it took much longer to suppress the relevance-driven activation of the cue, and it was equally, if not more difficult to localize and disengage from the cue when other distractors of equal salience were present than when the cue had a bottom-up salience advantage.
We also analyzed antisaccade latencies for error trials (light-shaded bars, figure 3B) in a 2-way ANOVA with factors of Group and Condition (table 2B[IV]). As for correct trials, mean antisaccade latencies on error trials were fastest in the classic condition, slower in the popout condition, and slowest in the search condition, leading to a significant main effect of Condition. However, latencies were similar in PSZ and HCS (nonsignificant main effect of Group and Group × Condition interaction).
Discussion
Nature of the Antisaccade Deficit in Schizophrenia
The present results indicate that antisaccade impairment in PSZ arises primarily because of difficulty in overcoming the top-down task relevance of the cue rather than by a deficit in overcoming its bottom-up, physical salience. We compared 3 variants of the task in which top-down relevance of the cue was constant and bottom-up salience varied, and also found that PSZ were impaired relative to HCS regardless of the presence of bottom-up attentional capture by the singleton cue (classic and pop-out conditions) or absence of such capture (search condition) indicating that this impairment is not purely driven by bottom-up physical salience but is instead driven by the task relevance of the cue. This is consistent with studies of college students in which difficulty of antisaccade performance increases as top-down attentional demands of cue discrimination increase.22,23
These findings are compatible with the broader idea that antisaccade task impairments reflect inhibitory control impairments.18,19That is, participants must exert considerable control to avoid fixating the task-relevant cue in all versions of the antisaccade task examined here. The results are also compatible with the recently proposed hyperfocusing hypothesis, which posits that schizophrenia is characterized by an unusually narrow but intense focusing of processing resources.35,36 Correct antisaccade performance presumably requires a precise titration of covert attention: Some attention must be allocated to the cue so that the correct antisaccade location can be determined, but an overcommitment of covert attention to the cue may trigger an eye movement to it. Thus, increased antisaccade error rates could reflect a more intense focusing of attention on the cue (leading to activation of the motor program for fixating the cue) rather than a deficit in inhibition per se. Indeed, a recent computational modeling study concluded that the antisaccade deficit in PSZ does not reflect a failure of inhibition but instead reflects exaggerated competition between prosaccade and antisaccade representations.37 However, there is no way to determine whether the present effects reflect hyperfocusing, a failure of inhibitory control, or both. This is an important question for future research.
Common Mechanisms Across Conditions
Our conclusions regarding the antisaccade deficit in PSZ presume that all versions of the antisaccade task tap into the same underlying mechanisms. If this assumption were incorrect, one could argue that the deficit exhibited by PSZ in the classic condition reflected the need to overcome the bottom-up salience of the cue, whereas the deficit in the search version reflected the need to overcome top-down attentional relevance of the cue. However, given the overall pattern of results, it is much more parsimonious to assume that the deficit in PSZ is driven by the same set of mechanisms in all 3 tasks.
To a first order of approximation, the only difference in results among conditions was the overall performance level. In both prosaccade and antisaccade versions of these tasks, the difficulty of finding the cue increased from the classic task to the popout task to the search task—error rates and saccadic latencies increased progressively across these 3 conditions. Both the pro- and anti-saccade versions of each condition required finding the cue and then programming the appropriate saccade, so that differences in task difficulty across conditions would have had equivalent impacts on prosaccade and antisaccade performance. Moreover, differences in search difficulty across conditions had similar impacts on prosaccade performance in PSZ and HCS. In addition, traditional hallmarks of antisaccade performance were present in antisaccade versions of all conditions: both error rates and saccadic latencies were increased in the antisaccade version of a given task compared with the corresponding prosaccade version, and saccadic reaction times were faster on error trials than on correct trials in the antisaccade versions. The decrement in performance in the antisaccade version relative to the prosaccade version of a given condition tended to increase slightly from the classic version to the search version, as did the magnitude of the antisaccade deficit in PSZ relative to HCS. However, these differences between conditions were small relative to the overall difference in performance between the pro- and anti-saccade versions within a condition.
The pattern of correlations was also consistent with the hypothesis that all 3 conditions tap into a common set of underlying processes. For both groups, both error rates and saccadic latencies were highly correlated across the classic, popout, and search conditions (especially for antisaccade versions, where most correlations were >0.8; supplementary table S1). In addition, correlations between antisaccade error rates and independent measures of executive control and WM were quite similar for the classic, popout, and search conditions (supplementary table S2). Previous studies using classic antisaccade tasks in PSZ have found that antisaccade performance correlated with measures of the overall cognitive ability, WM capacity, and executive function.38–41 Although a formal analysis of latent variables would require a larger sample size and a broader set of tasks, our results provide no reason to suspect that the 3 antisaccade tasks involve different underlying processes.
Impaired Inhibitory Function and Attention
The similarity between deficits found in the 3 versions of the antisaccade task suggests that they arise from the same disruption to neurocognitive networks in schizophrenia. Antisaccade performance impairments in SZ in our task are consistent with prior studies.9,42–44 Neural disruptions underlying these impairments in PSZ most consistently include under-activation of the dlPFC, a key region for cognitive control and WM.7,8,41,45 Similar to dlPFC lesion patients, PSZ may have a reduced ability to suppress the activity of saccade neurons in the frontal eye fields and superior colliculus on anti-saccade trials and a reduction in the rate of accumulation of activity for the correct anti-saccade.5 Consistent with our hyperfocusing formulation, it is likely that the posterior parietal cortex (PPC) is also implicated given its role in covert and overt visuospatial attention.46 Evidence from monkey neurophysiology, human functional neuroimaging, and focal lesion patients indicate that the PPC plays a fundamental role in directing attention to different locations in space.47–54 Studies examining presaccadic event-related potentials in human subjects also support the involvement of frontoparietal areas in antisaccade performance. Larger negative potentials at fronto-central and central sites, likely reflecting preparatory activity, have been observed prior to the execution of correct antisaccades compared with prosaccades and antisaccade errors.55–57 Critically, in the antisaccade task, PPC plays a role in both attentional disengagement from the cue and computation of the antisaccade vector.58 Our results suggest that deficits in disengaging from the cue may be critical for impairments observed in PSZ.
Conclusions
The antisaccade task is an important research tool to assay cognitive control. However, the classic version confounds bottom-up salience and attentional relevance of the cue, thereby clouding the interpretation of observed deficits. The present experimental approach allows for a more nuanced understanding of mechanisms underlying antisaccade deficits in PSZ. Moreover, these results open up the possibility of a different explanation of the antisaccade deficit: Rather than reflecting inhibitory control impairment, this deficit may instead (or in addition) be caused by a hyperfocusing of attention on the cue. The search version of the antisaccade task may serve as a valuable tool for understanding specific mechanisms underlying impaired cognitive performance in schizophrenia and other disorders.
Supplementary Material
Acknowledgment
The authors report no conflicts of interest.
Funding
This study was supported by the National Institutes of Health grant (R01MH065034 to J.M.G. and S.J.L.).
References
- 1. Levin S. Frontal lobe dysfunctions in schizophrenia–I. Eye movement impairments. J Psychiatr Res. 1984;18(1):27–55. [DOI] [PubMed] [Google Scholar]
- 2. Barch DM, Carter CS, Braver TS, et al. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58(3):280–288. [DOI] [PubMed] [Google Scholar]
- 3. Luck SJ, Gold JM. The construct of attention in schizophrenia. Biol Psychiatry. 2008;64(1):34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology 2011;36(1):316–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5(3):218. [DOI] [PubMed] [Google Scholar]
- 6. Hallett PE, Adams BD. The predictability of saccadic latency in a novel voluntary oculomotor task. Vision Res. 1980;20(4):329–339. [DOI] [PubMed] [Google Scholar]
- 7. Everling S, Fischer B. The antisaccade: a review of basic research and clinical studies. Neuropsychologia 1998;36(9):885–899. [DOI] [PubMed] [Google Scholar]
- 8. McDowell JE, Brown GG, Paulus M, et al. Neural correlates of refixation saccades and antisaccades in normal and schizophrenia subjects. Biol Psychiatry. 2002;51(3):216–223. [DOI] [PubMed] [Google Scholar]
- 9. Fukushima J, Fukushima K, Chiba T, Tanaka S, Yamashita I, Kato M. Disturbances of voluntary control of saccadic eye movements in schizophrenic patients. Biol Psychiatry. 1988;23(7):670–677. [DOI] [PubMed] [Google Scholar]
- 10. Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology 2006;43(3):302–313. [DOI] [PubMed] [Google Scholar]
- 11. Manoach DS, Lindgren KA, Cherkasova MV, et al. Schizophrenic subjects show deficient inhibition but intact task switching on saccadic tasks. Biol Psychiatry. 2002;51(10):816–826. [DOI] [PubMed] [Google Scholar]
- 12. Radant AD, Dobie DJ, Calkins ME, et al. Successful multi-site measurement of antisaccade performance deficits in schizophrenia. Schizophr Res. 2007;89(1-3):320–329. [DOI] [PubMed] [Google Scholar]
- 13. Sereno AB, Holzman PS. Antisaccades and smooth pursuit eye movements in schizophrenia. Biol Psychiatry. 1995;37(6):394–401. [DOI] [PubMed] [Google Scholar]
- 14. Guitton D, Buchtel HA, Douglas RM. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp Brain Res. 1985;58(3):455–472. [DOI] [PubMed] [Google Scholar]
- 15. Pierrot-Deseilligny CH, Ploner CJ, Müri RM, Gaymard B, Rivaud-Pechoux S. Effects of cortical lesions on saccadic; eye movements in humans. Ann New York Acad Sci. 2002;956(1):216–229. [DOI] [PubMed] [Google Scholar]
- 16. Pierrot-Deseilligny C, Müri RM, Ploner CJ, Gaymard B, Demeret S, Rivaud-Pechoux S. Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain 2003;126(6):1460–1473. [DOI] [PubMed] [Google Scholar]
- 17. Walker R, Husain M, Hodgson TL, Harrison J, Kennard C. Saccadic eye movement and working memory deficits following damage to human prefrontal cortex. Neuropsychologia 1998;36(11):1141–1159. [DOI] [PubMed] [Google Scholar]
- 18. Clementz BA. Psychophysiological measures of (dis)inhibition as liability indicators for schizophrenia. Psychophysiology 1998;35(6):648–668. [PubMed] [Google Scholar]
- 19. Crawford TJ, Bennett D, Lekwuwa G, Shaunak S, Deakin JF. Cognition and the inhibitory control of saccades in schizophrenia and Parkinson’s disease. Prog Brain Res. 2002;140:449–466. [DOI] [PubMed] [Google Scholar]
- 20. Dyckman KA, Lee AK, Agam Y, et al. Abnormally persistent fMRI activation during antisaccades in schizophrenia: a neural correlate of perseveration? Schizophr Res. 2011;132(1):62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Itti L, Koch C. A saliency-based search mechanism for overt and covert shifts of visual attention. Vision Res. 2000;40(10-12):1489–1506. [DOI] [PubMed] [Google Scholar]
- 22. Godijn R, Kramer AF. Prosaccades and antisaccades to onsets and color singletons: evidence that erroneous prosaccades are not reflexive. Exp Brain Res. 2006;172(4):439–448. [DOI] [PubMed] [Google Scholar]
- 23. Godijn R, Kramer AF. The effect of attentional demands on the antisaccade cost. Percept Psychophys. 2008;70(5): 795–806. [DOI] [PubMed] [Google Scholar]
- 24. Itti, L, Koch C, Niebur E. A model of saliency-based visual attention for rapid scene analysis. IEEE Trans Pattern Anal Mach Intell. 1998;20(11):1254–1259. [Google Scholar]
- 25. Gaspelin N, Luck SJ. The role of inhibition in avoiding distraction by salient stimuli. Trends Cogn Sci. 2018;22(1):79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jonides J, Yantis S. Uniqueness of abrupt visual onset in capturing attention. Percept Psychophys. 1988;43(4):346–354. [DOI] [PubMed] [Google Scholar]
- 27. Egeth HE, Yantis S. Visual attention: control, representation, and time course. Annu Rev Psychol. 1997;48:269–297. [DOI] [PubMed] [Google Scholar]
- 28. Folk C. Controlling spatial attention: lessons from the lab and implications for everyday life. In: Fawcett J, Risko E, Kingstone A, eds. The Handbook of Attention. Cambridge, MA: MIT Press; 2015:3–25. [Google Scholar]
- 29. Carr VJ, Dewis SA, Lewin TJ. Preattentive visual search and perceptual grouping in schizophrenia. Psychiatry Res. 1998;79(2):151–162. [DOI] [PubMed] [Google Scholar]
- 30. Mori S, Tanaka G, Ayaka Y, et al. Preattentive and focal attentional processes in schizophrenia: a visual search study. Schizophr Res. 1996;22(1):69–76. [DOI] [PubMed] [Google Scholar]
- 31. Bansal S, Robinson BM, Leonard CJ, Hahn B, Luck SJ, Gold JM. Failures in top-down control in schizophrenia revealed by patterns of saccadic eye movements. J Abnorm Psychol. 2019;128(5):415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fuller RL, Luck SJ, Braun EL, Robinson BM, McMahon RP, Gold JM. Impaired control of visual attention in schizophrenia. J Abnorm Psychol. 2006;115(2):266–275. [DOI] [PubMed] [Google Scholar]
- 33. Gold JM, Robinson B, Leonard CJ, et al. Selective attention, working memory, and executive function as potential independent sources of cognitive dysfunction in schizophrenia. Schizophr Bull. 2018;44(6):1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. First MB, Spitzer RL, Gibbon M, Williams JB. User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders SCID-I; Clinician Version. Washington, DC: American Psychiatric Pub; 1997. [Google Scholar]
- 35. Luck SJ, McClenon C, Beck VM, et al. Hyperfocusing in schizophrenia: evidence from interactions between working memory and eye movements. J Abnorm Psychol. 2014;123(4):783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luck SJ, Hahn B, Leonard CJ, Gold JM. The hyperfocusing hypothesis: a new account of cognitive dysfunction in schizophrenia. Schizophr Bull. 2019;45(5):991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cutsuridis V, Kumari V, Ettinger U. Antisaccade performance in schizophrenia: a neural model of decision making in the superior colliculus. Front Neurosci. 2014;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Radant AD, Claypoole K, Wingerson DK, Cowley DS, Roy-Byrne PP. Relationships between neuropsychological and oculomotor measures in schizophrenia patients and normal controls. Biol Psychiatry. 1997;42(9):797–805. [DOI] [PubMed] [Google Scholar]
- 39. Nieman DH, Bour LJ, Linszen DH, et al. Neuropsychological and clinical correlates of antisaccade task performance in schizophrenia. Neurology 2000;54(4):866–871. [DOI] [PubMed] [Google Scholar]
- 40. Hutton SB, Huddy V, Barnes TR, et al. The relationship between antisaccades, smooth pursuit, and executive dysfunction in first-episode schizophrenia. Biol Psychiatry. 2004;56(8):553–559. [DOI] [PubMed] [Google Scholar]
- 41. Levy DL, Mendell NR, Holzman PS. The antisaccade task and neuropsychological tests of prefrontal cortical integrity in schizophrenia: empirical findings and interpretative considerations. World Psychiatry. 2004;3(1):32–40. [PMC free article] [PubMed] [Google Scholar]
- 42. Crawford TJ, Haeger B, Kennard C, Reveley MA, Henderson L. Saccadic abnormalities in psychotic patients. I. Neuroleptic-free psychotic patients. Psychol Med. 1995;25(3):461–471. [DOI] [PubMed] [Google Scholar]
- 43. Maruff P, Danckert J, Pantelis C, Currie J. Saccadic and attentional abnormalities in patients with schizophrenia. Psychol Med. 1998;28(5):1091–1100. [DOI] [PubMed] [Google Scholar]
- 44. Kleineidam L, Frommann I, Ruhrmann S, et al. Antisaccade and prosaccade eye movements in individuals clinically at risk for psychosis: comparison with first-episode schizophrenia and prediction of conversion. Eur Arch Psychiatry Clin Neurosci. 2019;269(8):921–930. [DOI] [PubMed] [Google Scholar]
- 45. Thomas EH, Rossell SL, Myles JB, et al. Working memory and attention influence antisaccade error rate in schizophrenia. J Int Neuropsychol Soc. 2008;25(2):174–183. [DOI] [PubMed] [Google Scholar]
- 46. Constantinidis C. Posterior parietal mechanisms of visual attention. Rev Neurosci. 2006;17(4):415–427. [DOI] [PubMed] [Google Scholar]
- 47. Gottlieb J, Goldberg ME. Activity of neurons in the lateral intraparietal area of the monkey during an antisaccade task. Nat Neurosci. 1999;2(10):906–912. [DOI] [PubMed] [Google Scholar]
- 48. Ojeda N, Ortuño F, Arbizu J, et al. Functional neuroanatomy of sustained attention in schizophrenia: contribution of parietal cortices. Hum Brain Mapp. 2002;17(2):116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Batista AP, Andersen RA. The parietal reach region codes the next planned movement in a sequential reach task. J Neurophysiol. 2001;85(2):539–544. [DOI] [PubMed] [Google Scholar]
- 50. Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical independent or overlapping neural systems? Proc Nat Acad Sci U S A. 1998;95(3):831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Curr Opin Neurobiol. 2001;11(2):157–163. [DOI] [PubMed] [Google Scholar]
- 52. Husain M, Rorden C. Non-spatially lateralized mechanisms in hemispatial neglect Nat Rev Neurosci 2003;4(1):26. [DOI] [PubMed] [Google Scholar]
- 53. Kanwisher N, Wojciulik E. Visual attention: insights from brain imaging. Nat Rev Neurosci. 2000;1(2):91. [DOI] [PubMed] [Google Scholar]
- 54. Yantis S, Schwarzbach J, Serences JT, et al. Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci. 2002;5(10):995–1002. [DOI] [PubMed] [Google Scholar]
- 55. Everling S, Spantekow A, Krappmann P, Flohr H. Event-related potentials associated with correct and incorrect responses in a cued antisaccade task. Exp Brain Res. 1998;118(1):27–34. [DOI] [PubMed] [Google Scholar]
- 56. Klein C, Heinks T, Andresen B, Berg P, Moritz S. Impaired modulation of the saccadic contingent negative variation preceding antisaccades in schizophrenia. Biol Psychiatry. 2000;47(11):978–990. [DOI] [PubMed] [Google Scholar]
- 57. Richards JE. Cortical sources of event-related potentials in the prosaccade and antisaccade task. Psychophysiology 2003;40(6):878–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Doricchi F, Perani D, Incoccia C, et al. Neural control of fast-regular saccades and antisaccades: an investigation using positron emission tomography. Exp Brain Res. 1997;116(1):50–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.