Abstract
Background
Sensorimotor abnormalities precede and predict the onset of psychosis. Despite the practical utility of sensorimotor abnormalities for early identification, prediction, and individualized medicine applications, there is currently no dedicated self-report instrument designed to capture these important behaviors. The current study assessed and validated a questionnaire designed for use in individuals at clinical high-risk for psychosis (CHR).
Methods
The current study included both exploratory (n = 3009) and validation (n = 439) analytic datasets—that included individuals identified as meeting criteria for a CHR syndrome (n = 84)—who completed the novel Sensorimotor Abnormalities and Psychosis-Risk (SMAP-R) Scale, clinical interviews and a finger-tapping task. The structure of the scale and reliability of items were consistent across 2 analytic datasets. The resulting scales were assessed for discriminant validity across CHR, community sample non-psychiatric volunteer, and clinical groups.
Results
The scale showed a consistent structure across 2 analytic datasets subscale structure. The resultant subscale structure was consistent with conceptual models of sensorimotor pathology in psychosis (coordination and dyskinesia) in both the exploratory and the validation analytic dataset. Further, these subscales showed discriminant, predictive, and convergent validity. The sensorimotor abnormality scales discriminated CHR from community sample non-psychiatric controls and clinical samples. Finally, these subscales predicted to risk calculator scores and showed convergent validity with sensorimotor performance on a finger-tapping task.
Conclusion
The SMAP-R scale demonstrated good internal, discriminant, predictive, and convergent validity, and subscales mapped on to conceptually relevant sensorimotor circuits. Features of the scale may facilitate widespread incorporation of sensorimotor screening into psychosis-risk research and practice.
Keywords: sensorimotor, psychosis, clinical high risk, dyskinesia, coordination/physical activity
Introduction
Several signs of sensorimotor dysfunction have been found to precede and predict the onset of psychosis.1–8 These sensorimotor abnormalities are tied to cognitive deficits, functional outcomes, and other core features of psychotic disorders.9–14 A growing body of structural,15–18 connective, and functional imaging results,19–21 also has found that sensorimotor features reflect important vulnerability and disease driving mechanism.11–13,22 Relatedly, sensorimotor abnormalities may be used to form distinct subtypes among individuals meeting criteria for a clinical high-risk (CHR) syndrome.19 Further, these features may also be useful in monitoring side effects,23 promoting treatment planning,24 and tracking treatment outcome.25 In addition to sensorimotor abnormalities, sedentary behavior and level of aerobic activity are also important risk/protective indicators for individuals at CHR.26–29 Despite the clear importance of sensorimotor abnormalities and activity, at the present time, there is no dedicated self-report measure for assessing these symptoms in psychosis-risk populations. Clinician rated scales, designed for other clinical populations (eg, slowing in depression) and purposes (eg, medication side-effect scales) can be applied, but this approach requires highly specialized training. Instrumental methods are sensitive, but typically involve fragile, cumbersome, and/or expensive equipment.25,30–35 As a result, current options are impractical for widespread clinical use. Additionally, extant self-report and clinical sensorimotor assessments tend to assess a narrow range of sensorimotor signs, but psychotic disorders are characterized by a number of conceptually distinct sensorimotor abnormalities.35,36
Research has suggested that sensorimotor abnormalities reflect an early vulnerability for psychosis. For example, studies of infants that later develop psychosis in adulthood found that there are elevated levels of coordination deficits, delays, and dyskinesias when compared with controls.3 In addition, a growing body of evidence has indicated that adolescents and young adults meeting criteria for a CHR syndrome exhibit several mechanistically distinct sensorimotor signs. Indeed, coordination deficits and delays in sensorimotor learning may be reflected in cerebellar circuit dysfunction,17,21,37 whereas slowing as well as hyperkinetic movements related to basal ganglia circuit pathology.15 Notably, these circuits are relevant to prominent conceptual theories of psychosis,22,38–41 and relatedly, both domains predicted worsening course,1,2,21 poor functional outcomes,42,43 and ultimately conversion to psychosis.1,36,44–46 Further, neuroimaging studies in this population have refined our understanding of neural underpinnings, as well as the links with disease-relevant mechanisms.9,15,22,47–58 With respect to physical activity, those meeting criteria for a CHR syndrome exhibit decreased levels of physical activity.26,28,29,59 Furthermore, sedentary activity has been linked to abnormalities in the hippocampus among other regions26,60–62 and relatedly, one open-label exercise intervention has shown evidence of increased cognition, reduced symptoms, and improved functional connectivity in the hippocampus in this population.27
Due to the predictive quality, conceptual relevance, and mechanistic ties, sensorimotor abnormalities and physical activity may be particularly useful to assess in those with a CHR syndrome. Despite its promise, sensorimotor abnormalities and physical activity have been largely under-utilized in the early assessment of psychosis risk; some assessments lack sensorimotor items altogether63,64 and others only include one65,66 or 2 self-report items.66,67 The current study is intended to offer initial validation of a novel (designed specifically for the CHR syndrome) and convenient (self-report) sensorimotor tool, the Sensorimotor Abnormalities and Psychosis-Risk (SMAP-R) Scale.
Methods and Materials
Participants
All participants were recruited as a part of a large, multisite community sample known as the Multisite Assessment of Psychosis-Risk study.68 The primary focus of the study was to evaluate markers of risk for psychosis in a large, representative community sample across multiple study sites. Study sites included the greater catchment areas of Philadelphia (Temple University), Chicago (Northwestern University), and Baltimore (University of Maryland, Baltimore County). Recruitment occurred through various outlets, including ads on various Internet sites (eg, Craigslist, Facebook), student volunteer pools, refer-a-friend links, and flyers. Recruitment centered on non-clinical sources; therefore, no recruitment took place at clinical locations such as outpatient psychiatric clinics and hospitals in an attempt to keep the community sample relatively unbiased.
MAP Study—Screening Phase
The study contained 2 phases (figure 1). The first phase was completed by all 3448 participants and included an online battery of measures, including established psychosis risk questionnaires—eg, the prodromal questionnaire63 (PQ), the PRIME Screen65—and a variety of other questionnaires, including the SMAP-R Scale. The second phase included subjects that completed the first phase but included a second in-person visit described below. For the current analyses, the presence of a current psychotic diagnosis (n = 3, as assessed during an in-person visit) was also an exclusion criterion. To preserve the representativeness of the community sample, there were no other exclusion criteria.
Fig. 1.
Study sample: guide on data collection phases, sample recruitment overlap, and measure sample sizes.
MAP Study—Validation Phase
The second, validation phase was an in-person visit at the university study site that included a variety of convergent and discriminant measures to validate the current scale. These validation measures were collected within a larger study battery that included clinical assessments using both the Structured Interview for Psychotic Risk65 (SIPS) and the Structured Clinical Interview for DSM-5 Research Version67 (SCID-5-RV). During the clinical assessment, individuals were classified as clinical high-risk for psychosis (CHR) if they exhibited attenuated positive symptoms or genetic risk and deterioration of function. Attenuated positive symptom criteria were determined by the SIPS guidelines. In total, 85 individuals were classified as meeting criteria for a CHR syndrome, with all other individuals considered community sample non-psychiatric volunteers (CSV). The SCID provided comorbid diagnoses across the entire community sample. All diagnostic decisions were made by trained clinical staff under the direct supervision of each sites’ coauthors VAM, LE, and JS, with weekly cross-site diagnostic consultation calls. The second phase included the completion of the Pennsylvania Computerized Neurocognitive (PNC) battery and additional questionnaires. The PNC battery is a validated, reliable neurocognitive testing platform69 that has a demonstrated sensitivity to psychosis symptoms in patients with schizophrenia70 and in populations at CHR.30
Sensorimotor Abnormalities Psychosis Risk Scale
The SMAP-R scale is a 14-item questionnaire that included questions about early developmental motor delays, the frequency of abnormal sensorimotor experiences, general assessments of sensorimotor function, and frequency of physical activity. Questions were developed based on a qualitative review of extant literature12,22,35,48,71 regarding sensorimotor abnormalities linked to symptoms,4,10,19,21,55,72 conversion,1,5,36,44,45 and clinical/cognitive subtyping19 in those meeting criteria for a CHR syndrome. From this review of the literature, a set of items was compiled to examine relevant constructs that would also lend well to self-report questions (eg, ballistic movements, sway/balance). These items included several Likert-type scale responses, such as “On a scale of 1–10, how would you rate your current motor skills?” and “Do you enjoy hobbies where you use skilled hand movements (eg, art, crafts)?” (Responses: Never Sometimes Lots). For a version of the final measure and scoring guide, see supplementary materials.
SIPS Risk Calculator
The SIPS-RC is a Structured Interview on Prodromal Risk Symptoms (SIPS)-based risk calculator that provides practical, individualized risk assessments that can be easily implemented in a clinical setting.73,74 In this calculator, risk probability assessments are derived from 4 dimensions: positive and negative symptom severity, deterioration of function over the past 12-months, and low levels of dysphoric mood. In the current study, the global functioning scale75 was used to assess functional decline in the past year. Positive and negative symptom severity was assessed during the SIPS interview and quantified as a composite score of endorsed positive and negative symptoms.73 Finally, low levels of dysphoric mood were assessed during the general symptom assessment of the SIPS.73,74 This approach has been validated against external independent risk calculators.
Behavioral Validation and Discrimination
The Pennsylvania Neurocognitive Battery was completed at the in-person visit. This battery included a computerized finger tapping (CTAP) performance.76 During the CTAP task, participants were instructed to press the spacebar as quickly as possible, moving only the index finger for 10 seconds after their first button press. In this task, trial blocks alternated between the dominant and non-dominant hand for 10 blocks (5 blocks per hand). Median finger tapping and tapping variability (the standard deviation in taps between blocks) behavior was used as a convergent measure of motor performance to assess the sensitivity of the SMAP-R scale to motor performance. Variability in performance was included because a recent study demonstrated that it may be a sensitive and mechanistically relevant indicator among those meeting criteria for a CHR for psychosis.77
Analytical Strategy
Exploratory factory analyses were used to examine structure of responses in the 2 analytic datasets (ie, exploratory and validation). These 2 analytic datasets, with independent data-points include 2 analytic datasets: (1) an exploratory, screening analytic dataset (n = 3109) that completed only phase one of the study and (2) a scale validation analytic dataset (n = 439) that completed both phase 1 and phase 2.78 For each analysis, all participants with the data for that given analyses were included, rather than reducing the study sample size to only those individuals with a full and complete dataset; the intention in this approach was to both maximize power and transparency, see figure 1 for analyses sample sizes. Factor analyses and item reliability was assessed using the R v.4.0.079 and the Psych package; for more information on how factors were assessed, see supplementary material.80
All items that were grouped into a factor were examined as a composite subscale in the subsequent analyses. Items that were not included in the subscales (ie, items that were not related to other items) were treated as independent items and evaluated for discriminant validity and convergent validity. Independent items that failed to show discriminant and convergent validity were excluded from the final scale. These subsequent analyses probed the discriminant and predictive validity of these composite subscales and reduced the total number of comparisons.
In terms of discriminant validity, we examined the degree to which these scale factors differ between relevant CHR group (n = 84) compared other disorders (ie, depression; n = 35 and anxiety; n = 78), and a CSV (n = 78) samples among the validation analytic dataset, using general linear models for the composite scales and chi-square for independent items. In terms of predictive validity, scale factors were related to the calculated risk for psychosis (SIPS-RC) and current symptom severity (SIPS total scales) within the CHR group from the validation analytic dataset. To examine convergent validity, the sensorimotor scales were compared to the motor performance (ie, finger tapping task) in the validation analytic dataset. To examine discriminant validity of behavior, the sensorimotor scales were compared to the performance on an independent attentional task (ie, NL-CPT; see supplementary material for analyses and details).81 All validation analyses were completed in SPSS version 26.
Results
Participants
There were no significant differences in key demographic variables, including biological sex at birth, ethnicity, race, age, and income across exploratory and validation analytic datasets, as well as discriminatory clinical samples, table 1.
Table 1.
Demographic Metrics: A Comparison of Diagnostic Groups Within the Validation Sample for Discriminatory Analyses
| Exploratory Sample | Validation Sample | Sample Comparison Statistics | CHR | CHV | MDD | Anxiety | Discriminant Sample | Discriminant Group Statistics | |
|---|---|---|---|---|---|---|---|---|---|
| n = 3009 | n = 439 | n = 84 | n = 78 | n = 35 | n = 78 | n = 275 | |||
| Age -M(SD) | 20.31 (2.36) | 20.13 (1.84) | t(3436) = 1.523 P = .13 | ||||||
| Sex (% Female) | 67.75% | 77.47% | χ 2(1) = .285 P = .59 | 75.60% | 70.50% | 80% | 84.60% | 74.69% | χ 2 (3) = 4.70, P = .20 |
| Hispanic ethnicity | 10.38% | 10.25% | χ 2 (1) = .007 P = .93 | 7.31% | 8.97% | 14.29% | 5.26% | 8.98% | χ 2 (3) = 2.76, P = .43 |
| Race | χ 2 (5) = .852, P = .97 | ||||||||
| American Indian | 0.36% | 0.46% | 0.35% | 0.00% | 0.00% | 0.00% | 0% | χ 2 (3) = 2.31, P = .51 | |
| Asian | 24.06% | 25.06% | 6.36% | 8.13% | 2.47% | 8.13% | 28.98% | χ 2 (3) = 2.45, P = .49 | |
| Native Hawaiian | 0.03% | 0.00% | 0.35% | 0.35% | 0.00% | 0.00% | 0.82% | χ 2 (3) = 1.417, P = .70 | |
| Black/African American | 13.51% | 14.81% | 4.95% | 4.59% | 1.41% | 4.24% | 17.55% | χ 2 (3) = .629, P = .89 | |
| White | 51.34% | 50.57% | 16.61% | 13.43% | 7.42% | 13.07% | 58.37% | χ 2 (3) = 2.59, P = .46 | |
| Multiracial | 6.16% | 6.61% | 1.06% | 2.12% | 1.06% | 1.41% | 6.53% | χ 2 (3) = 1.78, P = .62 | |
| Unknown | 7.45% | 2.51% | 0.71% | 1.06% | 0.71% | 0.00% | 2.86% | χ 2 (3) = 3.978, P = .26 |
Note: CHR, clinical high-risk; CHV, community healthy volunteer; MDD, major depression disorder.
Exploratory Factor Analyses
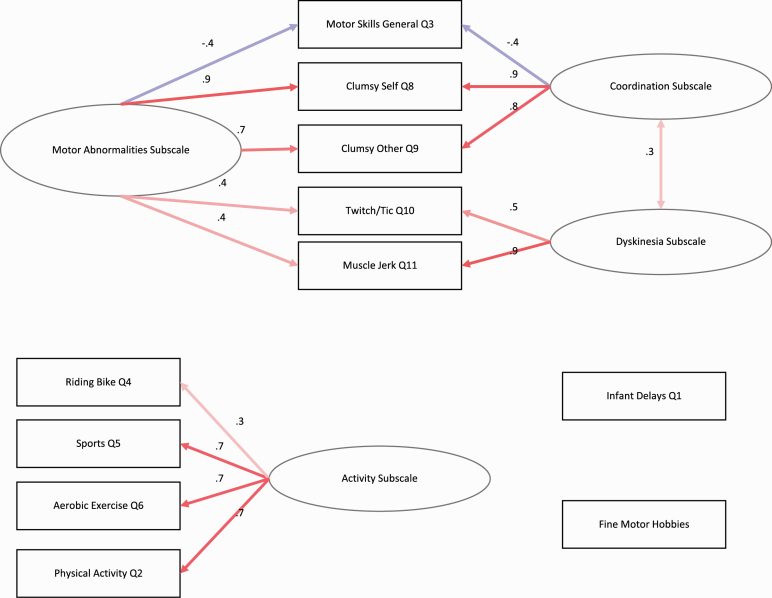
In the exploratory analytic dataset, the traditional Cattell’s scree analyses suggested that the intercorrelation matrix showed 4 components; scree analyses compared to simulated data suggested the presence of 3 components or 2 factors, supplementary figure 1. Both analytic datasets showed similar scale structures with 2 components dividing the scale into “Sensorimotor Abnormality” subscale items and “Physical Activity” subscale items; when reorganized into 3 factors, the Sensorimotor Abnormality items divided into facets “Coordination Abnormalities” facet items and “Dyskinesia” facet items, table 2. In the validation analyses, the traditional Cattell’s scree analyses suggested that the intercorrelation matrix showed 3 components; scree analyses compared to simulated data suggested the presence of 3 components or 2 factors (figure 2). There were 2 items that did not load onto these factors, including an item on sensorimotor delays (ie, “Are you aware of any delays in walking, talking, or toilet training when you were an infant or toddler?”) and an item on fine sensorimotor hobbies (ie, “Do you enjoy hobbies where you use skilled hand movements (art, crafts)?”). These items were treated as independent items in the following validation analyses. Both exploratory and validation analytic datasets showed similar internal consistency (table 3).
Table 2.
Scale Items Organized by Subscales and Facets
| Subscales | Facets | Definition | Items |
|---|---|---|---|
| Sensorimotor abnormalities | |||
| Dyskinesiaa | Involuntary movements most commonly seen in the limbs, mouth, or tongue resembling either chorea (ie, flinging, jerking) or athetosis (ie, writhing)15,43,56 | ||
| Do you experience urges to twitch or move suddenly, or make noises? | |||
| Do you ever notice that you have moved suddenly without planning it (eg, leg or arm jerking; head or neck jerking)? | |||
| Coordination abnormalitiesa | Abnormalities in sensory integration, motor coordination, motor sequencing, and reflexes6,82 | ||
| Do you feel clumsy? | |||
| Would other people say you are clumsy? | |||
| Physical activity | Physical activity, including exercise and general less strenuous activity, is reduced in psychosis and psychosis risk populations26,28 | ||
| How often do you participate in physical activities (jogging, team sports) each week? | |||
| On a scale of 1–10, how would you rate your current motor skills? | |||
| How secure/balanced is your current bicycle riding? | |||
| How often do you enjoy participating in sports? | |||
| Do you enjoy aerobic exercise? (jogging, running, swimming) |
Fig. 2.
Factor structure for both a 2-factor (Sensorimotor Abnormalities and Physical Activity Subscales) and 3-factor solution (Dyskinesia facets, Coordination Abnormalities facets, and Physical Activity Subscale).
Table 3.
Reliability of Scales Replicated Across Samples
| Factor Scales | Exploratory Sample | Validation Sample | ||
|---|---|---|---|---|
| Cronbach’s α | Guttman’s λ | Cronbach’s α | Guttman’s λ | |
| Sensorimotor abnormalities | .69 | 0.71 | .71 | 0.73 |
| Coordination abnormalities | .71 | 0.68 | .75 | 0.71 |
| Dyskinesia | .67 | 0.51 | .68 | 0.51 |
| Physical activity | .68 | 0.64 | .69 | 0.64 |
Discriminant Validity Among Clinical Samples
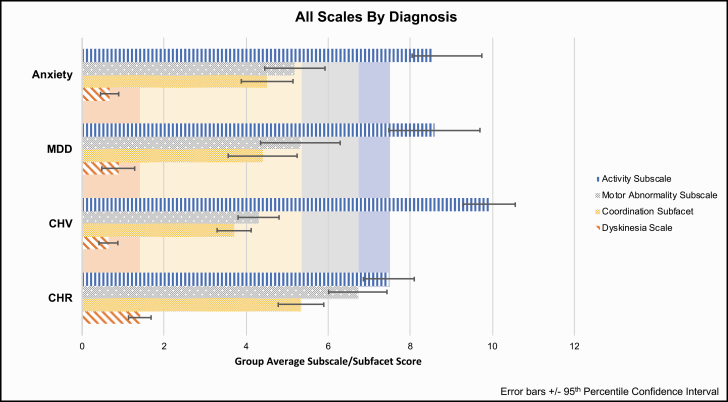
Composite subscales totals were compared across diagnostic groups in independent analyses of variance model (ANOVA) (figure 3). For each of the independent items, a limited set of Likert-responses were compared across groups using a chi-square analysis. Follow-up discriminant function analyses were conducted comparing CHR and CSV groups and showed converging results (supplementary materials).
Fig. 3.
Discriminant validation analyses: composite scales by group.
Sensorimotor Abnormalities Subscale by Diagnostic Group
The Sensorimotor Abnormality subscale significantly differed among groups, F(270,3) = 9.50, P < .001, partial = .10, such that the CHR group (M = 6.72, SD = 3.25, SEM = .36) showed increased Sensorimotor Abnormality score than individuals with depression (M = 5.32, SD = 2.78, SEM = .37, P = .02), anxiety (M = 5.18, SD = 3.20, SEM = .37, P = .006), and the CSV (M = 4.29, SD = 2.21, SEM = .25, P < .001),
Abnormalities Facets by Diagnostic Group
The Coordination Abnormalities facet significantly differed among groups, F(271,3) = 6.193, P < .001, partial = .07, such that the CHR group (M = 5.33, SD = 2.55, SEM = .28) showed increased Coordination Abnormalities facet scores compared to individuals with depression (M = 4.40, SD = 2.44, SEM = .412, P = .05), anxiety (M = 4.51, SD = 2.74, SEM = .317, P = .043), and the CSV (M = 3.70, SD = 1.83, SEM = .21, P < .001). The Dyskinesia facet significantly differed among groups, F(270,3) = 8.54, P < .001, partial=.09, such that CHR group (M = 1.41, SD = 1.26, SEM = 0.14) showed an increased Dyskinesia facet score than individuals with depression (M = 0.88, SD = 1.15, SEM = 0.20, P = .02), anxiety (M = 0.67, SD = 0.96, SEM = 0.11, P < .001), and the CSV (M = 0.636, SD = 1.01, SEM = 0.12, P < .001).
Physical Activity Subscale by Diagnostic Group
The Physical Activity subscale significantly differed among groups, F(250,3) = 8.536, P < .001, partial=.09, such that CHR (M = 7.47, SD = 2.67, SEM = 0.31) showed less physical activity than the CSV (M = 9.19, SD = 2.74, SEM = 0.32, P < .001). Although the CHR group showed less physical activity than individuals with depression (M = 8.59, SD = 3.18, SEM = 0.48, P = .076) and anxiety (M = 8.19, SD = 3.53, SEM = 0.45, P = .16); this difference was not significant.
Independent Items by Diagnostic Group
The motor delay response (no delays, some delays, a lot of delays) were compared across the diagnostic groups (CHR, CSV, depression, anxiety); groups differed in terms of motor delays χ 2 (223) = 9.45, P = .009. The CHR group showed the highest percentage of motor delays with 16.5% of the CHR group reporting some or severe motor delays, and 100% of individuals reporting severe motor delays were also members of the CHR group. The frequency of engagement in motor hobbies (never, sometimes, often) were compared across the diagnostic groups (CHR, CSV, depression, anxiety); groups did not differ in terms of their engagement in fine motor hobbies, χ 2 (275) = 8.94, P = .27.
Convergent Validity to Sensorimotor Performance
Composite scales were compared to performance on a finger tapping task performance. Within the CHR group, a repeated-measure general linear model compared motor speed across hands (dominant, non-dominant) by median and variance as the within-subject factor and the SMAP-R subscales, respectively, as between-subject factors. Finger tapping performance related to the “Sensorimotor Abnormalities” subscale, F(71) = 5.83, P = .016; higher self-reported Sensorimotor Abnormalities subscale related to lowered median taps on both the dominant (rpartial = −.23) and non-dominant hands (rpartial = −.18). Finger tapping performance also related to the Coordination Abnormalities facet, F(71) = 5.95, P = .017, such that increased Coordination Abnormalities facet scores were related to lowered median taps on both the dominant (rpartial= -.26) and non-dominant hands (rpartial= −.22). Finger tapping did not significantly relate to any other subscales or items, Ps > .13.
Divergent Validity to Non-Sensorimotor Behavior
Subscales were compared to efficiency on a number-letter continuous performance task. Within the CHR group, a general linear model predicted task efficiency with both SMAP-R subscales as between-subject factors. There were no significant relationships of any subscale, facet, or independent items to attentional task efficiency, Ps > .31.
Predictive and Convergent Validity to Clinical Features
Composite subscales totals significantly correlated with the psychosis risk score (SIPS-RC). Sensorimotor Abnormalities subscale related to SIPS-RC scores, r(312) = .15, P = .008. Coordination Abnormalities facet related to SIPS-RC scores, r(310) = .16, P = .006. Dyskinesia facet related to SIPS-RC scores, r(308) = .12, P = .04. “Physical Activity” subscale related to SIPS-RC scores, r(284) = −.150, P = .01. For each of the independent items a limited set of Likert-responses were compared across groups using a general linear model. SIPS-RC did not relate to infant delays (P = .23) or frequency of fine motor hobbies (P = .63).
Discussion
The SMAP-R scale showed a replicable structure that grouped items into Sensorimotor Abnormalities and Physical Activity subscales. Sensorimotor Abnormalities further divided into Coordination Abnormalities and Dyskinesia facet. Each of these factors is of significant interest to understanding, identifying, predicting, and treating individuals at CHR for psychosis. Therefore, a sensorimotor scale that differentiates distinct sensorimotor categories has significant potential. Further, the SMAPRS subscales discriminated individuals at CHR from anxiety and depression groups and a community sample of CSV. There was modest support for convergent validity. Specifically, the general Sensorimotor Abnormality subscale and the Coordination Abnormalities facet showed a small, but significant relationship to finger tapping performance, but not attentional performance on an unrelated task. Additionally, these scales related to a validated measure of risk for psychosis in the SIPS-RC. Collectively this scale may provide unique insight into sensorimotor abnormalities, which is largely under-utilized in current approaches to researching and treating the psychosis risk syndrome.35 This potential is especially important as sensorimotor abnormalities have demonstrated utility in domains predicting course,1,2,21 functional outcomes,42,43 and conversion to psychosis.1,36,44–46 As a result, the current self-report measure may be used as an initial screening to focus further clinical sensorimotor assessment and targeting individualized treatment.35
The exploratory and validation analytic dataset factor analyses suggested that items of the scale could be grouped into 2 subscales (Sensorimotor Abnormalities and Physical Activity subscales) with 2 facets (Coordination Abnormalities, Dyskinesia) in the exploratory analytic dataset.22 Scale structure was consistent across independent analytic datasets providing converging evidence of the stability and reliability of these subscale structures.78 It is also notable that both the exploratory and the validation analytic datasets reflected a diverse community sample, which should increase the generalizability of the current scale structures.83 The 2 subscales identified reflect distinct vulnerabilities/mechanisms/circuits that are central to the etiology of psychosis.22 For example, sedation and physical activity have been related to hippocampal dysfunction, a factor prominent among those identified as meeting criteria for a CHR syndrome,27,84–86 and are also a central component of prominent conceptual models.87 Further, poor coordination,18,21 as well as issues with balance and ataxia,10,21,50,88 are relevant to cerebellar-thalamic dysfunction and cognitive dysmetria49 and dyskinetic movements and basal ganglia circuit vulnerability are relevant to emerging psychosis as well as the revised dopamine hypothesis.9,15,38,53,54,89 Finally, the validation analyses provided initial evidence of the utility of these scales.
The validation analytic dataset was a diverse community sample83 that included CSV as well as individuals with psychiatric diagnoses, including major depression disorder and anxiety disorders, both known to be associated with lower rates of sensorimotor abnormalities (ie, psychomotor slowing/agitation).83,90 The CHR group showed significantly more Sensorimotor Abnormalities subscale, Coordination Abnormalities, and Dyskinesia facets, suggesting that these scales are particularly sensitive to the presence of psychosis liability, beyond general psychopathology present in depression and anxiety. In contrast, the Physical Activity subscale did not discriminate between the CSV and the CHR group. However, the Physical Activity subscale was sensitive to the presence of general psychopathology as the psychiatric groups did not differ from each other, but differed from the CSV sample.71,90 It is notable that both the Sensorimotor Abnormalities subscale and the Physical Activity subscale distinguished other anxiety and depression from CSV. This sensitivity to other psychopathology suggests that this scale may also have some utility in examining sensorimotor abnormalities in depression and anxiety. In summary, the scales showed both a specific relationship to psychosis risk and a general sensitivity to discriminate individuals with psychopathology from a CSV group.
Despite the value demonstrated in discriminating groups, the ability to distinguish those meeting criteria for a CHR syndrome from the comparison groups does not directly speak to the SMAPRS’s capacity to measure actual sensorimotor behavior.91–93 To this end, we have further validated these scales with the finger-tapping task, a measure known to reflect disturbances in the function of underlying neurocircuitry of sensorimotor systems.30,94,95 This task is particularly probative of cortico-cerebellar interactions that govern the execution of sub-second responses, but also motor slowing more generally. The Sensorimotor Abnormalities subscale related to the performance on this task, driven in large part by the Coordination Abnormalities facet. Although the relationship to finger tapping motor performance was small, it does suggest that individuals in the CHR group were able to faithfully report on their sensorimotor abnormalities (which likely includes other features in addition to motor slowing). Additionally, as expected, none of these subcscales related to performance on a foil (attentional) task.81 Collectively, these results demonstrated additional discriminant validity as the subscales specifically predicted task performance in the sensorimotor domain and not behavioral performance in unrelated domains.
All of the SMAP-R subscales related to the calculated risk for developing psychosis—a composite score that weights the predictive features of clinical high-risk for psychosis (ie, SIPS-RC score). The calculated risk scale serves as an estimation of the likelihood of an individual to transition to psychosis, but it also takes into account critical features of early clinical risk, eg, symptom severity and decreased global function.73,74 The relationship of these subscales with calculated risk may reflect the relevance of both Sensorimotor Abnormalities and Physical Activity subscales to emerging psychosis.
The motor developmental delays item distinguished between clinical groups with a larger proportion of individuals in the CHR group who endorsed minor motor developmental delays,3,96 and endorsements of major motor developmental delays were made up entirely of those in the CHR group. The motor delay item was included in future versions of the SMAP-R scale, but the fine motor hobby item will not be included in the final scale as it did it reflect group membership, clinical risk features, or motor performance.
This study included several strengths, but there were limitations that should be explored in future studies. First, the current study established the items based on a qualitative review, but future studies should consider implementing a search algorithm (eg, PRISMA) with established guidelines to create items. Although the current paper identified 3 scales that may reflect distinct vulnerabilities/mechanisms/circuits, we did not directly assess mechanistic ties and specificity in the current study. Similarly, while the current work assessed internal reliability, we did not assess test-retest reliability within subjects. Future studies will need to assess this critical aspect of reliability. Further, the original version of the scale omitted additional sensorimotor behaviors such as gestures and catatonia that are likely to tap into other distinct vulnerabilities.16,88,97–103
The current study reflects a major advancement in the accessibility and dispersal of sensorimotor assessment, but there is still room for important future work in this area. While the measure contains exploratory gesture items, this questionnaire could be administered with extant gesture self-report questionnaires,104 which have been validated in psychosis105 and major depression.106 Similarly, future studies should explore the use of extant assessments of motor slowing assessments in depression, eg, Salpetiere motor scale,107 to provide unique insight into motor slowing in CHR populations. Additionally, future studies would benefit from continuing to validate the SMAP-R, and to expand the scale further, using the number of available motor assessments. For example, future validation studies would benefit from incorporating instrumental sensorimotor assessments (eg, actigraphy, velocity scaling, force variability, postural sway, electromyography)82 as these tests are sensitive to subtle perturbations and tap into mechanisms overlapping with those addressed by several of the self-report subscales/facets. In addition, it will be important to validate future gesture items with rater-based inventories (eg, Tulia)103,108 and catatonia with instruments such as the Catatonia Rating Scale, Northoff Catatonia Scale, or Bush-Francis Catatonia Rating Scale 31,33,34 as more work continues to clarify the role of this domain in those at CHR for psychosis. In terms of diverse clinical samples, the current study shows some important potential of a self-report motor scale to provide transdiagnostic insight into sensorimotor abnormalities and levels of physical activity. Future work is needed to further explore this possibility with large and clinically diverse samples to further examine the sensitivity and specificity of the scale. In the absence of longitudinal data, it is unknown whether this particular Sensorimotor Abnormality subscale relates to clinical course or ultimate conversion to psychosis. Finally, the scale assesses several sensorimotor signs featured on the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) initiative’s new Sensorimotor Domain.22,71,90 Promising results in the present study hint the SMAP-R may serve as a useful transdiagnostic tool.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Supplemental Figure 1. Factor analyses and data structure for the validation analytic dataset: A. Depicts the intercorrelation structure of the items, B. Depicts the Cattell scree plot of the intercorrelation matrix, C. Depicts the factor analyses compared to simulated data in a scree plot, D. Depicts the factor structure for both a 2 factor (Sensorimotor Abnormalities and Physical Activity Subscales) and 3 factor solution (Dyskinesia facet, Coordination Abnormalities facet, and Physical Activity Subscale).
Supplemental Figure 2. Factor analyses and data structure for the exploratory analytic dataset: A. Depicts the intercorrelation structure of the items, B. Depicts the Cattell scree plot of the intercorrelation matrix, C. Depicts the factor analyses compared to simulated data in a scree plot, D. Depicts the factor structure for both a 2 factor (Sensorimotor Abnormalities and Physical Activity Subscales) and 3 factor solution (Dyskinesia facet, Coordination abnormalities facet, and Physical Activity Subscale).
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
This work was supported by the National Institutes of Mental Health (MH094650, MH112545, MH103231, MH094650, VM; 5R01MH112613-03, 3R01MH112613-02S1, and 5R01MH112613-02, LME; 5R01MH112612-03, 5R01MH112612-02, and 1R01MH112612-01).
References
- 1. Mittal VA, Walker EF. Movement abnormalities predict conversion to Axis I psychosis among prodromal adolescents. J Abnorm Psychol. 2007;116(4):796–803. [DOI] [PubMed] [Google Scholar]
- 2. Mittal VA, Tessner KD, Trottman HD, et al. Movement abnormalities and the progression of prodromal symptomatology in adolescents at risk for psychotic disorders. J Abnorm Psychol. 2007;116(2):260–267. [DOI] [PubMed] [Google Scholar]
- 3. Walker EF. Developmentally moderated expressions of the neuropathology underlying schizophrenia. Schizophr Bull. 1994;20(3):453–480. [DOI] [PubMed] [Google Scholar]
- 4. Mittal VA, Neumann C, Saczawa M, Walker EF. Longitudinal progression of movement abnormalities in relation to psychotic symptoms in adolescents at high risk of schizophrenia. Arch Gen Psychiatry. 2008;65(2):165–171. [DOI] [PubMed] [Google Scholar]
- 5. Schiffman J, Mittal V, Kline E, et al. Childhood dyspraxia predicts adult-onset nonaffective-psychosis-spectrum disorder. Dev Psychopathol. 2015;27(4 Pt 1):1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schiffman J, Sorensen HJ, Maeda J, et al. Childhood motor coordination and adult schizophrenia spectrum disorders. Am J Psychiatry. 2009;166(9):1041–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schiffman J, Walker E, Ekstrom M, Schulsinger F, Sorensen H, Mednick S. Childhood videotaped social and neuromotor precursors of schizophrenia: a prospective investigation. Am J Psychiatry. 2004;161(11):2021–2027. [DOI] [PubMed] [Google Scholar]
- 8. Schiffman J. Motor issues in the clinical high risk phase of psychosis. Schizophr Bull. 2017;43(5):937–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dean DJ, Mittal VA. Spontaneous parkinsonisms and striatal impairment in neuroleptic free youth at ultrahigh risk for psychosis. npj Schizophr. 2015;1:14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dean DJ, Kent JS, Bernard JA, et al. Increased postural sway predicts negative symptom progression in youth at ultrahigh risk for psychosis. Schizophr Res. 2015;162(1-3):86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hirjak D, Thomann PA, Kubera KM, Wolf ND, Sambataro F, Wolf RC. Motor dysfunction within the schizophrenia-spectrum: a dimensional step towards an underappreciated domain. Schizophr Res. 2015;169(1-3):217–233. [DOI] [PubMed] [Google Scholar]
- 12. Hirjak D, Meyer-Lindenberg A, Kubera KM, Thomann PA, Wolf RC. Motor dysfunction as research domain in the period preceding manifest schizophrenia: a systematic review. Neurosci Biobehav Rev. 2018;87:87–105. [DOI] [PubMed] [Google Scholar]
- 13. Walther S, Mittal VA. Motor System Pathology in Psychosis. Curr Psychiatry Rep. 2017;19(12):97. [DOI] [PubMed] [Google Scholar]
- 14. Ellman LM, Vinogradov S, Kremen WS, et al. Low maternal hemoglobin during pregnancy and diminished neuromotor and neurocognitive performance in offspring with schizophrenia. Schizophr Res. 2012;138(1):81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mittal VA, Daley M, Shiode MF, Bearden CE, O’Neill J, Cannon TD. Striatal volumes and dyskinetic movements in youth at high-risk for psychosis. Schizophr Res. 2010;123(1):68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stegmayer K, Bohlhalter S, Vanbellingen T, et al. Structural brain correlates of defective gesture performance in schizophrenia. Cortex. 2016;78:125–137. [DOI] [PubMed] [Google Scholar]
- 17. Dean DJ, Bernard JA, Orr JM, et al. Cerebellar morphology and procedural learning impairment in neuroleptic-naive youth at ultrahigh risk of psychosis. Clin Psychol Sci. 2014;2(2):152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bernard JA, Orr JM, Mittal VA. Cerebello-thalamo-cortical networks predict positive symptom progression in individuals at ultra-high risk for psychosis. Neuroimage Clin. 2017;14:622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dean DJ, Walther S, Bernard JA, Mittal VA. Motor clusters reveal differences in risk for psychosis, cognitive functioning, and thalamocortical connectivity: evidence for vulnerability subtypes. Clin Psychol Sci. 2018;6(5):721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Osborne KJ, Damme KSF, Gupta T, Dean DJ, Bernard JA, Mittal VA. Timing dysfunction and cerebellar resting state functional connectivity abnormalities in youth at clinical high-risk for psychosis. Psychol Med. 2020:1–10. doi: 10.1017/S0033291719004161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bernard JA, Dean DJ, Kent JS, et al. Cerebellar networks in individuals at ultra high-risk of psychosis: impact on postural sway and symptom severity. Hum Brain Mapp. 2014;35(8):4064–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mittal VA, Bernard JA, Northoff G. What can different motor circuits tell us about psychosis? An RDoC perspective. Schizophr Bull. 2017;43(5):949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caligiuri MP, Teulings HL, Dean CE, Niculescu AB III, Lohr JB. Handwriting movement kinematics for quantifying extrapyramidal side effects in patients treated with atypical antipsychotics. Psychiatry Res. 2010;177(1-2):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murck H, Laughren T, Lamers F, et al. Taking personalized medicine seriously: biomarker approaches in phase IIb/III studies in major depression and schizophrenia. Innov Clin Neurosci. 2015;12(3-4):26S–40S. [PMC free article] [PubMed] [Google Scholar]
- 25. Mittal VA, Hasenkamp W, Sanfilipo M, et al. Relation of neurological soft signs to psychiatric symptoms in schizophrenia. Schizophr Res. 2007;94(1-3):37–44. [DOI] [PubMed] [Google Scholar]
- 26. Mittal VA, Gupta T, Orr JM, et al. Physical activity level and medial temporal health in youth at ultra high-risk for psychosis. J Abnorm Psychol. 2013;122(4):1101–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dean DJ, Bryan AD, Newberry R, Gupta T, Carol E, Mittal VA. A supervised exercise intervention for youth at risk for psychosis: an open-label pilot study. J Clin Psychiatry. 2017;78(9):e1167–e1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Newberry RE, Dean DJ, Sayyah MD, Mittal VA. What prevents youth at clinical high risk for psychosis from engaging in physical activity? An examination of the barriers to physical activity. Schizophr Res. 2018;201:400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deighton S, Addington J. Exercise practices in individuals at clinical high risk of developing psychosis. Early Interv Psychiatry. 2015;9(4):284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Damme KSF, Osborne KJ, Gold JM, Mittal VA. Detecting motor slowing in clinical high risk for psychosis in a computerized finger tapping model. Eur Arch Psychiatry Clin Neurosci. 2020;270(3):393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bush G, Fink, M, Petrides G, Dowling F, Francis ACatatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129–136. [DOI] [PubMed] [Google Scholar]
- 32. Lund CE, Mortimer AM, Rogers D, McKenna PJ. Motor, volitional and behavioural disorders in schizophrenia. 1: assessment using the Modified Rogers Scale. Br J Psychiatry. 1991;158:323–327, 333. [DOI] [PubMed] [Google Scholar]
- 33. Northoff G, Koch A, Wenke J, et al. Catatonia as a psychomotor syndrome: a rating scale and extrapyramidal motor symptoms. Mov Disord. 1999;14(3):404–416. [DOI] [PubMed] [Google Scholar]
- 34. Starkstein SE, Petracca G, Tesón A, et al. Catatonia in depression: prevalence, clinical correlates, and validation of a scale. J Neurol Neurosurg Psychiatry. 1996;60(3):326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mittal VA, Walther S. As motor system pathophysiology returns to the forefront of psychosis research, clinical implications should hold center stage. Schizophr Bull. 2019;45(3):495–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Masucci MD, Lister A, Corcoran CM, Brucato G, Girgis RR. Motor dysfunction as a risk factor for conversion to psychosis independent of medication use in a psychosis-risk cohort. J Nerv Ment Dis. 2018;206(5):356–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gupta T, Dean DJ, Kelley NJ, Bernard JA, Ristanovic I, Mittal VA. Cerebellar transcranial direct current stimulation improves procedural learning in nonclinical psychosis: a double-blind crossover study. Schizophr Bull. 2018;44(6):1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35(3):549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Henriksen MG, Parnas J. Self-disorders and schizophrenia: a phenomenological reappraisal of poor insight and noncompliance. Schizophr Bull. 2014;40(3):542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Henriksen MG, Parnas J. Clinical manifestations of Self-disorders in schizophrenia spectrum conditions. Curr Prob Psychiat. 2017;18(3):177–183. [Google Scholar]
- 41. Northoff G, Duncan NW. How do abnormalities in the brain’s spontaneous activity translate into symptoms in schizophrenia? From an overview of resting state activity findings to a proposed spatiotemporal psychopathology. Progress Neurobiol. 2016;145–146:26–45. [DOI] [PubMed] [Google Scholar]
- 42. Osborne KJ, Vargas T, Mittal VA. Early childhood social communication deficits in youth at clinical high-risk for psychosis: associations with functioning and risk. Dev Psychopathol. 2020;32(2):559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mittal VA, Jalbrzikowski M, Daley M, Roman C, Bearden CE, Cannon TD. Abnormal movements are associated with poor psychosocial functioning in adolescents at high risk for psychosis. Schizophr Res. 2011;130(1-3):164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mittal VA, Walker EF, Bearden CE, et al. Markers of basal ganglia dysfunction and conversion to psychosis: neurocognitive deficits and dyskinesias in the prodromal period. Biol Psychiatry. 2010;68(1):93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Studerus E, Papmeyer M, Riecher-Rössler A. Neurocognition and motor functioning in the prediction of psychosis. Early Detection and Intervention in Psychosis. 2016;181:116–132. [Google Scholar]
- 46. Callaway DA, Perkins DO, Woods SW, Liu L, Addington J. Movement abnormalities predict transitioning to psychosis in individuals at clinical high risk for psychosis. Schizophr Res. 2014;159(2-3):263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ebel H, Gross G, Klosterkötter J, Huber G. Basic symptoms in schizophrenic and affective psychoses. Psychopathology. 1989;22(4):224–232. [DOI] [PubMed] [Google Scholar]
- 48. Dazzan P, Murray RM. Neurological soft signs in first-episode psychosis: a systematic review. Br J Psychiatry. 2002;181:50–57. [DOI] [PubMed] [Google Scholar]
- 49. Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24(2):203–218. [DOI] [PubMed] [Google Scholar]
- 50. Kent JS, Hong SL, Bolbecker AR, et al. Motor deficits in schizophrenia quantified by nonlinear analysis of postural sway. PLoS One. 2012;7(8):e41808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Petruzzelli MG, Margari L, Craig F, et al. Markers of neurodevelopmental impairments in early-onset psychosis. Neuropsychiatr Dis Treat. 2015;11:1793–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wilquin H, Delevoye-Turrell Y. Motor agency: a new and highly sensitive measure to reveal agency disturbances in early psychosis. PLoS One. 2012;7(2):e30449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dandash O, Pantelis C, Fornito A. Dopamine, fronto-striato-thalamic circuits and risk for psychosis. Schizophr Res. 2017;180:48–57. [DOI] [PubMed] [Google Scholar]
- 54. Dandash O, Fornito A, Lee J, et al. Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophr Bull. 2014;40(4):904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cortese L, Caligiuri MP, Malla AK, Manchanda R, Takhar J, Haricharan R. Relationship of neuromotor disturbances to psychosis symptoms in first-episode neuroleptic-naive schizophrenia patients. Schizophr Res. 2005;75(1):65–75. [DOI] [PubMed] [Google Scholar]
- 56. Mittal VA, Dean DJ, Pelletier A, Caligiuri M. Associations between spontaneous movement abnormalities and psychotic-like experiences in the general population. Schizophr Res. 2011;132(2-3):194–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bernard JA, Seidler RD. Cerebellar contributions to visuomotor adaptation and motor sequence learning: an ALE meta-analysis. Front Hum Neurosci. 2013;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bernard JA, Mittal VA. Dysfunctional activation of the cerebellum in schizophrenia: a functional neuroimaging meta-analysis. Clin Psychol Sci. 2015;3(4):545–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Koivukangas J, Tammelin T, Kaakinen M, et al. Physical activity and fitness in adolescents at risk for psychosis within the Northern Finland 1986 Birth Cohort. Schizophr Res. 2010;116(2):152–158. [DOI] [PubMed] [Google Scholar]
- 60. Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl). 2004;174(1):151–162. [DOI] [PubMed] [Google Scholar]
- 61. Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17(11):1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pajonk FG, Wobrock T, Gruber O, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67(2):133–143. [DOI] [PubMed] [Google Scholar]
- 63. Loewy RL, Pearson R, Vinogradov S, Bearden CE, Cannon TD. Psychosis risk screening with the Prodromal Questionnaire–brief version (PQ-B). Schizophr Res. 2011;129(1):42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Karcher NR, Barch DM, Avenevoli S, et al. Assessment of the Prodromal Questionnaire-Brief Child Version for measurement of self-reported psychoticlike experiences in childhood. JAMA Psychiatry. 2018;75(8):853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. [DOI] [PubMed] [Google Scholar]
- 66. Nakamura BJ, Ebesutani C, Bernstein A, Chorpita BF. A psychometric analysis of the child behavior checklist DSM-oriented scales. J Psychopathol Behav Assess. 2009;31(3):178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. First MB, Williams JB Structured Clinical Interview for DSM-5: Research Version. American Psychiatric Association; 2015. [Google Scholar]
- 68. Ellman LM, Schiffman J, Mittal VA. Community psychosis risk screening: an instrument development investigation. J Psychiatr Brain Sci. 2020;5:e200019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gur RC, Ragland JD, Moberg PJ, et al. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25(5):766–776. [DOI] [PubMed] [Google Scholar]
- 70. Gur RC, Ragland JD, Moberg PJ, et al. Computerized neurocognitive scanning: II. The profile of schizophrenia. Neuropsychopharmacology. 2001;25(5):777–788. [DOI] [PubMed] [Google Scholar]
- 71. Bernard JA, Mittal VA. Updating the research domain criteria: the utility of a motor dimension. Psychol Med. 2015;45(13):2685–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bermanzohn PC, Siris SG. Akinesia: a syndrome common to parkinsonism, retarded depression, and negative symptoms of schizophrenia. Compr Psychiatry. 1992;33(4):221–232. [DOI] [PubMed] [Google Scholar]
- 73. Osborne KJ, Mittal VA. External validation and extension of the NAPLS-2 and SIPS-RC personalized risk calculators in an independent clinical high-risk sample. Psychiatry Res. 2019;279:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang T, Li H, Tang Y, et al. Validating the predictive accuracy of the NAPLS-2 psychosis risk calculator in a clinical high-risk sample from the SHARP (Shanghai At Risk for Psychosis) program. Am J Psychiatry. 2018;175(9):906–908. [DOI] [PubMed] [Google Scholar]
- 75. Hall RCW. Global assessment of functioning: a modified scale. Psychosomatics. 1995;36(3):267–275. [DOI] [PubMed] [Google Scholar]
- 76. Gur RC, Richard J, Hughett P, et al. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 2010;187(2):254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dean DJ, Bernard JA, Damme KSF, O’Reilly R, Orr JM, Mittal VA. Longitudinal assessment and functional neuroimaging of movement variability reveal novel insights into motor dysfunction in clinical high risk for psychosis. Schizophr Bull. doi: 10.1093/schbul/sbaa072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Popper K The Logic of Scientific Discovery. Routledge; 2005. [Google Scholar]
- 79. R: The R Project for Statistical Computing. https://www.r-project.org/. Accessed June 8, 2020.
- 80. psych citation info. https://cran.r-project.org/web/packages/psych/citation.html. Accessed June 8, 2020.
- 81. Kurtz MM, Ragland JD, Bilker W, Gur RC, Gur RE. Comparison of the continuous performance test with and without working memory demands in healthy controls and patients with schizophrenia. Schizophr Res. 2001;48(2–3):307–316. [DOI] [PubMed] [Google Scholar]
- 82. van Harten PN, Walther S, Kent JS, Sponheim SR, Mittal VA. The clinical and prognostic value of motor abnormalities in psychosis, and the importance of instrumental assessment. Neurosci Biobehav Rev. 2017;80:476–487. [DOI] [PubMed] [Google Scholar]
- 83. Schiffman J, Ellman LM, Mittal VA. Individual differences and psychosis-risk screening: practical suggestions to improve the scope and quality of early identification. Front Psychiatry. 2019;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vargas T, Dean DJ, Osborne KJ, et al. Hippocampal subregions across the psychosis spectrum. Schizophr Bull. 2018;44(5):1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Damme KSF, Ristanovic I, Vargas T, Mittal VA. Timing of menarche and abnormal hippocampal connectivity in youth at clinical-high risk for psychosis. Psychoneuroendocrinology. 2020;117:104672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mittal VA, Walker EF. Advances in the neurobiology of stress and psychosis. Schizophr Res. 2019;213:1–5. [DOI] [PubMed] [Google Scholar]
- 87. Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204(2):295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Osborne KJ, Bernard JA, Gupta T, et al. Beat gestures and postural control in youth at ultrahigh risk for psychosis. Schizophr Res. 2017;185:197–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66(1):13–20. [DOI] [PubMed] [Google Scholar]
- 90. Shankman SA, Mittal VA, Walther S. An examination of psychomotor disturbance in current and remitted MDD: an RDoC study. J Psychiatr Brain Sci. 2020;5:e200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Baranowski T. Methodologic issues in self-report of health behavior. J Sch Health. 1985;55(5):179–182. [DOI] [PubMed] [Google Scholar]
- 92. Carol EE, Mittal VA. Self-reported cannabis use is inconsistent with the results from drug-screening in youth at ultra high-risk for psychosis in Colorado. Schizophr Res. 2014;157(1-3):317–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lindwall M, Ljung T, Hadžibajramović E, Jonsdottir IH. Self-reported physical activity and aerobic fitness are differently related to mental health. Mental Health and Physical Activity. 2012;5(1):28–34. [Google Scholar]
- 94. Buijink AWG, Broersma M, van der Stouwe AMM, et al. Rhythmic finger tapping reveals cerebellar dysfunction in essential tremor. Parkinsonism Relat Disord. 2015;21(4):383–388. [DOI] [PubMed] [Google Scholar]
- 95. Carroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia as measured by a repetitive finger tapping task. Brain Cogn. 2009;71(3):345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Keskinen E, Marttila A, Marttila R, et al. Interaction between parental psychosis and early motor development and the risk of schizophrenia in a general population birth cohort. Eur Psychiatry. 2015;30(6):719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mittal VA, Tessner KD, McMillan AL, Delawalla Z, Trotman HD, Walker EF. Gesture behavior in unmedicated schizotypal adolescents. J Abnorm Psychol. 2006;115(2):351–358. [DOI] [PubMed] [Google Scholar]
- 98. Millman ZB, Goss J, Schiffman J, Mejias J, Gupta T, Mittal VA. Mismatch and lexical retrieval gestures are associated with visual information processing, verbal production, and symptomatology in youth at high risk for psychosis. Schizophr Res. 2014;158(1-3):64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bernard JA, Millman ZB, Mittal VA. Beat and metaphoric gestures are differentially associated with regional cerebellar and cortical volumes. Human Brain Mapping. 2015;36(10):4016–4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Walther S, Mittal VA. Why we should take a closer look at gestures. Schizophr Bull. 2016;42(2):259–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Walther S, Eisenhardt S, Bohlhalter S, et al. Gesture performance in schizophrenia predicts functional outcome after 6 months. Schizophr Bull. 2016;42(6):1326–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Walther S, Vanbellingen T, Müri R, Strik W, Bohlhalter S. Impaired gesture performance in schizophrenia: particular vulnerability of meaningless pantomimes. Neuropsychologia. 2013;51(13):2674–2678. [DOI] [PubMed] [Google Scholar]
- 103. Walther S, Stegmayer K, Sulzbacher J, et al. Nonverbal social communication and gesture control in schizophrenia. Schizophr Bull. 2015;41(2):338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nagels A, Kircher T, Steines M, Grosvald M, Straube B. A brief self-rating scale for the assessment of individual differences in gesture perception and production. Learning and Individual Differences. 2015;39:73–80. [Google Scholar]
- 105. Nagels A, Kircher T, Grosvald M, Steines M, Straube B. Evidence for gesture-speech mismatch detection impairments in schizophrenia. Psychiatry Res. 2019;273:15–21. [DOI] [PubMed] [Google Scholar]
- 106. Suffel A, Nagels A, Steines M, Kircher T, Straube B. Feeling addressed! The neural processing of social communicative cues in patients with major depression. Hum Brain Mapp. 2020;41(13):3541–3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dantchev N, Widlöcher DJ. The measurement of retardation in depression. J Clin Psychiatry. 1998;59(suppl 14):19–25. [PubMed] [Google Scholar]
- 108. Vanbellingen T, Kersten B, Van Hemelrijk B, et al. Comprehensive assessment of gesture production: a New Test of Upper Limb Apraxia (TULIA). Eur J Neurol. 2010;17(1):59–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.