Abstract
Background
Schizophrenia (SCZ) and treatment-resistant schizophrenia (TRS) are associated with aberrations in immune-inflammatory pathways. Increased high mobility group protein 1 (HMGB1), an inflammatory mediator, and Dickkopf-related protein (DKK1), a Wnt/β-catenin signaling antagonist, affect the blood-brain barrier and induce neurotoxic effects and neurocognitive deficits.
Aim
The present study aims to examine HMGB1 and DDK1 in nonresponders to treatments (NRTT) with antipsychotics (n = 60), partial RTT (PRTT, n = 55), and healthy controls (n = 43) in relation to established markers of SCZ, including interleukin (IL)-6, IL-10, and CCL11 (eotaxin), and to delineate whether these proteins are associated with the SCZ symptom subdomains and neurocognitive impairments.
Results
HMGB1, DKK1, IL-6, and CCL11 were significantly higher in SCZ patients than in controls. DKK1 and IL-6 were significantly higher in NRTT than in PRTT and controls, while IL-10 was higher in NRTT than in controls. Binary logistic regression analysis showed that SCZ was best predicted by increased DDK1 and HMGB1, while NRTT (vs PRTT) was best predicted by increased IL-6 and CCL11 levels. A large part of the variance in psychosis, hostility, excitation, mannerism, and negative (PHEMN) symptoms and formal thought disorders was explained by HMGB1, IL-6, and CCL11, while most neurocognitive functions were predicted by HMGB1, DDK1, and CCL11.
Conclusions
The neurotoxic effects of HMGB1, DKK1, IL-6, and CCL11 including the effects on the blood-brain barrier and the Wnt/β-catenin signaling pathway may cause impairments in executive functions and working, episodic, and semantic memory and explain, in part, PHEMN symptoms and a nonresponse to treatment with antipsychotic drugs.
Keywords: schizophrenia, treatment resistance, neuro-immune, inflammation, cytokines, neurocognition
Introduction
The World Health Organization reported that schizophrenia (SCZ) patients die at a younger age as expected due to preventable physical diseases, such as cardiovascular disease, metabolic disease, and infections.1 These diseases have an immune-inflammatory etiology and, therefore, those comorbidities may be explained by the neuroimmune theory of SCZ. The first comprehensive neuroimmune theory of SCZ was introduced by Smith and Maes2 in 1995 as the “macrophage-T-lymphocyte theory” considering that activated macrophages and T lymphocytes are key phenomena in the pathophysiology of SCZ.2 Two years later, Maes and colleagues3 reported that SCZ is accompanied by an ongoing inflammatory response as indicated by increased plasma acute-phase proteins as well as complement components 3 and 4 and immunoglobulins G and M.In addition, these authors reported that increased plasma levels of interleukin (IL)-6, soluble IL-1 receptor antagonist (sIL-1RA), IL-8, and IL-10 are associated with treatment-resistant schizophrenia (TRS).4–6
Those findings on the immune-inflammatory response system (IRS) in SCZ are now well-replicated in meta-analytic studies.7,8 New studies showed that activated M1 macrophage (increased tumor necrosis factor-alpha [TNF-α] and IL-6), T helper (Th)-1 (increased interferon [IFN]-γ and IL-2), Th-2 (IL-4 and IL-5), Th-17 (IL-17), and T regulatory (Treg) cell activation (IL-10) coupled with increased immunoglobulin A levels to Gram-negative bacteria and neurotoxic tryptophan catabolites (TRYCATs) in (deficit) SCZ.9–14Furthermore, TRS is characterized by activated M1 and Th-1 phenotypes, increased IL-6 trans-signaling (increased IL-6 and sIL-6R), and elevated chemokines (CCL2, CCL3, and CCL11).13,14
SCZ phenotypes including first-episode psychosis, acute SCZ episodes, TRS, and chronic and deficit SCZ are not only accompanied by an activated IRS but also by the activation of the compensatory immune-regulatory system (CIRS)14 as indicated by enhanced Th-2 and Treg responses (see above) and increased levels of acute-phase proteins, sIL-2R, sIL-1RA, and sTNF-R2.14 These CIRS mechanisms are secondary to IRS activation and downregulate the primary IRS. M1, Th-1, and Th-2 products including IL-1β, TNF-α, IL-6, IFN-γ, CCL11, IL-4, IL-13, and TRYCATs have multiple neurotoxic effects and as such may cause deficits in executive and memory functions and negative and PHEM (psychosis, hostility, excitation, and mannerism) symptoms.11,15–19 Some cytokines/chemokines are state markers of SCZ (eg, IL-1β and IL-6), whereas other biomarkers are trait biomarkers (eg, sIL-2R and IFNγ).14
Severe IRS responses, as in sepsis, are mediated by high mobility group protein (HMGB)1, a damage-associated molecular pattern (DAMP) released by injured or necrotic cells, which acts as a pro-inflammatory cytokine promoting the release of IL-6, TNF-α, and IFN-γ.20,21 In neurological disorders, HMGB1 is a biomarker of neuroinflammation and neurodegeneration, which may cause blood-brain barrier (BBB) dysfunctions.22 Likewise, HMGB1 may impair memory and behaviors in mice mediated via the Toll-like receptor (TLR)4 complex and/or the receptor for advanced glycation end product (RAGE).23 Nevertheless, there are no data on whether HMGB1 is increased in patients with SCZ or TRS and whether this protein is associated with increased IL-6 and IL-10 and impaired cognitive functions.
Inflammation is also accompanied by an upregulation of Dickkopf-related protein 1 (DKK1), a pro-inflammatory glycoprotein secreted by platelets and endothelial cells.24 DKK1 antagonizes the canonical Wnt signaling transduction pathway and, therefore, may interfere with tissue regeneration and repair and, additionally, may induce a rapid disassembly of synapses in mature neurons.24,25 This is further underscored by the recent findings that circulating DKK1 is associated with cognitive decline in older adults.26 Moreover, in a Japanese population, DKK1 genetic variants are associated with SCZ.27 However, there are no data on whether increased DKK1 levels are associated with SCZ, TRS, and neurocognitive impairments and symptom severity.
Hence, the aims of the study are to (1) examine whether HMGB1 and DDK1 are increased in SCZ and TRS and (2) delineate the association between both proteins and established markers of SCZ (IL-6, IL-10, and CCL11), SCZ symptom subdomains, and neurocognitive impairments.
Participants and Methods
Participants
Sixty TRS patients and 55 non-TRS patients, as well as 43 healthy controls (both sexes, ages between 18 and 65 years), were recruited to participate in the current study. All patients were recruited at the Psychiatry Unit at Al-Imam Al-Hussain Medical City in Karbala Governorate-Iraq in 2019. All patients complied with the diagnostic criteria of SCZ according to the DSM-IV-TR. TRS is defined according to Conley and Kelly28 criteria as 2 periods of treatment nonresponse to 2 different antipsychotic treatments at adequate doses (600 mg chlorpromazine equivalents/day), each for 8 weeks in our study (this is 4–6 weeks in Conley and Kelly’s criteria), without a reduction in symptoms and no period of good social/occupational functioning and a Brief Psychiatric Rating Scale (BPRS) total score > 45 and a score of >4 on 2 out of 4 positive symptoms. We used an additional criterion based on the Clinical Global Impression (CGI) Improvement (CGI-I) scale29: nonresponders to treatment (NRTT) were those who did not show any change in the CGI-I or showed worse scores after treatment (minimally worse, much worse, and very much worse) and partial RTT (PRTT) were those with improved scores (minimally, much, or very much). We computed chlorpromazine dose equivalents for antipsychotic drugs using the dose equivalents for antipsychotic drugs method.30
Forty-three healthy controls participated in the study, namely family members or friends of the staff or patients. The normal controls were recruited from the same catchment area (Karbala city) and were all Arabic Shi′a Muslims with low-middle income. We excluded SCZ patients who showed axis-1 DSM-IV-TR diagnoses other than SCZ, including psycho-organic disorders, schizoaffective disorder, autism, major depression, and bipolar disorder. Healthy controls were excluded when they showed a lifetime or current diagnosis of axis I diagnosis or a positive family history of SCZ. Moreover, patients and controls were excluded when they (1) presented with neuro-immune, neuroinflammatory, or neurodegenerative disorders, including Parkinson’s disease, stroke, multiple sclerosis, and Alzheimer’s disease, and (2) suffered from medical illnesses, including diabetes type 1, psoriasis, chronic obstructive pulmonary disease, rheumatoid arthritis, and inflammatory bowel disease. Furthermore, we excluded patients and controls who had ever been using medications that interfere with immune functions, such as glucocorticoids, and immunosuppressive and anti-inflammatory drugs, including nonsteroidal anti-inflammatory drugs, or therapeutic doses of antioxidant supplements 3 months prior to the study. No other drugs were allowed during the study apart from the sporadic use of paracetamol. All subjects showed serum C-reactive protein (CRP) concentrations lower than 6 pg/ml excluding subjects with overt inflammation.
All controls and patients, as well as the guardians of patients (parents or the closest family members), gave written informed consent prior to participation in our study. The study was conducted according to International and Iraq ethics and privacy laws. Approval for the study was obtained from the Institutional Review Board of the University of Karbala (418/2019) and Karbala Health Department (1331/2019), which is in compliance with the International Guidelines for Human Research protection as required by the Declaration of Helsinki, The Belmont Report, Council for International Organizations of Medical Sciences (CIOMS) Guideline, and International Conference on Harmonization in Good Clinical Practice (ICH-GCP).
Measurements
Clinical Assessments.
The diagnosis of SCZ was made by a senior psychiatrist specialized in SCZ according to DSM-IV-TR diagnostic criteria using the Mini-International Neuropsychiatric Interview (M.I.N.I.), in a validated Arabic translation (Iraqi dialect). On the same day as the M.I.N.I., the same psychiatrist employed a semi-structured interview to assess demographics as well as clinical data in all participants and he also measured the CGI-I and Severity (CGI-S) scale.29 Not one of the patients showed complete remission after treatment as defined by the absence of significant remaining residual symptoms. We also assessed the Scale for the Assessments of Negative Symptoms (SANS) to assess the severity of negative symptoms.31 We computed scores reflecting PHEM (psychosis, hostility, excitation, and mannerism) symptoms, FTD (formal thought disorders), and PMR (psycho-motor retardation) as explained previously.16,19 Toward this end, we also assessed the BPRS,32 the Hamilton Depression Rating Scale,33 and the Positive and Negative Syndrome Scale (PANSS) for SCZ.34
On the same day, a well-trained research psychologist, blinded to the clinical diagnosis, completed the Brief Assessment of Cognition in SCZ (BACS)35 to assess episodic memory using the List Learning test, working memory with the Digit Sequencing Task, verbal fluency and semantic memory employing Category Instances and Controlled Word Association tests, attention using the Symbol Coding test, and executive functions using the Tower of London. DSM-IV-TR criteria were used to make the diagnosis of tobacco use disorder (TUD). Body mass index (BMI) was measured on the same day as the clinical interview and was scored as body weight (kg)/length (m2).
Assays.
After an overnight fast, 5 ml of venous blood were sampled, utilizing disposable needle and plastic syringes, between 8:00 and 9:00 am. The samples were transferred into a clean plain tube; blood was left at room temperature for 15 min for clotting, centrifuged at 3000 rpm for 10 min, and then serum was separated and transported into 2 Eppendorf tubes to be stored at −80°C until thawed and analyzed. Commercial enzyme linked immunosorbent assay (ELISA) sandwich kits were used to measure serum CCL11, DKK1, HMGB1, and IL-10 (Elabscience, Inc) and IL-6 (Melsin Medical Co.). All measured concentrations of CCL11 (sensitivity = 9.38 pg/ml), DKK1 (sensitivity = 18.75 pg/ml), HMGB1 (sensitivity = 18.75 pg/ml), and IL-6 (sensitivity = 0.1 pg/ml) were greater than the sensitivity of the assays. There was only 1 IL-10 concentration (4.05 pg/ml in a normal volunteer) that was lower than the sensitivity of the assay (sensitivity = 4.69 pg/ml). We did not apply left censoring and used the actual measurements in the statistical analyses.12 The procedures were followed exactly without modifications according to the manufacturer’s instructions. The intra-assay coefficient of variation (precision within an assay) was <10.0%. Serum CRP was measured using a kit supplied by Spinreact. The test is based on the principle of latex agglutination.
Statistical Analysis
Analysis of variance (ANOVA) was used to check differences in scale variables between categories, and analysis of contingency tables (χ2-test) was to assess associations between nominal variables. In order to assess associations among the biomarkers, clinical, and cognitive scores, we examined correlation matrices based on Pearson’s product-moment and Spearman’s rank-order correlation coefficients. We used multivariate general linear model (GLM) analysis to delineate the associations between diagnosis and the biomarkers while controlling for confounding variables, including nicotine dependence, sex, age, BMI, and education. Consequently, we carried out tests for between-subject effects to delineate the associations between diagnosis and each of the biomarkers. The effect size was estimated using partial eta-squared values. We also computed model generated (GLM analysis) estimated marginal mean (SE) values and performed protected pairwise comparisons among treatment means. In order to control for type-I errors, we employed the false-discovery rate (FDR) procedure.36 We employed binary logistic regression analysis to delineate the best predictors of NRTT (PRTT as reference group) and SCZ (controls as reference group) using the biomarkers as explanatory variables. Odd’s ratios with 95% confidence intervals were computed as well as Nagelkerke values as pseudo-R2 values. We used multiple regression analysis to assess the significant biomarkers, which predict the symptom domains and neurocognitive tests while allowing for possible effects of age, sex, and education. We used an automatic stepwise method with the inclusion of variables with a P-to-entry of .05 and P-to-remove of .06 while checking the R2 change. All regression analyses were checked for collinearity using tolerance and variance inflation factor values. Variables were also z-transformed, and the mean z scores were displayed in bar plots. Tests were 2-tailed and a P-value of .05 was used for statistical significance. All statistical analyses were performed using IBM SPSS windows version 25, 2017.
Results
Sociodemographic Data
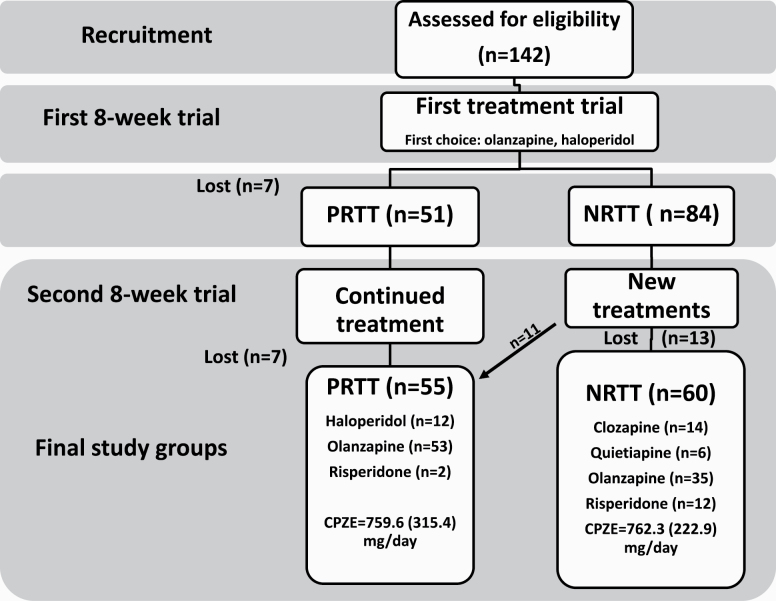
Table 1 shows the sociodemographic data of the NRTT, PRTT, and healthy controls. Figure 1 shows a flow diagram of both subjects and treatments. Finally, 55 PRTT and 60 NRTT were recruited to participate in the study.
Table 1.
Demographic and Clinical Data of Healthy Controls (HC) and Partial (PRTT) and Non (NRTT) Responders to Treatment
| Variables | HCx (n = 43) |
PRTTy (n = 55) |
NRTTz (n = 60) |
F/ψ/χ 2 | df | P |
|---|---|---|---|---|---|---|
| Age (years) | 33.2 (11.1) | 36.5 (9.5) | 36.2 (12.3) | 1.29 | 2/155 | .280 |
| Sex (female/male) | 19/24 | 15/40 | 22/38 | 3.08 | 2 | .214 |
| Single/married? | 12/31z | 35/30 | 32/28x | 6.69 | 2 | .035 |
| BMI (kg/m2) | 27.9 (4.1) | 29.6 (4.3) | 28.4 (4.9) | 1.90 | 2/155 | .153 |
| TUD (No/Yes) | 30/13 | 44/11 | 40/20 | 2.71 | 2 | .258 |
| Employment (No/Yes) | 17/26y,z | 36/19x | 43/17x | 11.63 | 2 | .003 |
| Education (years) | 11.1 (3.6)z | 10.8 (4.5)z | 8.9 (4.7)x,y | 4.21 | 2/155 | .017 |
| Age at onset (years) | — | 27.5 (7.5) | 29.3 (10.2) | 1.14 | 1/113 | .287 |
| List learninga | 54.9 (1.7) | 48.2 (1.5) | 21.4 (1.4) | 142.21 | 1/151 | <.001 |
| Digit sequencing taska | 18.1 (0.5) | 6.8 (0.4) | 2.7 (0.4) | 301.03 | 1/151 | <.001 |
| Category instancesa | 50.5 (1.6) | 41.4 (1.4) | 29.7 (1.3) | 52.09 | 1/151 | <.001 |
| COWAa | 49.1 (1.1) | 20.3 (0.9) | 6.5 (0.9) | 447.92 | 1/151 | <.001 |
| Symbol codinga | 76.4 (1.1) | 8.1 (0.9) | 3.3 (0.9) | 1564.46 | 1/151 | <.001 |
| Tower of Londona | 16.4 (0.5) | 8.6 (0.5) | 2.5 (0.5) | 198.70 | 1/151 | <.001 |
| SANS total scorea | 4.4 (0.3) | 52.5 (12.2) | 91.95 (16.9) | 591.70 | 2/155 | <.001 |
| CGI-I | — | 2.73 (0.45) | 4.20 (0.40) | 342.92 | 1/113 | <.001 |
| CGI-S | — | 4.38 (0.49) | 5.95 (0.70) | 190.63 | 1/113 | <.001 |
| Clozapine (No/Yes) | — | 55/0 | 46/14 | Ψ = 0.356 | =— | <.001 |
| Quetiapine (No/Yes) | — | 55/0 | 54/6 | Ψ = 0.225 | — | .016 |
| Haloperidol (No/Yes) | — | 43/12 | 60/0 | Ψ = 0.357 | — | <.001 |
| Olanzapine (No/Yes) | — | 2/53 | 25/35 | Ψ = 0.448 | — | <.001 |
| Risperidone | — | 53/2 | 48/12 | Ψ = 0.250 | — | .007 |
| Chlorpromazine equivalents (mg/daily) | — | 759.6 (315.4) | 762.3 (222.9) | 0.00 | 1/113 | .957 |
Note: BMI, body mass index; COWA, Controlled Oral Word Association Test; CGI-I, Clinical Global Impression-Improvement scale; CGI-S, Clinical Global Impression- Severity scale; SANS, Scale for the Assessment of Negative Symptoms; TUD, tobacco use disorder. Results are shown as mean (SD), except the neuropsychological test scores, which are shown as estimated marginal mean (SE) values after considering the effects of age, sex, and education. x, y, z: pairwise comparison
aThe test scores are significantly different between the 3 study groups.
Fig. 1.
Flow diagram of both patients and treatments with number of nonresponders to treatment (NRTT), partial responders to treatment (PRTT), and loss to follow-up. We recruited 142 schizophrenia (SCZ) patients treated with antipsychotic drugs during 2 trials with antipsychotic drugs. During the first trial, patients were treated for 8 weeks and after this trial divided into those without a clinical response (n = 84) and a partial response (n = 51) according to Clinical Global Impression Improvement (CGI-I) scores (loss to follow-up: n = 7). The partial responders continued to take the same medication for another 2 months, while we lost again 7 patients in the follow-up yielding a final PRTT study group of n = 55. The nonresponders to a first antipsychotic agent were switched to another antipsychotic treatment for another 8 weeks, and during this follow-up period, we lost 13 patients. Two months later, 11 patients showed a partial response to treatment. Finally, 55 PRTT or 60 NRTT according to CGI-I scores and Conley and Kelly28 criteria participated in this study. CPZE, chlorpromazine equivalents mg/day.
There were no significant differences in age, sex ratio, BMI, and TUD between PRTT and NRTT and normal controls. There were somewhat more NRTT who were single than normal controls. Significantly more SCZ patients were unemployed as compared with controls, while years of education were somewhat lower in NRTT. There were no differences in age at onset between both SCZ subgroups.
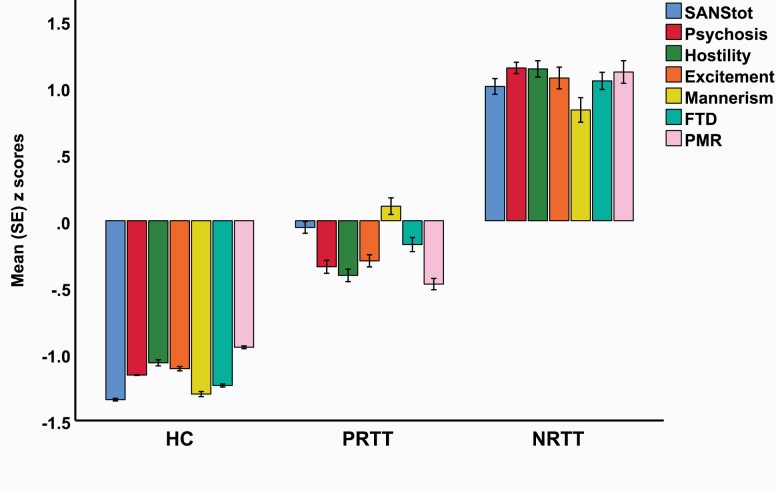
All 6 cognitive test scores were significantly different between the 3 study groups, and the scores decreased from controls to PRTT to NRTT. These differences remained significant after the FDR correction. The total SANS score was significantly different between the 3 study groups. Figure 2 displays a plot of all symptom domains examined in this study (shown as z scores). Psychosis (F = 772.55, df = 2/152, P < .001), hostility (F = 498.12, df = 2/152, P < .001), excitement (F = 320.71, df = 2/152, P < .001), mannerism (F = 204.41, df = 2/152, P < .001), FTD (F = 414.15, df = 2/152, P < .001), and PMR (F = 297.46, df = 2/152, P < .001) were significantly different between the 3 study groups and increased from controls to PRTT to NRTT. Table 1 also shows the measurement of the clinical global impression (CGI) score in the SCZ patients. Both the CGI-I and CGI-S scores were significantly higher in NRTT than in PRTT. All CGI-I scores in PRTT equaled 2 (much improved) or 3 (minimally improved) and in NRTT 4 (no change) or 5 (minimally worse). The table also shows the current medication patients were taking. Thus, NRTT were more often treated with clozapine, quetiapine, and risperidone than PRTT who were more often treated with olanzapine and haloperidol.
Fig. 2.
Bar plot displaying the scores on the SANS (Scale for the Assessment of Negative Symptoms) psychosis, hostility, excitement, mannerism, FTD (formal thought disorders), and PMR (psychomotor retardation) was significantly different between the 3 study groups and increased from healthy controls (HC) to partial responders to treatment (PRTT) to nonresponders to treatment (NRTT).
Biomarkers Between the Study Groups
In the total study group, there were significant correlations between IL-6 and DKK1 (r = .641, P < .001, n = 158) and HMGB1 (r = .249, P = .002, n = 158) and a significant association between IL-10 and HMGB1 (r = .465, P < .001, n = 158). The correlation between IL-6 and HMGB1 was established in controls (r = .649, P < .001, n = 43) and SCZ patients (r = .561, P < .001, n = 115). A significant correlation between IL-10 and DKK1 was detected in controls (r = .395, P = .009, n = 43) and SCZ patients (r = .430, P < .001, n = 115).
Table 2 shows the results of multivariate GLM analyses comparing the differences in the biomarkers between the 3 study groups while adjusting for sex, age, BMI, and TUD. There were highly significant differences in the biomarkers between the groups with an effect size of 0.245, while the 4 covariates had no significant effects. Tests for between-subject effects and table 3, which shows the estimated marginal means, indicate that DKK1 and IL-6 were significantly higher in NRTT than in PRTT and controls and that HMGB1 was significantly different between SCZ and controls. IL-10 was higher in NRTT than in controls while PRTT patients occupied an intermediate position. The intergroup differences in the 5 biomarkers remained significant after FDR correction. Supplementary material shows that extraneous variables including the drug state had no significant effects on the biomarkers.
Table 2.
Results of Multivariate General Linear Model (GLM) Analysis Showing the Associations Between Biomarkers and Diagnosis While Adjusting for Background Variables
| Type | Dependent Variables | Explanatory Variables | F | df | P | Partial η 2 |
|---|---|---|---|---|---|---|
| Multivariate | DKK1, HMGB1, IL-6, IL-10, CCL11 | Diagnosis | 9.52 | 10/294 | <.001 | 0.245 |
| Sex | 1.52 | 5/147 | .186 | 0.049 | ||
| TUD | 0.45 | 5/147 | .814 | 0.015 | ||
| Age | 0.12 | 5/147 | .989 | 0.004 | ||
| BMI | 0.71 | 5/147 | .617 | 0.024 | ||
| Tests for between-subject effects | DKK1 | Diagnosis | 8.41 | 1/151 | <.001 | 0.100 |
| HMGB1 | Diagnosis | 35.02 | 1/151 | <.001 | 0.317 | |
| IL-6 | Diagnosis | 14.66 | 1/151 | <.001 | 0.163 | |
| IL-10 | Diagnosis | 3.53 | 1/151 | .032 | 0.045 | |
| CCL11 | Diagnosis | 4.52 | 1/151 | .012 | 0.056 |
Note: BMI, body mass index; CCL11, CC-motif chemokine 11 or eotaxin; DKK1, Dickkopf protein 1; HMGB1, high mobility group box 1 protein; IL, interleukin; TUD, tobacco use disorder. Diagnosis: partial responders to treatment vs nonresponders to treatment vs healthy controls.
Table 3.
Model-Generated Estimated Marginal Means Values (SE) of the Biomarkers in Partial Responders to Treatment (PRTT), Nonresponders to Treatment (NRTT), and Healthy Controls (HC)
| Biomarkers | HCa | PRTTb | NRTTc |
|---|---|---|---|
| DKK1 pg/ml | 702.1 (92.2)c | 848.3 (93.5)c | 1120 (79.4)a,b |
| HMGB1 ng/ml | 7.90 (1.72)b,c | 18.90 (1.74)a | 22.06 (1.48)a |
| IL-6 pg/ml | 4.95 (0.91)c | 5.95 (0.92)c | 7.91 (0.78)a,b |
| IL-10 pg/ml | 10.67 (0.91)c | 12.31 (0.93) | 13.96 (0.79)a |
| CCL11 pg/ml | 179.0 (10.0)c | 198.3 (10.1)c | 222.1 (8.6)a |
Note: CCL11, CC-motif chemokine 11 or eotaxin; DKK1, Dickkopf protein 1; HMGB1, high mobility group box 1 protein; IL, interleukin.
a–cPairwise comparisons between group means.
Table 4 shows the results of 2 binary logistic regression analyses examining the best predictors of SCZ (vs controls) and NRTT (vs PRTT) using an automatic stepwise method with biomarkers as explanatory variables while allowing for the effects of age, sex, and education. The first regression analysis showed that SCZ was best predicted by increased levels of DDK1 and HMGB1 (χ 2 = 60.58, df = 2, P < .001, Nagelkerke = 0.462) while the accuracy was 74.7% with a sensitivity of 72.2% and a specificity of 81.4%. The second regression shows that IL-6 combined with CCL11 were the best predictors of NRTT vs PRTT (χ 2 = 25.84, df = 2, P < .001, Nagelkerke = 0.268).
Table 4.
Results of 2 Different Binary Logistic Regression Analyses With Schizophrenia (vs healthy controls) and Nonresponders to Treatment (NRTT) vs Partial Responders to Treatment (PRTT) as Dependent Variables and the Biomarkers as Explanatory Variables
| Dichotomies | Explanatory Variables | B | SE | Wald | df | P | OR | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Schizophrenia/controls | DKK1 | 0.459 | 0.240 | 5.60 | 1 | .018 | 1.77 | 1.10–2.83 |
| HMGB1 | 1.602 | 0.282 | 32.26 | 1 | <.001 | 4.96 | 2.86–8.63 | |
| NRTT/PRTT | IL-6 | 1.039 | 0.221 | 16.44 | 1 | <.001 | 2.83 | 1.71–4.67 |
| CCL11 | 0.512 | 0.211 | 5.37 | 1 | .020 | 1.67 | 1.08–2.57 |
Note: OR, odds ratio; 95% CI, 95% confidence intervals; CCL11, CC-motif chemokine 11 or eotaxin; DKK1, Dickkopf protein 1; HMGB1, high mobility group box 1 protein; IL, interleukin.
Prediction of Symptom Domains by Biomarkers
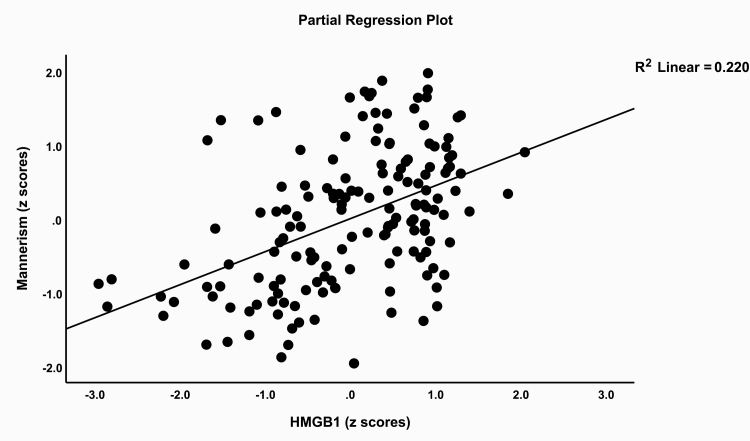
Table 5 shows stepwise multiple regression analyses with the symptom domains as dependent variables and the 5 biomarkers as explanatory variables while allowing for the effects of age, sex, and education. Regression #1 shows that 35.6% of the variance in the SANS score was explained by HMGB1, IL-6, and CCL11. Regressions #2, #3, and #4 show that these variables explained a considerable part of the variance in psychosis (32.2%), hostility (30.7%), and excitation (29.9%). Regression #5 shows that 32.0% of the variance in mannerism was explained by HMGB1, DKK1, and CCL11. Figure 3 shows the partial regression plot of the mannerism scores on HMGB1 levels. IL-6 and HMGB1 together explained 22.2% of the variance in PMR (regression #6). Regression #7 shows that 36.9% of the variance in FTD was explained by HMGB1, IL-6, CCL11, and education.
Table 5.
Results of Multiple Regression Analysis With Schizophrenia Symptom Domains as Dependent Variables
| Dependent variables | Explanatory Variables | β | t | P | F model | df | P | R 2 |
|---|---|---|---|---|---|---|---|---|
| #1. SANS | Model | 28.38 | 3/154 | <.001 | .356 | |||
| HMGB1 | .443 | 6.57 | <.001 | |||||
| IL-6 | .224 | 3.35 | .001 | |||||
| CCL11 | .179 | 2.74 | .007 | |||||
| #2. Psychosis | Model | 24.34 | 3/154 | <.001 | .322 | |||
| HMGB1 | .401 | 5.79 | <.001 | |||||
| IL-6 | .250 | 3.65 | <.001 | |||||
| CCL11 | .158 | 2.36 | .020 | |||||
| #3. Hostility | Model | 22.74 | 3/154 | <.001 | .307 | |||
| HMGB1 | .370 | 5.29 | <.001 | |||||
| IL-6 | .271 | 3.91 | <.001 | |||||
| CCL11 | .158 | 2.34 | .021 | |||||
| #4. Excitation | Model | 21.87 | 3/154 | <.001 | .299 | |||
| HMGB1 | .382 | 5.42 | <.001 | |||||
| IL-6 | .238 | 3.42 | .001 | |||||
| CCL-11 | .168 | 2.46 | .015 | |||||
| #5. Mannerism | Model | 24.21 | 3/154 | <.001 | .320 | |||
| HMGB1 | .448 | 6.60 | <.001 | |||||
| DKK1 | .195 | 2.90 | .004 | |||||
| CCL11 | .173 | 2.58 | .011 | |||||
| #6. PMR | Model | 22.16 | 2/155 | <.001 | .222 | |||
| IL-6 | .299 | 4.09 | <.001 | |||||
| HMGB1 | .298 | 4.07 | <.001 | |||||
| #7. FTD | Model | 22.36 | 4/153 | <.001 | .369 | |||
| HMGB1 | .424 | 6.32 | <.001 | |||||
| IL-6 | .201 | 2.99 | .003 | |||||
| CCL11 | .194 | 2.98 | .003 | |||||
| Education | −.138 | −2.10 | .037 |
Note: CCL11, CC-motif chemokine 11 or eotaxin; DKK1, Dickkopf protein 1; FTD, formal thought disorders; HMGB1, high mobility group box 1 protein; IL, interleukin; PMR, psychomotor retardation; SANS, Scale for the Assessment of Negative Symptoms.
Fig. 3.
Partial regression plot of the mannerism scores on high mobility group box (HMGB)1 plasma concentrations.
Prediction of Cognitive Impairments by Biomarkers
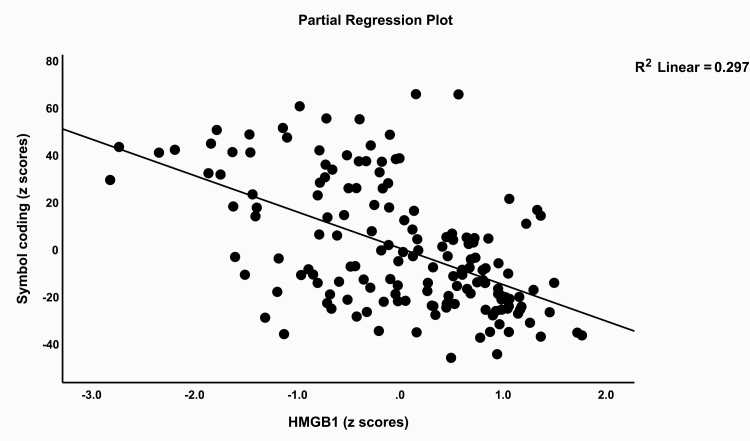
Table 6 shows multiple regression analyses with the cognitive test results as dependent variables and biomarkers as explanatory variables while allowing for the effects of age, sex, and education. We found that (regression #1) 20.6% of the variance in List Learning scores was explained by the regression on HMGB1, DKK1, and CCL11 (all inversely associated) and education (positively associated). Up to 33.5% of the variance in Digit Sequencing Task scores (regression #2) was explained by the combined effects of HMGB1, IL-6, CCL11 (inversely), and education (positively). Part of the variance (20.7%) in Category Instances scores was explained by HMGB1, DKK1 (negatively), and education (positively). We found that 33.4% of the variance in the COWA test (regression #4) scores was explained by the cumulative effects of HMGB1 and IL-6 (both negatively), while 39.5% of the variance in Symbol Coding scores (#5) was associated with HMGB1 and DKK1. Figure 4 shows the partial regression plot association between Symbol Coding scores and HMGB1 levels. Regression #6 shows that part of the variance in the Tower of London scores was explained by HMGB1 and DKK1 (inversely) and education (positively).
Table 6.
Results of Multiple Regression Analysis With Neurocognitive Test Scores as Dependent Variables
| Dependent Variables | Explanatory Variables | β | t | P | F model | df | P | R 2 |
|---|---|---|---|---|---|---|---|---|
| #1. List learning | Model | 9.95 | 4/153 | <.001 | .206 | |||
| HMGB1 | −.228 | −3.08 | .002 | |||||
| Education | .244 | 3.35 | .001 | |||||
| DKK1 | −.179 | −2.45 | .015 | |||||
| CCL11 | −.152 | −2.08 | .039 | |||||
| #2. Digit sequencing task | Model | 19.30 | 4/153 | <.001 | .335 | |||
| HMGB1 | −.402 | −5.84 | <.001 | |||||
| IL-6 | −.192 | −2.78 | .006 | |||||
| Education | .163 | 2.42 | .017 | |||||
| CCL11 | −.160 | −2.40 | .018 | |||||
| #3. Category instances | Model | 13.37 | 3/154 | <.001 | .207 | |||
| HMGB1 | −.292 | −4.01 | <.001 | |||||
| DKK1 | −.242 | −3.33 | .001 | |||||
| Education | .165 | 2.28 | .024 | |||||
| #4. COWA | Model | 38.83 | 2/155 | <.001 | .334 | |||
| HMGB1 | −.490 | −7.23 | <.001 | |||||
| IL-6 | −.208 | −3.07 | .003 | |||||
| #5. Symbol coding | Model | 50.34 | 2/154 | <.001 | .395 | |||
| HMGB1 | −.583 | −9.20 | <.001 | |||||
| DKK1 | .165 | −2.61 | .010 | |||||
| #6. Tower of London | Model | 26.36 | 3/154 | <.001 | .339 | |||
| HMGB1 | −.436 | −6.56 | <.001 | |||||
| Education | .279 | 4.32 | <.001 | |||||
| DKK1 | −.153 | −2.30 | .023 |
Note: CCL11, CC-motif chemokine 11 or eotaxin; DKK1, Dickkopf protein 1; FTD, formal thought disorders; HMGB1, high mobility group box 1 protein; IL, interleukin; PMR, psychomotor retardation; SANS, Scale for the Assessment of Negative Symptoms.
Fig. 4.
Partial regression plot of the Symbol Coding test scores on high mobility group box (HMGB)1 plasma concentrations.
Discussion
The first major finding of this study is that SCZ is characterized by increased levels of HMGB1, DKK1, IL-6, and CCL11 as compared with healthy controls. Our results that IL-6 and CCL11 are increased in SCZ are in accordance with findings in previous studies.5,12,13,17–19,37,38
HMGB1 is a transcriptional modifier that acts as a pro-inflammatory cytokine promoting the release of other cytokines, including IL-6.21 HMGB1 is normally localized in the nucleus but following immune signals, including lipopolysaccharides (LPS) and TNF-α, HMGB1 is translocated to the cytoplasm and may aggregate and accumulate in secretory lysosomes to be secreted from activated monocytes and natural killer cells.39–41 Extracellular HMGB1 engages membrane receptors leading to immune activation and neuroinflammation42 by activating TLR2 and TLR4 signaling.20 Moreover, hemoglobin released from lysed red blood cells may synergize with HMGB1 to stimulate the production and release of pro-inflammatory cytokines.43 Haptoglobin may form a complex with HMGB1, thereby increasing IL-10.43 In this respect, we found that HMGB1 levels are significantly and positively associated with IL-6 and IL-10, indicating that increased HMGB1 is part in the immune-inflammatory pathophysiology of SCZ.
This is also the first report that serum DKK1 concentrations are significantly increased in SCZ. DKK1 is secreted by endothelial cells and platelets,24,44 and increased peripheral levels are observed in acute infections.45 In mice, LPS may increase the expression of DKK1 and IL-6,46 while in the present study, there were significant and positive correlations between IL-6 and DKK1. Nevertheless, one study reported lowered DKK1 and upregulated mRNA expression of Wnt signaling pathway genes in SCZ.47 Both DKK1 and IL-6 and other cytokines, which are increased in SCZ (including TNF-α), may inhibit the Wnt-β-catenin signaling pathway.47
The second major finding of our study is that NRTT was associated with increased DKK1, IL-6, and CCL11 as compared with PRTT and that IL-10 was significantly increased in NRTT as compared with controls. Increased IL-6 and IL-10 levels were previously reported in patients with TRS.4,6 Our results further extend the findings that TRS is accompanied by IRS activation as indicated by increased levels of sIL-6R, IL-8, CCL2, and CCL3 and by CIRS activation as indicated by elevated levels of sIL-1RA, sTNFR1, and sTNFR2.4–6,13,14 Noto et al48 reported that drug-naïve first-episode psychosis is characterized by significant IRS (M1 + Th-1 + Th-17) and CIRS (Th-2 and Treg) responses and that treatment with risperidone attenuates both IRS and CIRS responses, while increased baseline levels of some CIRS biomarkers may predict clinical improvement. All in all, the above results and the current study indicate that antipsychotic agents may attenuate the immune response in some SCZ patients, namely in PRTT, thereby improving symptoms and neurocognitive deficits.
The third major finding of this study is that PHEM and negative symptoms and FTD are highly significantly predicted by increased HMGB1, IL-6, and CCL11, while impairments in executive functions, working memory and episodic and semantic memory, and attention are predicted by increased levels of especially HMGB1 and DKK1 and CCL11. Previously, we reported that CCL11 and IL-6 and other neurotoxic immune compounds (see Introduction) significantly predict PHEM and negative symptoms, FTD, and PMR, suggesting that immune-inflammatory pathways are involved in the pathophysiology of SCZ.12,18,19
There is now evidence that HMGB1 plays an important role not only in the propagation of immune-inflammatory responses (see above) but also in neuroinflammatory and neurodegenerative processes and the associated memory impairments in Parkinson’s and Alzheimer’s disease and multiple sclerosis.49 HMGB1 released from necrotic neurons or inflamed microglia may act on microglia macrophage antigen complex 1, thereby stimulating the production of multiple neurotoxic factors.50 HMGB1 may cause neurite degeneration via the TLR4 complex and phosphorylation of Myristoylated Alanine Rich C-Kinase Substrate via mitogen-activated protein kinases.51 On the other hand, HMGB1-specific antibodies protect against lethal endotoxaemia20 and preserve BBB integrity, thereby attenuating glial activation, oxidative stress, and elevated inflammatory gene expression, including IL-6, damage to hippocampal neurons, neuronal degeneration, and brain damage.52,53 Furthermore, blocking of HMGB1 signaling improves neuroprotection in neurodegenerative disorders.54 Clinical studies show that sepsis survivors have permanent cognitive deficits, which are probably mediated via elevated HMGB1 levels.55
As described above, DKK1 is a natural antagonist of the Wnt/β-catenin signaling pathway,56 which is a key regulator of BBB function and contributes to its formation, maturation, and function.57–59 Elevating β-catenin signaling leads to lowered permeability of the endothelial cells of the BBB,59 whereas DKK1-induced aberrations in Wnt/β-catenin signaling may induce BBB breakdown.60,61 Moreover, the administration of DKK1 to a mixture of human neurons and astrocytes in culture results in the downregulation of neuronal processes explaining that lowering DKK1 protects against neurotoxicity.62 DKK1 mediates amyloid-ß-associated synaptic loss, causes a rapid disassembly of synapses in mature neurons,25 induces BCL2 associated X (BAX), and decreases B-cell lymphoma 2, thereby causing cell death.63 Moreover, loss of DKK1 may counteract the downregulation of hippocampal neurogenesis and accompanying cognitive impairments that are associated with increasing DKK1 levels with age.64 This may explain that DKK1-deficient mice show improved working memory, memory consolidation, and affective behaviors.64 It is important to note that the effects of HMGB1 and DKK1 affecting BBB functions may aggravate the effects of CCL11, neurotoxic TRYCATs, and LPS, which all lead to the breakdown of the BBB in SCZ.11
Limitation of the Study
The results of our study should be discussed with respect to its limitations. First, we performed a case-control study and, therefore, no firm causal inferences may be established. Second, it would have been more interesting if we had examined haptoglobin and hemoglobin in relation to HMGB1 as well as a broader panel of cytokines and downstream targets of the Wnt and HMGB1 pathways. Third, we found that treatment with olanzapine was associated with increased CCL11 levels. Nevertheless, our intergroup analyses were adjusted for the drug state, which did not affect the differences in CCL11 between the study groups. Finally, although all subjects were drawn from the same community and catchment area, there were differences in education and employment between patients and controls. In our studies, lower education is a consistent predictor of the severity of SCZ, which may be explained by the knowledge that better cognitive reserve is inversely associated with the severity of SCZ.65 Higher unemployment rates are consistently reported in SCZ as a consequence of mainly socioeconomic pressures.66
Conclusions
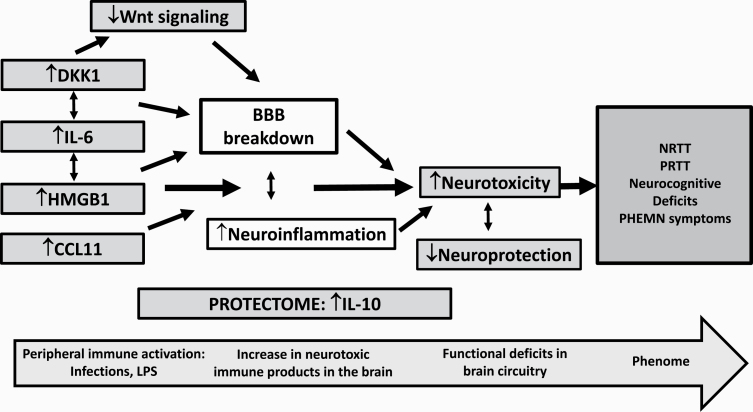
HMGB1, DKK1, IL-6, and CCL11 were significantly increased in SCZ, whereas a nonresponse to antipsychotic treatment was associated with increased DKK1, IL-6, and CCL11. HMGB1 and DKK1 participate in the immune pathophysiology of SCZ and may explain, in part, the phenome of SCZ (neurocognitive impairments and various symptom clusters) via their detrimental effects on the BBB and neurotoxic effects. The results show that the immune variables measured here are biomarkers of a nonresponse to treatment. Figure 5 displays the proposed mechanism of action of the different pathways leading to the phenome features of SCZ. Future research should examine whether TRS is a qualitative different class as compared with non-TRS and should delineate the neurocognitive and symptomatic features of TRS.
Fig. 5.
The proposed mechanisms of action of the different pathways leading to the phenome features of schizophrenia, including neurocognitive deficits and psychosis, hostility, excitation, mannerism, and negative symptoms (PHEMN) and a nonresponse to treatment (NRTT). We suggest that an infectious process or increased LPS from Gram-negative bacteria may activate peripheral immune-inflammatory pathways, including interleukin-6 (IL-6),14 high mobility box protein 1 (HMGB1), Dickkopf 1 (DKK1), and CCL11 (eotaxin). Increased IL-6 levels associate with increased HMGB1 and DKK1. The latter may cause a downregulation of Wnt signaling, and all factors together may cause the breakdown of the blood-brain barrier (BBB) and, consequently, neuroinflammation. NRTT patients also show marginally increased levels of IL-10, a negative immune-regulatory cytokine, which has protective functions and is part of the protectome. Nevertheless, impairments are detected in other parts of the protectome (eg, lowered natural protective immunoglobulin M) leading to lowered (neuro)protection,14,17 which together with increased neurotoxicity may cause the phenome of schizophrenia.
Supplementary Material
Acknowledgment
We acknowledge the staff of the Psychiatry Unit at Al-Imam Al-Hussain Medical City in Karbala city for their help in the collection of samples and the high-skilled staff members of Asia Clinical Laboratory, Najaf city, for their help in the ELISA measurements .The authors have no conflict of interest with any commercial or other association in connection with the submitted article.
Funding
There was no specific funding for this specific study.
Authors’ Contribution
All the contributing authors have participated in the preparation of the manuscript.
References
- 1. World Health Organization (WHO). Schizophrenia. In: World Health Organization. World Health Organization; 2018. https://www.who.int/news-room/fact-sheets/detail/schizophrenia. Accessed February 4, 2020. [Google Scholar]
- 2. Smith RS, Maes M. The macrophage-T-lymphocyte theory of schizophrenia: additional evidence. Med Hypotheses. 1995;45:135–141. [DOI] [PubMed] [Google Scholar]
- 3. Maes M, Delange J, Ranjan R, et al. Acute phase proteins in schizophrenia, mania and major depression: modulation by psychotropic drugs. Psychiatry Res. 1997;66(1):1–11. [DOI] [PubMed] [Google Scholar]
- 4. Lin A, Kenis G, Bignotti S, et al. The inflammatory response system in treatment-resistant schizophrenia: increased serum interleukin-6. Schizophr Res. 1998;32(1):9–15. [DOI] [PubMed] [Google Scholar]
- 5. Maes M, Bocchio Chiavetto L, Bignotti S, et al. Effects of atypical antipsychotics on the inflammatory response system in schizophrenic patients resistant to treatment with typical neuroleptics. Eur Neuropsychopharmacol. 2000;10(2):119–124. [DOI] [PubMed] [Google Scholar]
- 6. Maes M, Bocchio Chiavetto L, Bignotti S, et al. Increased serum interleukin-8 and interleukin-10 in schizophrenic patients resistant to treatment with neuroleptics and the stimulatory effects of clozapine on serum leukemia inhibitory factor receptor. Schizophr Res. 2002;54(3):281–291. [DOI] [PubMed] [Google Scholar]
- 7. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noto C, Ota VK, Gouvea ES, et al. Effects of risperidone on cytokine profile in drug-naïve first-episode psychosis. Int J Neuropsychopharmacol. 2014;18:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li H, Zhang Q, Li N, et al. Plasma levels of Th17-related cytokines and complement C3 correlated with aggressive behavior in patients with schizophrenia. Psychiatry Res. 2016;246:700–706. [DOI] [PubMed] [Google Scholar]
- 11. Maes M, Sirivichayakul S, Kanchanatawan B, Vodjani A. Breakdown of the paracellular tight and adherens junctions in the gut and blood brain barrier and damage to the vascular barrier in patients with deficit schizophrenia. Neurotox Res. 2019;36(2):306–322. [DOI] [PubMed] [Google Scholar]
- 12. Al-Hakeim, HK, Al-Mulla, AF, Maes, M. The neuro-immune fingerprint of major neuro-cognitive psychosis or deficit schizophrenia: a supervised machine learning study. Neurotox Res. 2020;37(3):753–771. [DOI] [PubMed] [Google Scholar]
- 13. Noto C, Maes M, Ota VK, et al. High predictive value of immune-inflammatory biomarkers for schizophrenia diagnosis and association with treatment resistance. World J Biol Psychiatry. 2015;16(6):422–429. [DOI] [PubMed] [Google Scholar]
- 14. Roomruangwong C, Noto C, Kanchanatawan B, et al. The role of aberrations in the Immune-Inflammatory Response System (IRS) and the Compensatory Immune-Regulatory Reflex System (CIRS) in different phenotypes of schizophrenia: the IRS-CIRS theory of schizophrenia. Mol Neurobiol. 2020;57(2):778–797. [DOI] [PubMed] [Google Scholar]
- 15. Sirivichayakul, S, Kanchanatawan, B, Thika, S, Carvalho, AF, Maes, M. A new schizophrenia model: immune activation is associated with induction of different neurotoxic products which together determine memory impairments and schizophrenia symptom dimensions. CNS Neurol Disord Drug Targets. 2019;18:124–140. [DOI] [PubMed] [Google Scholar]
- 16. Maes M, Kanchanatawan B, Sirivichayakul S, Carvalho AF. In schizophrenia, increased plasma IgM/IgA responses to gut commensal bacteria are associated with negative symptoms, neurocognitive impairments, and the deficit phenotype. Neurotox Res. 2019;35(3):684–698. [DOI] [PubMed] [Google Scholar]
- 17. Maes, M, Sirivichayakul, S, Kanchanatawan, B, Carvalho, AF. In schizophrenia, psychomotor retardation, with executive and memory impairments, negative and psychotic symptoms, neurotoxic immune products and lower natural IgM to malondialdehyde. World J Biol Psychiatry. 2020; 21(5):383–401. [DOI] [PubMed] [Google Scholar]
- 18. Al-Hakeim, HK, Almulla, AF, Al-Dujaili, AH, Maes, M. Construction of a neuro-immune-cognitive pathway-phenotype underpinning the phenome of deficit schizophrenia. Curr Top Med Chem. 2020;20(9):747–758. [DOI] [PubMed]
- 19. Sirivichayakul S, Kanchanatawan B, Thika S, Carvalho AF, Maes M. Eotaxin, an endogenous cognitive deteriorating chemokine (ECDC), is a major contributor to cognitive decline in normal people and to executive, memory, and sustained attention deficits, formal thought disorders, and psychopathology in schizophrenia patients. Neurotox Res. 2019;35:122–138. [DOI] [PubMed] [Google Scholar]
- 20. Yang H, Wang H, Levine YA, et al. Identification of CD163 as an anti-inflammatory receptor for HMGB1-haptoglobin complexes. JCI Insight. 2016;1:e85375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5(4):331–342. [DOI] [PubMed] [Google Scholar]
- 22. Festoff BW, Sajja RK, van Dreden P, Cucullo L. HMGB1 and thrombin mediate the blood-brain barrier dysfunction acting as biomarkers of neuroinflammation and progression to neurodegeneration in Alzheimer’s disease. J Neuroinflammation. 2016;13(1):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mazarati A, Maroso M, Iori V, Vezzani A, Carli M. High-mobility group box-1 impairs memory in mice through both toll-like receptor 4 and receptor for advanced glycation end products. Exp Neurol. 2011;232(2):143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chae WJ, Bothwell ALM. Dickkopf1: an immunomodulatory ligand and Wnt antagonist in pathological inflammation. Differentiation. 2019;108:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dickins EM, Salinas PC. Wnts in action: from synapse formation to synaptic maintenance. Front Cell Neurosci. 2013;5:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ross RD, Shah RC, Leurgans S, Bottiglieri T, Wilson RS, Sumner DR. Circulating Dkk1 and TRAIL are associated with cognitive decline in community-dwelling, older adults with cognitive concerns. J Gerontol A Biol Sci Med Sci. 2018;73(12):1688–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aleksic B, Kushima I, Ito Y, et al. Genetic association study of KREMEN1 and DKK1 and schizophrenia in a Japanese population. Schizophr Res. 2010;118(1-3):113–117. [DOI] [PubMed] [Google Scholar]
- 28. Conley RR, Kelly DL. Management of treatment resistance in schizophrenia. Biol Psychiatry. 2001;50(11):898–911. [DOI] [PubMed] [Google Scholar]
- 29. Guy W. Clinical Global Impressions. In: ECDEU Assessment Manual for Psychopharmacology—Revised. Rockville, MD: U.S. Department of Health, Education, and Welfare; National Institute of Mental Health; Psychopharmacology Research Branch; Division of Extramural Research Programs;1976: 218–222. [Google Scholar]
- 30. Leucht S, Samara M, Heres S, Davis JM. Dose equivalents for antipsychotic drugs: the DDD method. Schizophr Bull. 2016;42 (Suppl 1:S90–S94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989;7:49–58. [PubMed] [Google Scholar]
- 32. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 33. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 35. Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2-3):283–297. [DOI] [PubMed] [Google Scholar]
- 36. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B (Methodol). 1995;57:289–300. [Google Scholar]
- 37. Maes M, Meltzer HY, Bosmans E. Immune-inflammatory markers in schizophrenia: comparison to normal controls and effects of clozapine. Acta Psychiatr Scand. 1994;89(5):346–351. [DOI] [PubMed] [Google Scholar]
- 38. Teixeira AL, Reis HJ, Nicolato R, et al. Increased serum levels of CCL11/eotaxin in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(3):710–714. [DOI] [PubMed] [Google Scholar]
- 39. Gardella S, Andrei C, Ferrera D, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3(10):995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Semino C, Ceccarelli J, Lotti LV, Torrisi MR, Angelini G, Rubartelli A. The maturation potential of NK cell clones toward autologous dendritic cells correlates with HMGB1 secretion. J Leukoc Biol. 2007;81(1):92–99. [DOI] [PubMed] [Google Scholar]
- 41. Zhao G, Zhang J, Nie D, et al. HMGB1 mediates the development of tendinopathy due to mechanical overloading. PLoS One. 2019;14(9):e0222369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fossati S, Chiarugi A. Relevance of high-mobility group protein box 1 to neurodegeneration. Int Rev Neurobiol. 2007;82:137–148. [DOI] [PubMed] [Google Scholar]
- 43. Lin T, Sammy F, Yang H, et al. Identification of hemopexin as an anti-inflammatory factor that inhibits synergy of hemoglobin with HMGB1 in sterile and infectious inflammation. J Immunol. 2012;189(4):2017–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guo Y, Mishra A, Howland E, et al. Platelet-derived Wnt antagonist Dickkopf-1 is implicated in ICAM-1/VCAM-1-mediated neutrophilic acute lung inflammation. Blood 2015;126(19):2220–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mazon M, Larouche V, St-Louis M, Schindler D, Carreau M. Elevated blood levels of Dickkopf-1 are associated with acute infections. Immun Inflamm Dis. 2018;6(4):428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang R, Real CI, Liu C, et al. Hepatic expression of oncogenes Bmi1 and Dkk1 is up-regulated in hepatitis B virus surface antigen-transgenic mice and can be induced by treatment with HBV particles or lipopolysaccharides in vitro. Int J Cancer. 2017;141(2):354–363. [DOI] [PubMed] [Google Scholar]
- 47. Malysheva K, de Rooij K, Lowik CW, et al. Interleukin 6/Wnt interactions in rheumatoid arthritis: interleukin 6 inhibits Wnt signaling in synovial fibroblasts and osteoblasts. Croat Med J. 2016;57(2):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Noto MN, Maes M, Nunes SOV, et al. Activation of the immune-inflammatory response system and the compensatory immune-regulatory system in antipsychotic naive first episode psychosis. Eur Neuropsychopharmacol. 2019;29(3):416–431. [DOI] [PubMed] [Google Scholar]
- 49. Fang P, Schachner M, Shen YQ. HMGB1 in development and diseases of the central nervous system. Mol Neurobiol. 2012;45(3):499–506. [DOI] [PubMed] [Google Scholar]
- 50. Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, Hong JS. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci. 2011;31(3):1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fujita K, Motoki K, Tagawa K, et al. HMGB1, a pathogenic molecule that induces neurite degeneration via TLR4-MARCKS, is a potential therapeutic target for Alzheimer’s disease. Sci Rep. 2016;6:31895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hei Y, Chen R, Yi X, Long Q, Gao D, Liu W. HMGB1 neutralization attenuates hippocampal neuronal death and cognitive impairment in rats with chronic cerebral hypoperfusion via suppressing inflammatory responses and oxidative stress. Neuroscience 2018;383:150–159. [DOI] [PubMed] [Google Scholar]
- 53. Yang L, Wang F, Yang L, et al. HMGB1 a-box reverses brain edema and deterioration of neurological function in a traumatic brain injury mouse model. Cell Physiol Biochem. 2018;46(6):2532–2542. [DOI] [PubMed] [Google Scholar]
- 54. Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. [DOI] [PubMed] [Google Scholar]
- 57. Liebner S, Corada M, Bangsow T, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183(3):409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Artus C, Glacial F, Ganeshamoorthy K, et al. The Wnt/planar cell polarity signaling pathway contributes to the integrity of tight junctions in brain endothelial cells. J Cereb Blood Flow Metab. 2014;34(3):433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang Y, Sabbagh MF, Gu X, Rattner A, Williams J, Nathans J. Beta-catenin signaling regulates barrier-specific gene expression in circumventricular organ and ocular vasculatures. eLife 2019;8:e43257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu L, Wan W, Xia S, Kalionis B, Li Y. Dysfunctional Wnt/β-catenin signaling contributes to blood-brain barrier breakdown in Alzheimer’s disease. Neurochem Int. 2014;75:19–25. [DOI] [PubMed] [Google Scholar]
- 61. Na KS Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277–86. [DOI] [PubMed] [Google Scholar]
- 62. Orellana JA, Sáez JC, Bennett MV, Berman JW, Morgello S, Eugenin EA. HIV increases the release of Dickkopf-1 protein from human astrocytes by a Cx43 hemichannel-dependent mechanism. J Neurochem. 2014;128(5):752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Scali C, Caraci F, Gianfriddo M, et al. Inhibition of Wnt signaling, modulation of Tau phosphorylation and induction of neuronal cell death by DKK1. Neurobiol Dis. 2006;24(2):254–265. [DOI] [PubMed] [Google Scholar]
- 64. Seib DR, Corsini NS, Ellwanger K, et al. Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell. 2013;12(2):204–214. [DOI] [PubMed] [Google Scholar]
- 65. Herrero P, Contador I, Stern Y, Fernández-Calvo B, Sánchez A, Ramos F. Influence of cognitive reserve in schizophrenia: a systematic review. Neurosci Biobehav Rev. 2020;108:149–159. [DOI] [PubMed] [Google Scholar]
- 66. Marwaha S, Johnson S. Schizophrenia and employment – a review. Soc Psychiatry Psychiatr Epidemiol. 2004;39(5):337–349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.