Abstract
Introduction
Clarifying the role of neuroinflammation in schizophrenia is subject to its detection in the living brain. Free-water (FW) imaging is an in vivo diffusion-weighted magnetic resonance imaging (dMRI) technique that measures water molecules freely diffusing in the brain and is hypothesized to detect inflammatory processes. Here, we aimed to establish a link between peripheral markers of inflammation and FW in brain white matter.
Methods
All data were obtained from the Australian Schizophrenia Research Bank (ASRB) across 5 Australian states and territories. We first tested for the presence of peripheral cytokine deregulation in schizophrenia, using a large sample (N = 1143) comprising the ASRB. We next determined the extent to which individual variation in 8 circulating pro-/anti-inflammatory cytokines related to FW in brain white matter, imaged in a subset (n = 308) of patients and controls.
Results
Patients with schizophrenia showed reduced interleukin-2 (IL-2) (t = −3.56, P = .0004) and IL-12(p70) (t = −2.84, P = .005) and increased IL-6 (t = 3.56, P = .0004), IL-8 (t = 3.8, P = .0002), and TNFα (t = 4.30, P < .0001). Higher proinflammatory signaling of IL-6 (t = 3.4, P = .0007) and TNFα (t = 2.7, P = .0007) was associated with higher FW levels in white matter. The reciprocal increases in serum cytokines and FW were spatially widespread in patients encompassing most major fibers; conversely, in controls, the relationship was confined to the anterior corpus callosum and thalamic radiations. No relationships were observed with alternative dMRI measures, including the fractional anisotropy and tissue-related FA.
Conclusions
We report widespread deregulation of cytokines in schizophrenia and identify inflammation as a putative mechanism underlying increases in brain FW levels.
Keywords: diffusion-weighted imaging, magnetic resonance imaging/DWI/MRI, cytokines
Introduction
Evidence from genetics, epidemiology, and molecular studies of brain and blood implicate inflammation in the etiology and pathophysiology of schizophrenia.1,2 Numerous studies report increased blood levels of proinflammatory cytokines in individuals with schizophrenia compared with controls, including increased levels of IL-8, tumor necrosis factor-alpha, and IL-6,3,4 which exert effects on the peripheral and central nervous system (CNS). Brain changes induced by cytokines are most prominent with infection, tissue damage, or neurodegeneration, where large-scale infiltration of white blood cells, phagocytosis, and tissue repair is needed. However, the neuropathology of schizophrenia is, by comparison, subtler and the involvement of cytokines is less clear.
Direct evidence for neuroinflammation in schizophrenia comes from postmortem studies examining cytokine messenger RNA expression levels.5,6 Evidence from in vivo data are less compelling owing to mixed findings in positron emission tomography (PET) studies investigating putative microglial activation via binding rates to the translocator protein.7 The disparity in current findings may reflect interindividual variation across patients and the existence of neuroinflammatory subsets driven by, for example, acute symptomatic epochs and/or specific brain regions. Alternatively, current PET measures may not adequately capture neuroinflammatory processes in schizophrenia.5 For example, microglial activation reflects one aspect of the inflammatory cascade characterized by the acute response to immune dysfunction or brain injury and thus may be evident only immediately following an acute psychotic episode.8 Other in vivo neuroimaging measures may capture different neuroinflammatory events that are relevant in schizophrenia and could, in turn, clarify the role of neuroinflammation in schizophrenia.
Free-water (FW) imaging is an in vivo diffusion-weighted magnetic resonance imaging (dMRI) technique that extends the popular diffusion tensor imaging (DTI) analysis to quantify freely diffusing water molecules mostly found in extracellular spaces: blood, CSF, and interstitial fluid. FW imaging produces voxel-wise estimates of the fractional volume of FW and FW-corrected DTI maps. Excess FW has been reported in brain white matter of individuals with schizophrenia,9–11 and inflammatory processes are hypothesized to contribute to this increase.10 For example, altered cytokines—produced within the brain or that cross the blood-brain barrier—activate microglia and astrocytes in the brain parenchyma through action at cell surface receptors.12,13 Glial activation can, in turn, promote microvessel permeability and cause vasodilatation, which increases blood flow.14 In addition, glial secretion of hydrolytic enzymes and reactive oxygen and nitrogen species can break down extracellular matrix (ECM) proteins,15 which promotes water molecule diffusion.16 Despite established links between inflammation and extracellular water content in animals, this relationship has not been directly tested in living humans.
Here, we apply a cross-sectional multimodal study design to explicitly test the hypothesized link between white matter FW and peripheral inflammation, as measured by levels of circulating cytokines. We aimed to establish whether such a relationship would be specific to schizophrenia or evident among healthy individuals as well. To this end, we first examined 8 pro-/anti-inflammatory peripheral cytokine levels and dMRI-derived properties of white matter microstructure, including (1) FW; (2) the conventional DTI metric, fractional anisotropy (FA); and (3) FW-corrected, tissue-related FA (FAT), in schizophrenia patients and healthy controls drawn from the Australian Schizophrenia Research Bank (ASRB). Sophisticated data harmonization allowed us to achieve a large sample for neuroimaging analyses. We next examined the relationship between these cytokine levels and dMRI measures, hypothesizing that a relationship would be specific to the FW parameter, but not FAT and FA parameters. Given that these cytokines are widely implicated in schizophrenia pathophysiology, we expected that the association in patients would be stronger, and/or extend across larger portions of white matter, compared with controls. This study includes the largest investigations of systematic inflammation and advanced dMRI properties in schizophrenia and the first to test whether variation in cytokine levels specifically covary with brain FW.
Methods
Participants
This study comprised a total of 1143 participants: 497 individuals diagnosed with schizophrenia or schizoaffective disorder and 646 healthy controls (table 1A). Diffusion-weighted MRI data were available in a subset of subjects (n = 308; table 1B). All data were collected by the ASRB across 5 Australian states and territories. Full details related to recruitment procedures have been published elsewhere.17 Exclusion criteria and procedures for assessing clinical status and symptoms are described in supplementary material. Approval for data analysis was provided by the Melbourne Health Human Research Ethics Committee (MHREC: 2010.250), and written informed consent was obtained from all participants, according to the Declaration of Helsinki.
Table 1.
Sample Characteristics
| (a) Entire Sample (n = 1143) | Schizophrenia | Healthy Controls | ||||
|---|---|---|---|---|---|---|
| n = 497 | n = 646 | |||||
| M | SD | M | SD | t or χ2 | P | |
| Age, y | 40 | 11 | 43 | 13 | −0.11 | <.0001 |
| Gender, M/F | 325/172 | 257/389 | 73.71 | <.0001 | ||
| Storage sample time (y) | 7.06 | 1.28 | 6.58 | 0.97 | 7.18 | <.0001 |
| Characterizing illness | ||||||
| Age of onset, y | 23.83 | 7.10 | ||||
| Illness duration, y | 15.73 | 10.27 | ||||
| Positive symptoms (DIP Lifetime) | 7.30 | 3.06 | ||||
| Positive symptoms (DIP Current) | 2.34 | 2.76 | ||||
| Negative symptoms (SANS Total) | 28.01 | 19.24 | ||||
| (b) Imaging Subset (n = 308) | Schizophrenia | Healthy Controls | ||||
| n = 199 | n = 109 | |||||
| M | SD | M | SD | t or χ2 | P | |
| Age, y | 42 | 13 | 39 | 10 | −2.03 | .044 |
| Gender, M/F | 135/64 | 53/56 | 10.93 | .001 | ||
| Storage sample time (y) | 7.56 | 1.01 | 7.40 | 0.91 | 1.40 | .162 |
| Characterizing illness | ||||||
| Age of onset, y | 23.37 | 6.53 | ||||
| Illness duration, y | 15.38 | 9.95 | ||||
| Positive symptoms (DIP Lifetime) | 7.66 | 3.41 | ||||
| Positive symptoms (DIP Current) | 2.69 | 3.03 | ||||
| Negative symptoms (SANS Total) | 27.06 | 18.86 | ||||
Note: DIP, Diagnostic Interview for Psychosis; SANS, Scale for the Assessment of Negative Symptoms.
Cytokine Measurement
Whole blood was collected from participants comprising the entire sample (table 1A) into Serum Separator Tubes (SST, BD Biosciences). The tubes were inverted and left at room temperature for 30 minutes (to allow clotting) and centrifuged at 2000g for 5 minutes at 4°C. Serum was then removed and stored frozen until sent to a central repository and inventoried, aliquoted, and stored at −80°C. Eight cytokines (interferon gamma [IFNγ], IL-10, IL-12(p70), IL-1β, IL-2, IL-6, IL-8, and TNFα) from the Human High Sensitivity T-Cell panel (HSTCMAG-28K, Merck Millipore) were assayed using a Luminex Magpix-based assay (Luminex corporation). Serum samples were thawed at 4 °C and centrifuged at 1400g to remove any aggregate protein. The supernatant was then transferred to a fresh low-binding microfuge tube and diluted 1:2 in assay buffer according to the manufacturer’s instructions. A 10-point standard curve with serial dilutions of 1:4 was generated using reconstituted stock standards supplied by the manufacturer; quality controls (QCs) supplied by the manufacturer were also used to determine assay accuracy. The data were generated against a 5-parameter logistic standard curve and corrected for background readings using Millipore Analyst Software (Merck Millipore, Billerica, MA, USA). Samples were run randomly across a total of eighteen 96-well plates in singles, and a pooled serum sample was used as an internal control on all plates. The average coefficient of variance for duplicate values (n = 160) across analytes was minimal (0.75%).
The average minimum detectable value across all plates was 0.11 pg/ml for IFNγ, 0.12 pg/ml for IL-10, 0.03 pg/ml for IL-12, 0.04 pg/ml for IL-1β, 0.09 pg/ml for IL-2, 0.03 pg/ml for IL-6, 0.20 pg/ml for IL-8, and 0.04 pg/ml for TNFα. Values below the range of detection were replaced by the respective minimum detectable value (entire sample: average 17 samples/cytokine = <1.5%; imaging subset: average 3.3 samples/cytokine = 1.1%), and values above the range of detection were replaced by the maximum detectable value (entire sample: average 7 samples/cytokine = <0.6%; imaging subset: average 3.1 samples/cytokine = 1.0%). The average variance for all cytokines across the plates in the QCs were 10% and 10.8%, respectively, for the entire sample and 10.1% and 11.0%, respectively, for the imaging subset. The average variance in the internal control was 31.3% across all 17 plates.
Diffusion MRI Acquisition and Harmonization Across Study Sites
Diffusion-weighted MRI scans were acquired in each participant comprising the imaging subset (table 1B) with a Siemens Avanto 1.5-Tesla system (Siemens) in 64 directions using a spin-echo echo planar imaging sequence (see supplementary material for parameters) across 5 different Australian sites (supplementary table 1). The same model of MRI scanner, acquisition sequences, and exclusion/inclusion criteria were used at each site. No scanner upgrades were performed during the study lifetime. Furthermore, a robust retrospective harmonization procedure18 was applied to the raw diffusion-weighted images (DWIs) across the 5 study sites to remove any site-related differences (see supplementary material).
White Matter Image Processing
DWIs were preprocessed with FMRIB Software Library (FSL, version 5.11; see supplementary material).19 FA volumes were computed by fitting a single-diffusion tensor to the DWIs, and FW and FAT volumes were computed by FW imaging, which applies a regularization framework to fit a 2-compartment model to the DWIs.20 This model separates the contribution of FW from water molecules diffusing in the vicinity of tissue. For the echo time used here, FW, the fractional volume of which is quantified by FW, requires large enough spaces that constitute unrestricted extracellular water molecules around and between myelin and axolemma. Water molecule diffusion in the tissue compartment is modeled by a diffusion tensor, from which FAT is calculated, reflecting the anisotropy of the signal originating from the tissue compartment.
ENIGMA-DTI protocols (http://enigma.ini.usc.edu/protocols/dti-protocols/) were used to extract average dMRI measure (FW, FAT, and FA) estimates from voxels on a white matter skeleton (see supplementary material).21 Statistical inference was performed on the resulting skeletonized maps (FW, FAT, and FA).
Statistical Modeling
Demographics.
An independent t-test and Chi-square test assessed differences in age and sex proportions between patient and control groups comprising the entire study sample (table 1A) as well as the imaging subset (table 1B).
Between-Group Difference in Cytokines.
Cytokine comparisons were examined in the entire ASRB sample (n = 1143), as well as in the imaging subset (n = 308). To satisfy normality assumptions, cytokine levels were quantile normalized prior to analyses. A general linear model (GLM) was formulated to test the main effect of diagnosis on individual variation in cytokine levels (dependent variable) while controlling for the confounding effects of age, sex, and freezer storage time (in days). Each of the 8 cytokines was tested independently. Multiple comparison correction for the 8 tests was performed by controlling the false discovery rate (FDR)22 at 5%.
Between-Group Difference in White Matter Microstructure.
Between-group comparisons in white matter dMRI parameters, FW, FAT, and FA were performed using Randomise running in FSL.23 On a voxel-by-voxel basis, a GLM was conducted to assess the null hypothesis of equality in FW, FAT, and FA between the schizophrenia and healthy control groups, while controlling for age, the square of age, and sex. Each of the 3 dMRI measures was tested independently. Multiple comparison correction for the set of all skeleton voxels was performed with threshold-free cluster enhancement (TFCE).24
Association Between Serum Cytokine Levels on White Matter Microstructure.
We next sought to test the impact of each cytokine on skeleton-averaged white matter dMRI metrics (FW, FA, and FAT) in the imaging subset (table 1B). To this end, the following regression model was fitted:
where denotes the skeleton-averaged FW for the participant with index ; is a binary indicator of the diagnostic status of participant (patient: 1, control: 0); and represents a matrix of nuisance confounds for participant n, including age (linear), the square of age (quadratic), sex, and serum freezer storage time. The model coefficients can be understood as follows: β1 is the intercept term; β2 models the between-group difference in the dependent variable (FW); β3 models the impact of cytokine level; β4 models the interaction between diagnosis and cytokine level; and is a matrix modeling the confound effects. The GLM was repeated with FA or FAT as the dependent variable to test whether relationships were generalized to conventional DTI (ie, FA) or cellular (ie, FAT) measures of white matter microstructure. Error control across the 8 GLMs (for each cytokine tested) was achieved by controlling the FDR at 5%. We additionally fit the above model separately to the patient and control groups (removing diagnosis and interaction terms from the model), to comprehensively assess the strength and nature of the relationships in the respective groups.
Voxel-Wise Associations Between FW and Cytokines.
To localize the impact of cytokine levels on white matter microstructure, correlations between significant cytokines and dMRI measures (as determined by the GLM analyses above) were performed using Randomise running in FSL (v5.11).23 On a voxel-by-voxel basis, a GLM assessed the null hypothesis that β3( and were equal to zero. Each cytokine-of-interest was tested independently. Correction for multiple comparisons for the set of all skeleton voxels was performed with TFCE.24 Nuisance confounds included age, the square of age, sex, and serum freezer storage time (in days). Voxel-wise associations were also examined separately in patients and controls using the same model but with the removal of diagnosis and interaction term.
Results
Demographics
Table 1 displays sample demographics, statistics, and patient symptom ratings for the entire sample (table 1A) and the imaging subset (table 1B). Compared with controls, patients comprised a significantly greater proportion of males and differed in age; patients were significantly younger in the entire sample and older in the imaging subset. Sex and age were, therefore, included as nuisance covariates. In addition, the main correlation analysis was repeated separately in males and females.
Altered Serum Cytokine Profiles in Schizophrenia
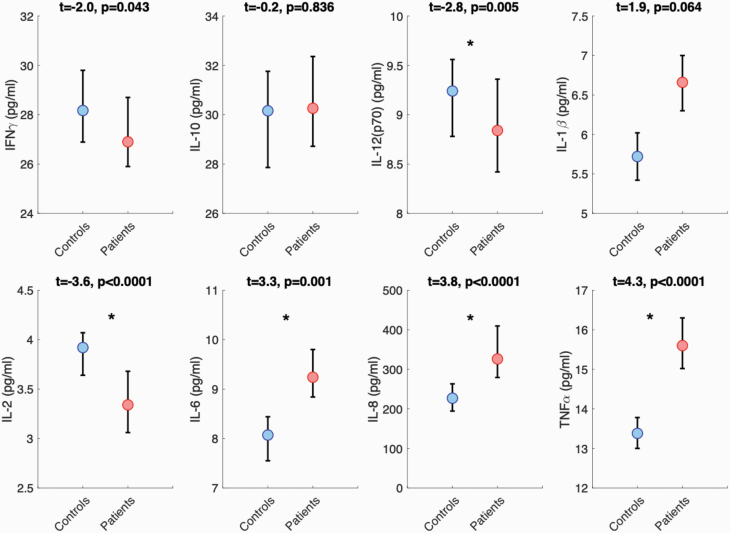
Figure 1 displays results from cytokine serum level comparisons between the entire sample of patients (n = 497) and controls (n = 646). Compared with controls, patients displayed significantly lower concentrations of IL-2 and IL-12(p70) and significantly higher concentrations of IL-6, IL-8, and TNFα (figure 1 and supplementary table 3). Between-group comparisons in the imaging subset (n = 308) were largely consistent with the main analysis; however, IL-6 did not survive FDR correction (P = .09; supplementary table 4).
Fig. 1.
Between-group comparisons in serum cytokine levels. Plots display the median and 95% confidence intervals for patients and controls. Asterisks denote significant differences. Analyses corrected for age, sex, and freezer storage time. Refer to supplementary material for statistics after controlling for diagnosis, medication class, and general health conditions.
In supplemental analyses, these results were reproduced in between-group comparisons comprising only patients diagnosed with schizophrenia (n = 410; supplementary table 5) and after excluding cytokine values outside the detectable range (supplementary tables 6 and 7). Furthermore, concentrations of IL-6, IL-8, and TNFα remained significantly higher in patients compared with controls after controlling for medication class (supplementary tables 7 and 8) and self-reported health conditions including smoking and obesity/overweight status (supplementary tables 9 and 10). In contrast to the main findings, IL-1β concentration was significantly higher in patients compared with controls after controlling for medication class and health conditions. In comparison, no between-group differences were seen for IL-2 and IL-12(p70) after controlling for potential confounds (see supplementary material).
Altered White Matter Microstructure in Schizophrenia
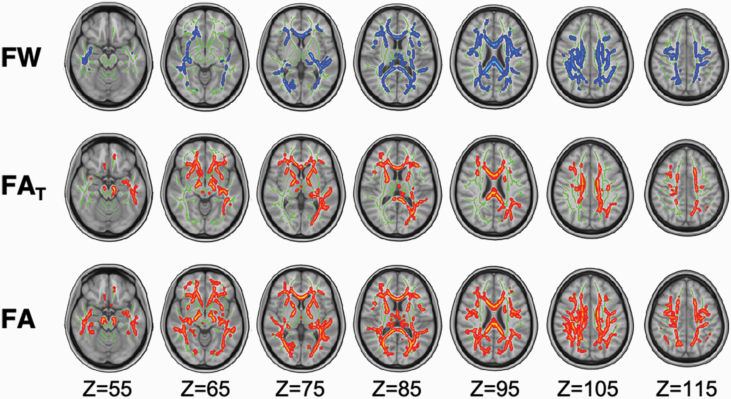
Figure 2 displays results from group comparisons of white matter microstructure, after controlling for age and sex. Compared with controls, patients displayed significantly higher FW values (tMAX = 4.8; tMEAN = 1.6; P < .01), as well as reduced FA (tMAX = 5.3; tMEAN = 1.7; P < .007) and FAT (tMAX = 5.1; tMEAN = 1.7; P < .009; figure 2). Alterations were widespread across all diffusion metrics (FW = 30%, FAT = 20%, and FA = 39% of the white matter skeleton), affecting areas with commissural, association, and projection fibers, including the corpus callosum, inferior longitudinal fasciculus, cingulum, and corona radiata, which are commonly implicated in schizophrenia pathophysiology (figure 2).
Fig. 2.
Between-group comparisons in white matter microstructure. Voxel-wise analyses revealed significantly higher free water (FW) and significantly decreased tissue-specific fractional anisotropy (FAT) and conventional fractional anisotropy (FA) in patients relative to controls. Significant voxels were dilated by 1 voxel to clearly demarcate significant regions. All voxel-wise analyses controlled for age and sex.
Levels of IL-6 and TNFα Contribute to FW Levels in Patients and Controls
Combined Group Analyses.
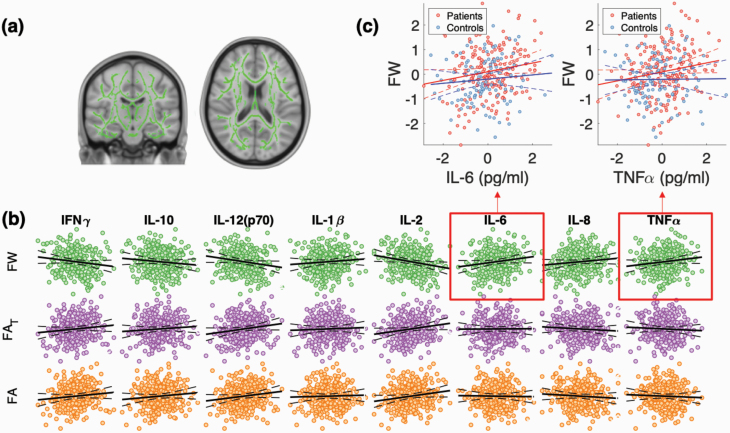
Figure 3 displays the relationships between cytokines and skeleton-averaged white matter diffusion metrics. As shown, there were significant main effects of IL-6 (: t = 3.4, Cohen’s d = 0.20; P = .0007) and TNFα (: t = 2.7; Cohen’s d = 0.16; P = .0007) on skeleton-averaged FW, with higher IL-6 and TNFα levels associated with higher FW (figure 3B). There was no significant interaction between diagnosis and cytokine levels in either analysis after FDR correction (P > .05), indicating similar effects of IL-6 and TNFα levels on FW across patients and controls. With regard to nuisance confounds, there was no main effect of sex (P > .05); to confirm, results were reproduced separately in males and females (supplementary figure 2). Furthermore, there was no impact of serum freezer storage time (P > .05); however, there was a significant impact of age on skeleton-averaged FW (t = 3.0; P = .003 for the quadratic age term; supplementary figure 3).
Fig. 3.
Cytokine-diffusion-weighted magnetic resonance imaging associations are specific to FW. (a) General linear models (GLMs) examined the association between the average free water (FW), tissue-specific fractional anisotropy (FAT), and conventional fractional anisotropy (FA) extracted from voxels comprising a white matter skeleton, with serum levels of 8 cytokines. (b) Across the pooled sample of patients and controls, variation in IL-6 and TNFα was significantly and positively associated with FW. Dashed lines indicate the 95% confidence intervals for the regression line. (c) Separate analyses in patients and controls revealed that IL-6 and TNFα were significantly associated with FW in schizophrenia but not controls. Each GLM is controlled for age, sex, and freezer storage time. Error control across the 8 GLMs (for each cytokine tested) was achieved by controlling the false discovery rate at 5%.
No significant cytokine or cytokine by diagnosis effects were found for FAT or FA (P > .05). Hence, these parameters were not examined further. Taken together, variation in specific proinflammatory cytokines, namely, IL-6 and TNFα, selectively covary with white matter FW levels.
Exploratory Within-Group Analyses.
We additionally examined the impact of IL-6 and TNFα on skeleton-averaged FW in patients and controls separately. No significant associations were found in the healthy control group (P = .06, d = 0.08 for IL-6 and P = .2, d = 0.09 for TNFα). In contrast, patients showed significant effects of IL-6 (t = 2.7, d = 0.15; P = .008) and TNFα (t = 2.2, d = 0.13; P = .027) on skeleton-averaged FW (figure 3C). This finding was not due to the increased sample size of patients (n = 199) relative to controls (n = 109), with the impact of IL-6 and TNFα on FW replicated in 4 random patient subsamples of equal size to the healthy group (n = 109; IL-6: mean t = 2.7, mean d = 0.16, and mean P = .01; TNFα: mean t = 2.2, mean d = 0.13, and mean P = .03). Thus, cytokine-related increases in FW were stronger—albeit not significantly (given the nonsignificant group by cytokine interaction)—in patients compared with controls.
Association Between Cytokines and FW Differs Spatially Between Patients and Controls
Combined Group Analyses.
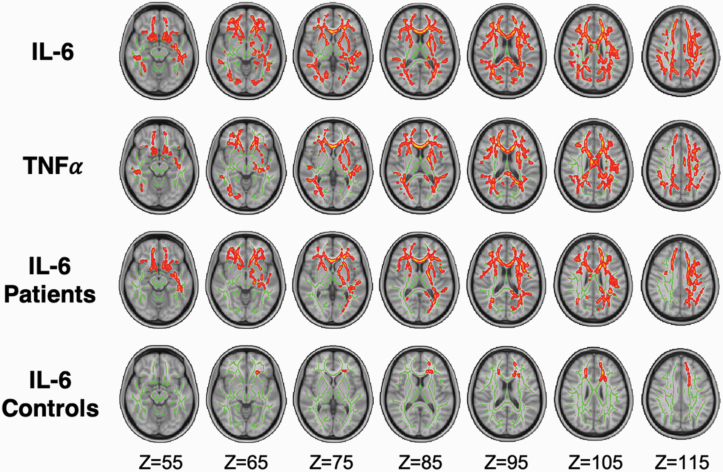
Figure 4 displays results from voxel-wise correlations between cytokines-of-interest (IL-6 and TNFα) and FW. There was a significant positive correlation between IL-6 levels and FW across 40% of the white matter skeleton, traversing major commissural (forceps minor and major), association (bilateral inferior-frontal occipital fasciculi, inferior longitudinal fasciculus [IFOF]), and projection (anterior thalamic radiation) fibers (tMAX = 5.1; tMEAN = 1.6; P = .009). In addition, there was a significant positive correlation between TNFα levels and FW in 24% of skeleton voxels across similar regions (tMAX = 4.3; tMEAN = 1.5; P = .037). The highest effect-size was located in the genu of the corpus callosum for both IL-6 and TNFα correlations with FW. No significant cytokine by diagnosis effects were found.
Fig. 4.
Voxel-wise associations between free water (FW) and cytokines, IL-6, and TNFα. The impact of cytokines on voxel-wise FW revealed significant effects of IL-6 (first row) and TNFα (second row), with higher cytokine levels associated with higher FW. The impact of IL-6 and voxel-wise FW was significant in patients (third row) and controls (fourth row). Significant voxels were dilated by 1 voxel to clearly demarcate significant regions. All analyses, including within-group associations, controlled for age, sex, and freezer storage time.
Exploratory Within-Group Analyses.
Figure 4 displays results from separate voxel-wise correlation analyses in patients and controls. There was a significant positive correlation between IL-6 and FW in healthy controls, which was circumscribed (1% of skeleton) to the genu of the corpus callosum and anterior thalamic radiation (tMAX = 4.8; tMEAN = 2.2; P = .045 . In patients, there was a significant positive correlation between IL-6 on FW that was spatially widespread (22% of skeleton), encompassing the forceps minor and major, IFOF, and the anterior thalamic radiations (tMAX = 4.8; tMEAN = 1.6; P = .018; figure 4). This finding was not due to the increased sample size of the patient group (supplementary figure 4). Thus, reciprocal increases in serum IL-6 and white matter FW were more widespread in patients compared with controls. With regard to TNFα, a positive correlation with FW trended toward significance in patients (20% of skeleton; tMAX = 4.6; tMEAN = 2.0; P = .075) and healthy controls (20% of skeleton; tMAX = 4.4; tMEAN = 1.9; P = .069). Thus, higher TNFα relates to diffuse FW elevations (ie, skeleton-averaged FW) in a regionally nonselective manner.
Discussion
In this study comprising the largest sample of circulating cytokines in schizophrenia to date, we first verified the widespread deregulation of serum cytokine levels in people with schizophrenia. We then provided evidence linking higher proinflammatory signaling of IL-6 and TNFα to increased FW levels in brain white matter.10,25,26 As hypothesized, this relationship was more pronounced and widely distributed in individuals with schizophrenia compared with controls.
We observed aberrant blood levels in 5 out of 8 cytokine components, including IL-2, IL-12(p70), IL-6, IL-8, and TNFα, suggesting that schizophrenia is characterized by immune dysregulation governed by both anti-and pro-inflammatory processes. Importantly, these cytokine alterations are unlikely to be solely due to select confounds, including medication class, smoking, and the presence of health conditions (see supplementary material). Our findings are consistent with a growing number of meta-analyses3,4 and systematic reviews,27,28 which provide evidence for low-grade peripheral inflammation in schizophrenia, with upregulation of proinflammatory cytokines. Our clinical cohort comprised patients with protracted illness durations and thus implicates persistent immune activation with prolonged illness, possibly due to the effects of chronic stress.4
We observed significant associations between white matter FW with levels of IL-6 and TNFα. These results may suggest a specificity of select pro-inflammatory cytokines on brain FW levels (although the direction of this relationship remains an open question). Both IL-6 and TNFα constitute the main drivers of acute-phase protein (eg, C-reactive protein) production that accompany inflammatory states29 and have known mechanisms of altering CNS function.30,31 IL-6 and TNFα levels selectively covaried with extracellular FW, as cytokine levels showed no significant correlation to FA or FAT. This is despite widespread abnormalities spanning all 3 white matter measures (FA, FAT, and FW) in schizophrenia compared with control subjects. These results expand upon recent evidence that FW levels relate to inflammatory states, including a study that linked FW to levels of the antioxidant, glutathione.32 The selectivity of the cytokine associations with FW underscores the benefit of parsing classical DTI-derived anisotropy measures into distinct components to aid biological interpretation. Collectively, our findings lend support to the hypothesis that FW reflects a highly sensitive and physiologically meaningful biomarker of inflammation, providing impetus to examine possible causal relations between inflammation and FW.
Despite not showing an overall difference in cytokine-FW associations between patients and controls, our exploratory within-group analyses revealed that these associations were more pronounced in people with schizophrenia. This finding may reflect co-amplified inflammatory and FW signals in patients or the impact of possible chronically elevated inflammation in patients compared with controls. In the schizophrenia group, IL-6 correlated with widely distributed FW, whereas, in healthy controls, the association between IL-6 on FW was confined to select anterior callosal and thalamic radiation fibers. Despite this spatial discrepancy, the strongest cytokine-related increase in patients also traversed anterior callosal white matter. While speculative, it is possible that frontal white matter is particularly sensitive to elevated inflammatory cytokines. This hypothesis is supported by recent evidence that excess FW selectively impacts frontal white matter in adult rats prenatally exposed to maternal immune challenge.26 The reproducibility of fiber-specific sensitivities to systemic inflammation between this animal work and across both our study groups further affirms a biological process underlying the associations between inflammation and excess FW.
The primary strengths of this study include the unique sample, enabling the largest study of systemic inflammatory markers in schizophrenia to date, while controlling for a range of medication and health-related confounds. Furthermore, this study represents the largest analysis of advanced diffusion measures in schizophrenia and is the first study to explore links between cytokine levels and these advanced diffusion white matter measures. Notably, dMRI data were carefully and nonlinearly harmonized across sites, thereby eliminating site/scanner confounds.
Nonetheless, our findings are subject to several limitations. Although supplemental cytokine comparisons controlled for smoking and obesity/overweight status, these are blunt measures that may not fully ameliorate the effects of smoking and weight as captured by the duration/frequency of smoking and body-mass index. These data were unavailable. Antipsychotic dose and cumulative medication use were also unavailable. Furthermore, while drug or alcohol dependence were exclusion criteria, alcohol/cannabis use below the dependence threshold may have affected cytokine levels and white matter indices as well as their relationship. Comprehensive study designs that collect continuous weight, drug/smoking, and medication variables are needed to examine these questions in future work. In addition, with the single-b shell DWI data available here, FW imaging relies on spatial regularization and may be biased by T2 effects as well as perfusion and pseudo diffusion effects from blood.
Conclusions
Our findings substantiate previous findings for widespread cytokine dysregulation in schizophrenia and demonstrate a direct relationship between inflammation in the body and FW in the brain, as measured by dMRI. This cytokine-related FW increase is spatially widespread in patients, whereas in controls, the relationship is confined to the anterior corpus callosum and thalamic radiations, which may represent core regions affected by or vulnerable to immune dysregulation. The peripheral inflammatory markers related to FW (IL-6 and TNFα) are major proinflammatory cytokines. Thus, our findings point to inflammation as a mechanism underlying increases in FW, which is sensitive to schizophrenia pathophysiology and may thus represent a clinically feasible biomarker.
Supplementary Material
Acknowledgments
We gratefully acknowledge all participants for making this study possible. We also thank Trish Collinson who organized aliquoting and shipping for serum samples. This study used data from the Australian Schizophrenia Research Bank (ASRB), funded by an NHMRC Enabling Grant (386500; Carr V, Schall U, Scott R, Jablensky A, Mowry B, Michie P, Catts S, Henskens F, Pantelis C, and Loughland C), the Pratt Foundation, Ramsay Health Care, the Viertel Charitable Foundation, and the Schizophrenia Research Institute, using an infrastructure grant from the NSW Ministry of Health. C.S.W. is on an advisory board for Lundbeck, Australia Pty Ltd and in collaboration with Astellas Pharma Inc, Japan. All authors report no financial relationships with commercial interests.
Funding
This study was supported by National Health and Medical Research Council (NHMRC) Investigator grants (1175754 to M.A.D. and 1177370 to V.L.C.), NHMRC Senior Research Fellowship (1136649 to A.Z.), NMHRC Senior Principal Research Fellowship (1105825 to C.P. and 1117079 to C.S.W.), National Institute of Mental Health (R01MH108574 to O.P.) and the NSW Ministry of Health, Office of Health and Medical Research (C.S.W.).
Data Availability
Genetic, clinical, neuropsychological, and brain imaging data can be accessed from the Australian Schizophrenia Research Bank (ASRB), subject to the approval of the ASRB Access Committee. Further details are available online (https://www.neura.edu.au/discovery-portal/asrb/).
References
- 1. Réus GZ, Fries GR, Stertz L, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 2015;300:141–154. [DOI] [PubMed] [Google Scholar]
- 2. Aricioglu F, Ozkartal CS, Unal G, Dursun S, Cetin M, Müller N. Neuroinflammation in schizophrenia: a critical review and the future. Klin Psikofarmakol B. 2016;26(4):329–444. [Google Scholar]
- 3. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206–214. [DOI] [PubMed] [Google Scholar]
- 6. Fillman SG, Weickert TW, Lenroot RK, et al. Elevated peripheral cytokines characterize a subgroup of people with schizophrenia displaying poor verbal fluency and reduced Broca’s area volume. Mol Psychiatry. 2016;21(8):1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laskaris LE, Di Biase MA, Everall I, et al. Microglial activation and progressive brain changes in schizophrenia. Br J Pharmacol. 2016;173(4):666–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84(4):932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pasternak O, Westin CF, Bouix S, et al. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci. 2012;32(48):17365–17372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pasternak O, Kubicki M, Shenton ME. In vivo imaging of neuroinflammation in schizophrenia. Schizophr Res. 2016;173(3):200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lyall AE, Pasternak O, Robinson DG, et al. Greater extracellular free-water in first-episode psychosis predicts better neurocognitive functioning. Mol Psychiatry. 2018;23(3):701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, Jacque C. Cytokine signals propagate through the brain. Mol Psychiatry. 2000;5(6):604–615. [DOI] [PubMed] [Google Scholar]
- 13. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206–214. [DOI] [PubMed] [Google Scholar]
- 14. Xanthos DN, Sandkühler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci. 2014;15(1):43–53. [DOI] [PubMed] [Google Scholar]
- 15. Kawabori M, Yenari MA. The role of the microglia in acute CNS injury. Metab Brain Dis. 2015;30(2):381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Knauss R, Schiller J, Fleischer G, Kärger J, Arnold K. Self-diffusion of water in cartilage and cartilage components as studied by pulsed field gradient NMR. Magn Reson Med. 1999;41(2):285–292. [DOI] [PubMed] [Google Scholar]
- 17. Loughland C, Draganic D, McCabe K, et al. Australian Schizophrenia Research Bank: a database of comprehensive clinical, endophenotypic and genetic data for aetiological studies of schizophrenia. Aust N Z J Psychiatry. 2010;44(11):1029–1035. [DOI] [PubMed] [Google Scholar]
- 18. Cetin Karayumak S, Bouix S, Ning L, et al. Retrospective harmonization of multi-site diffusion MRI data acquired with different acquisition parameters. Neuroimage 2019;184:180–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage 2012;62(2):782–790. [DOI] [PubMed] [Google Scholar]
- 20. Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009;62(3):717–730. [DOI] [PubMed] [Google Scholar]
- 21. Jahanshad N, Kochunov PV, Sprooten E, et al. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. Neuroimage 2013;81:455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 2002;15(4):870–878. [DOI] [PubMed] [Google Scholar]
- 23. Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage 2014;92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009;44(1):83–98. [DOI] [PubMed] [Google Scholar]
- 25. Di Biase MA, Zhang F, Lyall A, et al. Neuroimaging auditory verbal hallucinations in schizophrenia patient and healthy populations. Psychol Med. 2019;50(3):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Di Biase MA, Katabi G, Piontkewitz Y, Cetin-Karayumak S, Weiner I, Pasternak O. Increased extracellular free-water in adult male rats following in utero exposure to maternal immune activation. Brain Behav Immun. 2020;83:283–287. [DOI] [PubMed] [Google Scholar]
- 27. Rodrigues-Amorim D, Rivera-Baltanás T, Spuch C, et al. Cytokines dysregulation in schizophrenia: a systematic review of psychoneuroimmune relationship. Schizophr Res. 2018;197:19–33. [DOI] [PubMed] [Google Scholar]
- 28. Kroken RA, Sommer IE, Steen VM, Dieset I, Johnsen E. Constructing the immune signature of schizophrenia for clinical use and research; an integrative review translating descriptives into diagnostics. Front Psychiatry. 2018;9:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Banks RE, Forbes MA, Storr M, et al. The acute phase protein response in patients receiving subcutaneous IL-6. Clin Exp Immunol. 1995;102(1):217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Banks W, Moinuddin A, Morley J. Regional transport of TNF-alpha across the blood-brain barrier in young ICR and young and aged SAMP8 mice. Neurobiol Aging. 2001;22:671–676. [DOI] [PubMed] [Google Scholar]
- 31. Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci Lett. 1994;179(1-2):53–56. [DOI] [PubMed] [Google Scholar]
- 32. Lesh TA, Maddock RJ, Howell A, et al. Extracellular free water and glutathione in first-episode psychosis—a multimodal investigation of an inflammatory model for psychosis. Mol Psychiatry. 2019:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genetic, clinical, neuropsychological, and brain imaging data can be accessed from the Australian Schizophrenia Research Bank (ASRB), subject to the approval of the ASRB Access Committee. Further details are available online (https://www.neura.edu.au/discovery-portal/asrb/).