Abstract
Cognitive impairment is a hallmark of schizophrenia and a robust predictor of functional outcomes. Impairments are found in all phases of the illness and are only moderately attenuated by currently approved therapeutics. Neurophysiological indices of sensory discrimination (ie, mismatch negativity (MMN) and P3a amplitudes) and gamma-band auditory steady-state response (ASSR; power and phase locking) are translational biomarkers widely used in the development of novel therapeutics for neuropsychiatric disorders. It is unclear whether laboratory-based EEG measures add explanatory power to well-established models that use only cognitive, clinical, and functional outcome measures. Moreover, it is unclear if measures of sensory discrimination and gamma-band ASSR uniquely contribute to putative causal pathways linking sensory discrimination, neurocognition, negative symptoms, and functional outcomes in schizophrenia. To answer these questions, hierarchical associations among sensory processing, neurocognition, clinical symptoms, and functional outcomes were assessed via structural equation modeling in a large sample of schizophrenia patients (n = 695) and healthy comparison subjects (n = 503). The results showed that the neurophysiologic indices of sensory discrimination and gamma-band ASSR both significantly contribute to and yield unique hierarchical, “bottom-up” effects on neurocognition, symptoms, and functioning. Measures of sensory discrimination showed direct effects on neurocognition and negative symptoms, while gamma-band ASSR had a direct effect on neurocognition in patients. Continued investigation of the neural mechanisms underlying abnormal networks of MMN/P3a and gamma-band ASSR is needed to clarify the pathophysiology of schizophrenia and the development of novel therapeutic interventions.
Keywords: mismatch negativity (MMN), gamma-band auditory steady-state response (ASSR), schizophrenia, functional outcomes, cognitive function
Introduction
Cognitive impairment is a robust predictor of functional outcomes in schizophrenia,1,2 is found in all phases of the illness,3 and is only moderately attenuated by currently available therapeutics.4,5 Recent work has clarified that abnormalities in early auditory information processing are associated with widespread impairments in cognitive and psychosocial functioning.6–10 These data encourage targeting low-level information processing impairments with medications or cognitive training as a strategy for improving outcomes in both cognitive and psychosocial functioning.11–18 For example, Thomas et al19 found via structural equation modeling that information processing deficits measured by electroencephalographic (EEG) biomarker of sensory discrimination, predicted poor functional outcomes via impaired neurocognition and increased negative symptoms in a large cohort of patients who participated in the Consortium on the Genetics of Schizophrenia-2 (COGS-2) study.
EEG biomarkers are widely used in translational investigations in schizophrenia to assess multiple stages, neural substrates of information processing. Some EEG biomarkers are robust, reliable, relatively inexpensive, scalable, and also show acute sensitivity to interventions, underscoring their potential utility in precision medicine trials.17,20–24 In Thomas et al,19 two EEG biomarkers were used to operationalize sensory discrimination: mismatch negativity (MMN) and P3a. MMN is an event-related potential (ERP) typically measured in the context of a passive auditory oddball paradigm where a series of identical standard tones are interspersed with less frequent “oddball” stimuli that differ in some physical characteristic such as stimulus duration, pitch or loudness. MMN is a negative-going peak (post-stimulus 135–205 ms) reflecting the differences between scalp-level ERP responses to deviant vs standard stimuli and is thought to reflect an automatic deviance detection process.25 MMN is followed by a positive ERP component P3a (250–300 ms) that is thought to reflect an automatic shift of attention toward infrequent novel or salient stimuli.26 Previous studies have found large effect size MMN/P3a impairments in schizophrenia,15,27–39 as well as relationships with neurocognition, negative symptoms, and psychosocial functioning.15,28,32,34,40,41 Recent findings also suggest that MMN/P3a have utility in predicting conversion to psychosis in clinical high-risk populations.42–44
Of course, other translational EEG biomarkers also capture important aspects of early auditory information processing and neural substrates underlying fundamental neural abnormalities in schizophrenia. In particular, the auditory state-steady response (ASSR) is a neurophysiologic biomarker that tests the capacity of distributed neural circuits to generate and support synchronized gamma oscillations under optimized, stimulus-driven conditions.45,46 This neural “entrainment” to 40 Hz stimulation is also presumed to reflect the critical ability of the neural system to temporally integrate information across low-level distributed sensory processing networks.47–49 Reduced gamma-band ASSR has independently been associated with cognitive impairment and functional outcomes in schizophrenia patients.31,50–65
Despite the demonstrated utility of MMN, P3a, and ASSR to separately assess sensory discrimination and neural synchrony in translational and clinical studies, individual biomarkers assessed in isolation may not be able to fully capture the heterogeneity of neurophysiological deficits underlying cognitive impairment in schizophrenia. Although MMN, P3a, and ASSR are conceptually related measures of early information processing in response to simple auditory stimuli, inspection of individual studies suggests divergent neural substrates and distinct correlates with important outcome domains. We have recently identified bivariate correlations of MMN and gamma-band ASSR with functional outcomes in this cohort.66 It is unclear, however, whether these biomarkers uniquely contribute to complex, causal multivariate pathways involving cognition, negative symptoms, and functional outcomes when studied in conjunction.
MMN, P3a, and gamma-band ASSR measures are generated by a highly distributed and dynamic/interactive networks of cortical sources that reflect important core processes of neural functioning such as code formation, prediction error signaling,67–72 deviance detection,25,73 and the synchronization of oscillatory information across brain regions74,75; the fluid network coordination underlying these prerequisite operations ultimately support integrative higherorder cognitive functioning.22,46,76–80 In addition to auditory cortices such as the Heschl’s gyrus and the superior temporal gyrus, the frontal cortex and the cingulate have been reported as contributing sources of MMN.22,36,40,67–72,81–90 Regarding gamma oscillations, Tada et al75 investigated the temporal response dynamics of gamma-band ASSR across spatially distributed cortical surfaces in humans using electrocorticography (ECoG). They demonstrated prominent increases of gamma oscillations in the primary auditory cortex (A1) and sensorimotor cortex as well as increases of activity at the prefrontal gyrus. The reduced gamma-band oscillations at the prefrontal gyrus reflect cognitive dysfunction in patients with schizophrenia.80 Recently, we reported abnormal temporofrontal networks of MMN and gamma-band ASSR in patients with schizophrenia of this cohort using a novel effective connectivity and computational modeling framework.91,92 We also showed associations of abnormal MMN network connectivity at the prefrontal gyrus and negative symptoms in the prior study.91 Thus, in addition to abnormalities detected at scalp sites, abnormal source and source-network dynamics appear to underlie at least some cognitive, clinical, and psychosocial outcomes.
This study aimed to (1) extend the previous findings19 that deficits in measures of early auditory information processing lead to poor functional outcomes via impaired neurocognition and increased negative symptoms, using a non-overlapping cohort of schizophrenia patients who did not participate in the COGS-2 and (2) determine whether measures of gamma synchronization uniquely contribute to pathways linking sensory discrimination, neurocognition, negative symptoms, and functional outcomes using hierarchical information processing model in a large sample of schizophrenia patients and healthy comparison subjects.
Methods
Subjects
Participants were 503 healthy comparison subjects and 695 patients diagnosed with schizophrenia (supplementary method S1, supplementary table S1). Data acquisition was described previously.40,54,55,66,83,91–93 Written informed consent was obtained from each subject. The Institutional Review Board of University of California San Diego approved all experimental procedures (071128, 071831, 170147).
Measures
Mismatch Negativity and P3a
Subjects were presented with binaural tones (1 kHz, 85 dB, with 1 ms rise/fall, stimulus onset asynchrony 500 ms) via insert earphones (Aearo Company Auditory Systems, Indianapolis, IN; Model 3A). A duration-deviant auditory oddball paradigm where the deviant stimuli differed in duration was employed following our established procedures.40 Standard (P = .90, 50-ms duration) and deviant (P = .10, 100-ms duration) tones were presented in a pseudorandom order with a minimum of six standard stimuli presented between each deviant stimulus. The ERP waveform in response to standard stimuli was subtracted from the ERP waveform in response to deviant stimuli to show the MMN/P3a waveform. The MMN and P3a amplitude at Fz were measured using the mean voltage from 135 to 205 ms and 250 to 300 ms post stimuli in accordance with previous studies, respectively.25,29,31,32,94 During the MMN/P3a and ASSR sessions, participants watched a silent cartoon video. EEG recording and preprocessing are shown in supplementary method S2.
Auditory Steady-State Response Paradigm
Auditory steady-state stimuli were 1 ms, 93 dB clicks presented at 40 Hz in 500 ms trains. A block typically contained 200 trains of the clicks with 500 ms intervals. The ASSR at Fz was used for analysis. We performed time-frequency analyses with a short-term Fourier transformation (STFT) and then calculated inter-trial phase coherence (ITC) and event-related spectral perturbation (ERSP) as indices of ASSR. The ITC indicates phase consistency across trials, and ranges between 0 (random phase across trials) and 1 (identical phase across trials). The ERSP indicates event-related changes in power contained in the ERP average relative to a prestimulus baseline. We used the ITC and ERSP as measures of phase and power, respectively, because these parameters provide information about the temporal dynamics of ASSR. A power measure and phase measures are assumed to be independent, although signal amplitude can partially bias phase measures in actual. While ERSP includes both evoked power by stimulus and spontaneous power, ITC reflects purely phase synchrony associated with stimulus onset. Both ITC and ERSP have been described as two critical features of the gamma-band ASSR evoked response in previous studies in schizophrenia patients.65 Decreases in ITC and/or ERSP reflect reduced neural responses to auditory steady-state stimulation. We calculated the mean ITC and ERSP by averaging the data over stimulation time (0–500 ms) and response frequency (35–45 Hz) as per previous reports.6,58,59
Neurocognition
Measures of neurocognition included total correct scores from the Total Learning (list A trials 1–5) and Recognition Hits subscales from the California Verbal Learning Test-Second Edition (CVLT-II)95 for the assessment of verbal learning, and Letter Number Span test scores and Letter Number Sequencing test scores96 for the assessment of working memory. For all cognitive measures, higher scores indicate better performance, and standard scores were employed.
Negative Symptoms
We assessed negative symptoms using the Scale for the Assessment of Negative Symptoms (SANS).97 The SANS includes five interviewer-rated global ratings: Affective Flattening or Blunting, Alogia, Avolition or Apathy, Anhedonia or Asociality, and Attention. Higher scores indicate more symptoms for all SANS items. Attention ratings were not included in the analyses.
Functional Outcomes
We identified items in the Scale of Functioning (SOF)98 that are most reflective of daily activities and outcomes using factor analysis among the 15 interviewer-rated items (supplementary method S3). In the analysis, we extracted four items of the SOF as the first factor that best represents functional outcomes: independence of living arrangements (Independence), short-term planning (Plans), financial management/independence (Finances), and overall level of functioning (Impairment). Because the remaining three factors yielded relatively lower eigenvalues and were comprised of items that reflective of clinical symptoms, they were not carried forward for use in subsequent analyses. Higher scores indicate better functioning for the items.
Statistical Analysis
We employed independent t tests to compare MMN, P3a, gamma-band ASSR, and cognitive measures between healthy comparison subjects and schizophrenia patients. The significance level was set at P < .05. Cohen’s d effect sizes (absolute values) were calculated from the overall group contrast to compare ERP, gamma-band ASSR, and cognitive measures.
We used structural equation modeling (SEM) to summarize associations among measures described in the previous sections using latent variables.99,100 Latent variables are not directly observed but rather are inferred from associations among observed (directly measured) variables. Our latent variable measurement model (M0) included latent variables for Sensory Discrimination, Gamma-band ASSR, Cognition, Negative Symptoms, and Functional Outcomes in schizophrenia patients. The latent Sensory Discrimination variable was indicated by MMN and P3a; the latent Gamma-band ASSR variable was indicated by gamma ITC and ERSP; the latent Cognition variable was indicated by CVLT-II Total Learning and Recognition Hits, Letter Number Span, and Letter Number Sequencing; the latent Negative Symptoms variable was indicated by Affective Flattening or Blunting, Alogia, Avolition or Apathy, and Anhedonia or Asociality; the latent Functional Outcomes variable was indicated by SOF Independence, Plans, Finances, and Impairment.
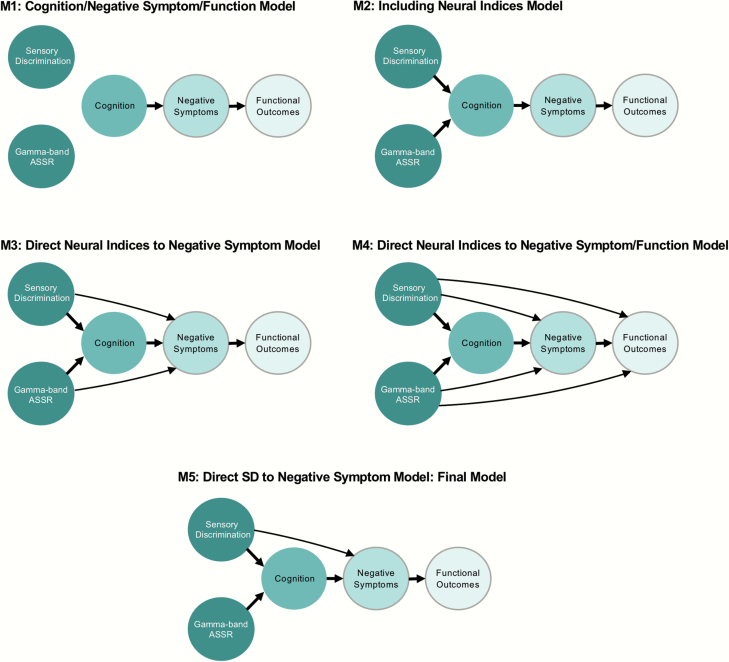
Next, we fitted a series of path models that explored casual pathways emanating from the neurophysiological indices (Sensory Discrimination and Gamma-band ASSR). These models are shown in figure 1. The first model (M1) assumed a casual pathway from Cognition to Negative Symptoms and from Negative Symptoms to Functional Outcomes, which is a well-established theoretical model of schizophrenia but does not include causal pathways from EEG variables. The second model (M2) added causal paths between the neurophysiological indices and Cognition, the third model (M3) added causal paths between neurophysiological indices and Negative Symptoms, and the fourth model (M4) added causal paths between neurophysiological indices and Functional Outcomes.
Fig. 1.
Path models for the associations among Sensory Discrimination, Gamma-band ASSR, Cognition, Negative Symptoms and Functional Outcomes constructs. In these models, we used negative symptoms as one latent variable because we intended to simply focus on the hierarchical organization from neurophysiological indices to functional outcomes via neurocognition and negative symptoms, but not on complex clinical symptoms in schizophrenia patients. Thus, these models are different from our previous work19 as the addition of Gamma-band ASSR into the neurophysiologic model of Sensory Discrimination allows a clearer resolution of the spectrum of neurophysiologic impairments as their causal “up stream” effects on Cognition, Negative Symptoms, and Functional Outcomes. ASSR, auditory steady-state response (Colored figure is available online).
Model parameters were estimated using the latent variable analysis (lavaan) package for R.101 Models were compared using difference chi-squared tests and the Akaike information criterion (AIC). Smaller AIC value indicates a better fit. Model fit was evaluated using comparative fit index (CFI) and root mean square error of approximation (RMSEA). Comparative fit index values near 0.95 or greater and RMSEA values near 0.06 and lower are typically considered acceptable.102 Inferential tests were two-tailed with the significance level set to 0.05. Additional information on the estimation approach is shown in Supplementary Method 4.
Results
Difference of Sensory Discrimination Indices, Gamma-Band ASSR Indices, and Cognition Between Healthy Comparison Subjects and Schizophrenia Patients
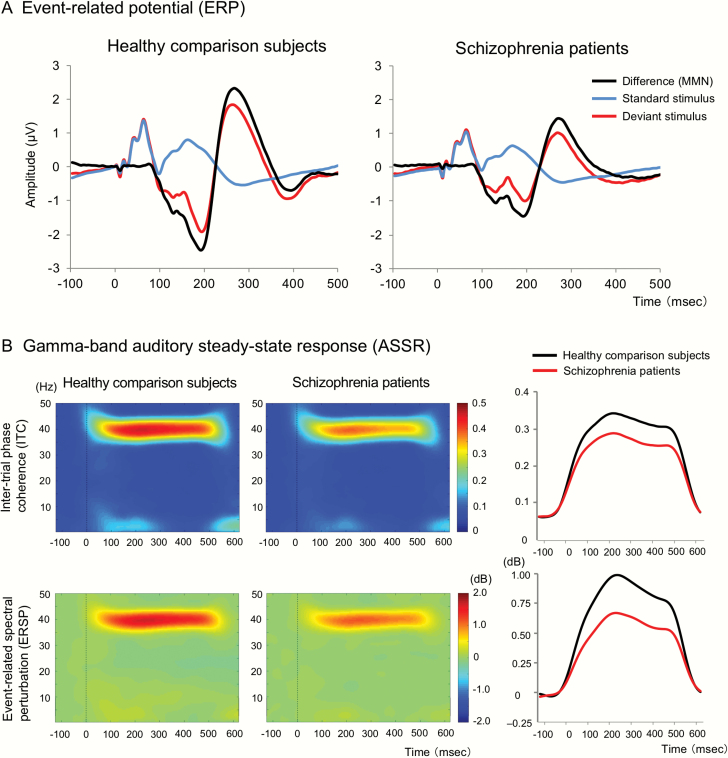
Sensory discrimination as measured by MMN (d = 0.69) and P3a amplitude (d = 0.67), Gamma-band ASSR measured by ITC (d = 0.44) and ERSP (d = 0.39), CVLT-II Total Learning scores (d = 1.21), CVLT-II Recognition Hits (d = 0.58), Letter Number Span (d = 0.76), and Letter Number Sequencing (d = 1.15) were all significantly lower in schizophrenia patients compared with healthy comparison subjects (figure 2, supplementary table S1).
Fig. 2.
Event-related potentials (A), inter-trial phase coherence, and event-related spectral perturbation (B) at Fz. The inter-trial phase coherence indicates phase consistency across trials and ranges between 0 (random phase across trials) and 1 (identical phase across trials). The time-course figures on the right side (B) show mean of inter-trial phase coherence and event-related spectral perturbation between 35 and 45 Hz, respectively (Colored figure is available online).
Structural Equation Modeling in Schizophrenia Patients
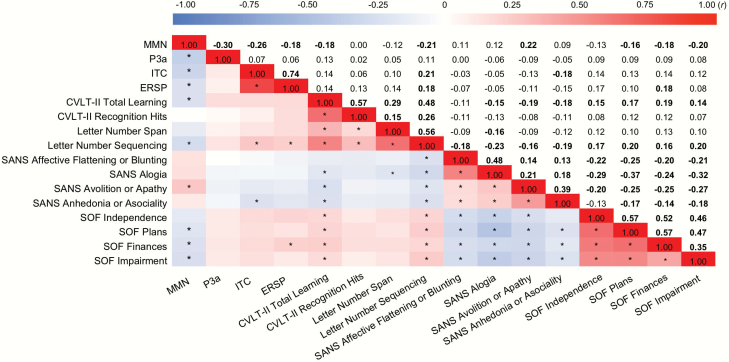
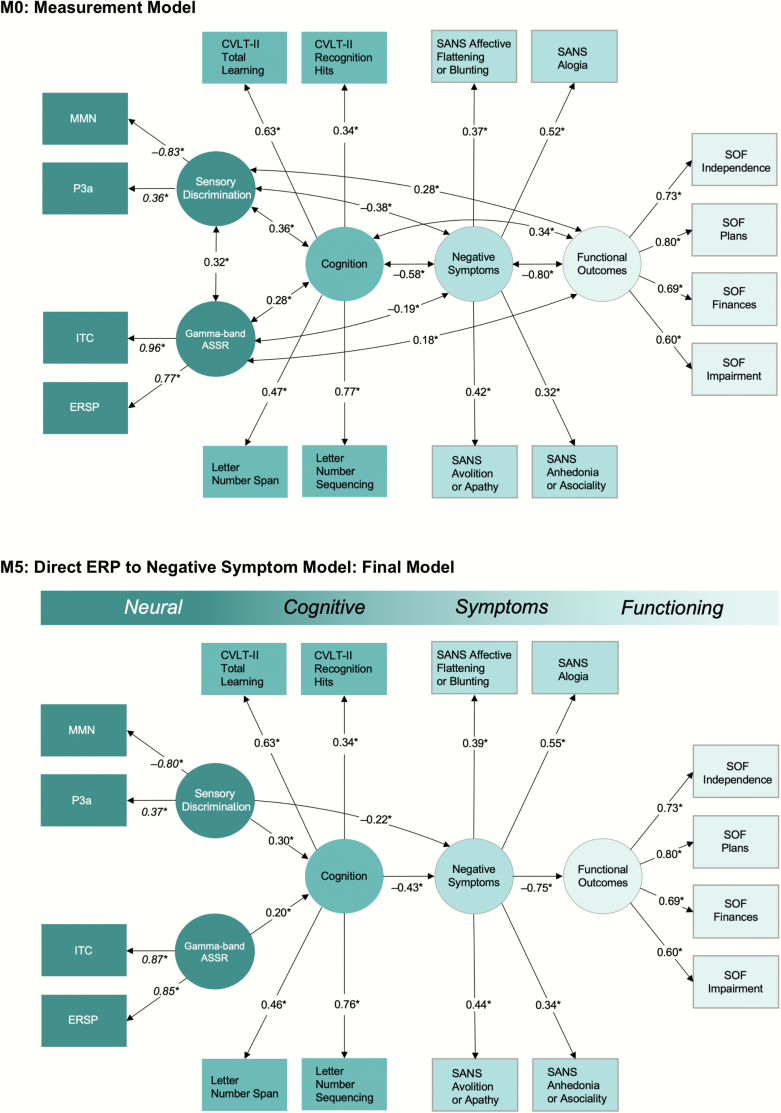
Correlations among the indices are shown in figure 3. Descriptive statistics are shown in supplementary table S1. Model fit statistics are shown in table 1. The M0 model provided acceptable fit. The M0 parameter estimates are shown in figure 4. Our initial path model with neuropsychological measures (M1) also provided an acceptable fit (supplementary figure S1). Therefore, we continued with tests of directed paths.
Fig. 3.
Correlations among observed indices of Sensory Discrimination indices, Gamma-band ASSR indices, Cognition, Negative Symptoms, and Functional Outcomes. Asterisks and bold values indicate statistical significance P < .00042 (0.05/120; 120 indices) adjusted with Bonferroni correction. CVLT-II, California Verbal Learning Test-Second Edition; ERSP, Event-Related Spectral Perturbation; ITC, Inter-Trial Coherence; MMN, Mismatch Negativity; SANS, Scale for the Assessment of Negative Symptoms; SOF, Scale of Functioning (Colored figure is available online).
Table 1.
Model Fit Statistics
| Difference with M1 | Each Model | |||||||
|---|---|---|---|---|---|---|---|---|
| χ 2 | df | Direction | χ 2 | df | AIC | CFI | RMSEA | |
| M0: Measurement Model | NA | NA | NA | 129.2 | 90 | NA | 0.985 | 0.025 |
| M1: Cognition/Negative Symptom/ Function Model | NA | NA | NA | 206.5 | 98 | 26695.3 | 0.958 | 0.040 |
| M2: Including Neural Indices Model | 49.23 | 2 | M2 > M1, P = 2.0×10−11* | 147.1 | 96 | 26639.9 | 0.980 | 0.028 |
| M3: Direct Neural Indices to Negative Symptom Model | 62.70 | 4 | M3 > M1, P = 7.9×10−−13* | 136.3 | 94 | 26633.1 | 0.984 | 0.025 |
| M4: Direct Neural Indices to Negative Symptom/Function Model | 64.61 | 6 | M4 > M1, P = 5.2×10−12* | 135.8 | 92 | 26636.7 | 0.983 | 0.026 |
| M5: Direct SD to Negative Symptom Model: Final Model | 59.94 | 3 | M5 > M1, P = 6.0×10−13* | 136.7 | 95 | 26631.5 | 0.984 | 0.025 |
AIC, Akaike information criterion; CFI, comparative fit index; ERP, event-related potential; NA, not applicable; RMSEA, root mean square error of approximation; SD, sensory discrimination.
*Statistical significance of P-value < .05.
Fig. 4.
Measurement model (M0) and final path model (M5). Associations between nodes (observed variables [rectangles] and latent variables [ovals]) are represented by edges (lines) that can be either directed (single-headed arrow) or undirected (double-headed arrow). Coefficients for the completely standardized solution are reported in the figure. Information in italics indicates constrained loadings (supplementary method S4). *P < .05. CVLT-II, California Verbal Learning Test-Second Edition; SANS, Scale for the Assessment of Negative Symptoms; ASSR, Auditory Steady-State Response; SOF, Scale of Functioning (Colored figure is available online).
Models 2 through 4 all fitted the data better than M1, thus indicating that causal pathways from EEG variables are necessary. Of these models, M3 best fit the data. In this model, significant associations were observed between all observed indicators and latent variables, between Sensory Discrimination and Cognition; between Sensory Discrimination and Negative Symptoms; between Gamma-band ASSR and Cognition; between Cognition and Negative Symptoms; and between Negative Symptoms and Functional Outcomes. Conversely, non-significant associations were observed between Gamma-band ASSR and Negative Symptoms. Our final model, M5, all non-significant associations in M3 were omitted. M5 ultimately provided the best fit for the data.
Using M5 as our final model, the effect of Sensory Discrimination on Cognition was estimated to be β = 0.30 (P = .001), the total effect of Sensory Discrimination (direct effect plus indirect effect through Cognition) on Negative Symptoms was estimated to be β = −0.35 (P < .001), and the indirect effect of Sensory Discrimination on Functional Outcomes through Cognition and Negative Symptoms was estimated to be β = 0.10 (P = .001). The effect of Gamma-band ASSR on Cognition was estimated to be β = 0.20 (P = .004), the indirect effect of Gamma-band ASSR on Negative Symptoms through Cognition was estimated to be β = −0.09 (P = .016), the indirect effect of Gamma-band ASSR on Functional Outcomes through Cognition and Negative Symptoms was estimated to be β = 0.06 (P = .017).
Discussion
The present study supports previous findings that laboratory-based EEG biomarkers of early auditory information processing significantly add to our understanding of the cognitive, clinical, and functional disability of schizophrenia patients. Furthermore, results show that the EEG measures of both Sensory Discrimination as well as Gamma-band ASSR can be placed in a hierarchical framework linking these impairments in low-level sensory processing to higherorder cognitive, clinical, and psychosocial outcomes in schizophrenia patients. The present results not only provided independent replication and validation of previous studies using similar SEM approaches but also demonstrated a unique contribution of Gamma-band ASSR. Specifically, Sensory Discrimination (MMN/P3a) had a direct effect on Cognition and Negative Symptoms, while Gamma-band ASSR had a direct and unique effect on Cognition. Furthermore, both Sensory Discrimination and Gamma-band ASSR indirectly impacted Functional Outcomes.
The present integrative multivariate results obtained from n = 695 schizophrenia patients also confirm previous findings of bivariate associations among MMN, P3a, ASSR, neurocognition, symptoms, and functional outcomes in schizophrenia patients.19,32,34,41,55,57 We also found that both Sensory Discrimination and Gamma-band ASSR have significant direct effects on the Cognition and significant indirect (mediating) effects on Functional Outcomes. Consistent with our previous study, we found that MMN/P3a indirectly mediated Functional Outcomes through a direct influence on Negative Symptoms.19 Whereas our previous study showed that cognition was more strongly associated with functional outcomes than with negative symptoms,19 the current study showed that Cognition was more prominently associated with Negative Symptoms than with Functional Outcomes (figure 4, M0). These important, but nuanced differences in relative path strengths among cognition, symptoms, and functional measures observed across the two studies could be due to the use of different Cognition and Functional Outcomes measures. Both the study of Thomas et al19 and the current study are consistent with previous studies which found that negative symptoms mediate relationships between cognitive dysfunction and lower functional outcomes in patients with schizophrenia.103–106 Moreover, results clearly indicate that EEG-based measures of Sensory Discrimination and Gamma-band ASSR significantly add explanatory power to these well-established models with strong evidence for “bottom-up” effects on important cognitive, clinical, and functional outcomes, supporting the use of these translational biomarkers in the context of the development of novel therapeutic interventions for schizophrenia patients.
In contrast to MMN/P3a measures, a direct effect on Negative Symptoms was not observed for the Gamma-band ASSR in the current study. The dissociation between MMN/P3a and ASSR on negative symptoms may be related to differences in the neuropathological substrates of MMN and gamma-band ASSR impairment in schizophrenia patients. MMN reflects N-methyl-d-aspartate (NMDA) receptor function107,108 and it is known that both positive and negative symptoms are associated with impaired NMDA receptor function by ketamine.109,110 Differential effects of NMDA receptor dysfunction on cortical microcircuitry may play a role in the different effects of MMN and gamma-band ASSR on negative symptoms in schizophrenia patients.
Complementary but distinct EEG biomarkers of MMN/P3a and gamma-band ASSR improve our understanding of the hierarchical/bottom-up nature of clinically relevant deficits in schizophrenia; specifically, the present model provides evidence that multiple EEG metrics can be used in synergistic ways to better understand the complex biological mechanisms underlying cognitive and functional outcomes in schizophrenia. Furthermore, the present model may provide valuable information for new treatment development. For example, we have shown that MMN/P3a assessed at the outset of treatment predicts cognitive and clinical gains after a full course of targeted cognitive training.17 Similarly, a recent study by Medalia et al111 showed that low-level auditory training exercises were primarily useful in patients with schizophrenia with pre-existing deficits in early auditory processing (lower score in Tone Matching Test) and enabled such individuals to benefit from cognitive remediation aimed at higher-order deficits. Thus, EEG and behavioral measures of auditory system fidelity may be helpful for novel pharmacologic, non-pharmacologic, and combined pharmacologic augmentation of cognitive therapeutic strategies112 that leverage validated pathophysiological models of disease as well as reliable biomarkers for predicting and/or monitoring therapeutic response.11–18,113
Limitations
The results of the present study should be considered in the context of several limitations. First, this was a cross-sectional cohort study of a heterogeneous sample of schizophrenia patients, the majority of whom were receiving complex medication regimens. As is the case for most large-scale studies of schizophrenia patients, the medication, psychosocial environments, and other important factors that could potentially influence brain function or interacting pathways from brain function across domains could not be experimentally parsed in the present study. Second, the schizophrenia patients in this study had a chronic illness; results therefore may not generalize to at-risk or early-illness psychosis patients. In this context, separate studies suggest that the stage of illness appears to moderate the effect of MMN32 and evoked gamma oscillations58 on global functioning. Third, the SEM approach used here does not prove a causal association. Rather, it is used as a statistical framework for comparing the plausibility of models. Additional replication and refinement in longitudinal and experimental data sets is necessary to support these findings. Finally, we showed a “bottom up” effect of EEG-based measures of early auditory information processing on functional outcomes. While the current study did not have a cognitive test battery that was adequately balanced to determine whether the EEG measures of early auditory information processing provide unique pathways via auditory vs visual domains of cognitive function, Thomas et al19 explicitly tested this possibility and found that impairments in early auditory processing are comparably associated with both auditory and visual domains of cognitive functioning. Since early auditory processing arises from a broadly distributed network91,92 results lend support to the view that these neurophysiological measures generally reflect impaired brain functioning rather than a specific deficit in auditory information processing.
Conclusions
The results of this study show that the use of complementary but distinct EEG biomarkers, MMN/P3a and gamma-band ASSR, improves our understanding of the hierarchical/bottom-up nature of deficits in schizophrenia. Specifically, multiple EEG metrics may be used in synergistic ways to better understand cognitive, clinical, and psychosocial impairments in schizophrenia patients. Investigation of the neural mechanisms underlying the abnormal networks of MMN/P3a and gamma-band ASSR focusing on the hierarchical nature of deficits in schizophrenia patients will strengthen the utility of MMN/P3a and gamma-band ASSR as translational brain markers for clarifying the pathophysiology and the development of novel therapeutic interventions.
Supplementary Material
Acknowledgments
Swartz Center for Computational Neuroscience is supported by a generous gift of Swartz Foundation (New York). The funders had no role in the study design, data collection and analysis, publication decision, or manuscript preparation.
Funding
This study was supported by JSPS Overseas Research Fellowships (D. Koshiyama), the Sidney R. Baer, Jr. Foundation, NIMH grant MH042228 (D. Braff), NIMH grant MH079777 (G. Light), and the VISN-22 Mental Illness Research, Education, and Clinical Center.
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153(3):321–330. [DOI] [PubMed] [Google Scholar]
- 2. Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119–136. [DOI] [PubMed] [Google Scholar]
- 3. Lieberman JA, Perkins D, Belger A, et al. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50(11):884–897. [DOI] [PubMed] [Google Scholar]
- 4. Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav. 2011;99(2):245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robbins TW. Pharmacological treatment of cognitive deficits in nondementing mental health disorders. Dialogues Clin Neurosci. 2019;21(3):301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koshiyama D, Fukunaga M, Okada N, et al. Subcortical association with memory performance in schizophrenia: a structural magnetic resonance imaging study. Transl Psychiatry. 2018;8(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koshiyama D, Fukunaga M, Okada N, et al. Role of subcortical structures on cognitive and social function in schizophrenia. Sci Rep. 2018;8(1):1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koshiyama D, Fukunaga M, Okada N, et al. Role of frontal white matter and corpus callosum on social function in schizophrenia. Schizophr Res. 2018;202:180–187. [DOI] [PubMed] [Google Scholar]
- 9. Javitt DC, Sweet RA. Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nat Rev Neurosci. 2015;16(9):535–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uhlhaas PJ, Singer W. Oscillations and neuronal dynamics in schizophrenia: the search for basic symptoms and translational opportunities. Biol Psychiatry. 2015;77(12):1001–1009. [DOI] [PubMed] [Google Scholar]
- 11. Braff DL, Light GA. Preattentional and attentional cognitive deficits as targets for treating schizophrenia. Psychopharmacology (Berl). 2004;174(1):75–85. [DOI] [PubMed] [Google Scholar]
- 12. Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajós M. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7(1):68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lavoie S, Murray MM, Deppen P, et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33(9):2187–2199. [DOI] [PubMed] [Google Scholar]
- 14. Light GA, Swerdlow NR. Future clinical uses of neurophysiological biomarkers to predict and monitor treatment response for schizophrenia. Ann N Y Acad Sci. 2015;1344:105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Light GA, Swerdlow NR, Thomas ML, et al. Validation of mismatch negativity and P3a for use in multi-site studies of schizophrenia: characterization of demographic, clinical, cognitive, and functional correlates in COGS-2. Schizophr Res. 2015;163(1–3):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perez VB, Tarasenko M, Miyakoshi M, et al. Mismatch negativity is a sensitive and predictive biomarker of perceptual learning during auditory cognitive training in schizophrenia. Neuropsychopharmacology. 2017;42(11):2206–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hochberger WC, Joshi YB, Thomas ML, et al. Neurophysiologic measures of target engagement predict response to auditory-based cognitive training in treatment refractory schizophrenia. Neuropsychopharmacology. 2019;44(3):606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Swerdlow NR, Bhakta S, Chou HH, Talledo JA, Balvaneda B, Light GA. Memantine effects on sensorimotor gating and mismatch negativity in patients with chronic psychosis. Neuropsychopharmacology. 2016;41(2):419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thomas ML, Green MF, Hellemann G, et al. Modeling deficits from early auditory information processing to psychosocial functioning in schizophrenia. JAMA Psychiatry. 2017;74(1):37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perez VB, Miyakoshi M, Makeig SD, Light GA. Mismatch negativity reveals plasticity in cortical dynamics after 1-hour of auditory training exercises. Int J Psychophysiol. 2019;145:40–47. [DOI] [PubMed] [Google Scholar]
- 21. Hochberger WC, Thomas ML, Joshi YB, et al. Oscillatory biomarkers of early auditory information processing predict cognitive gains following targeted cognitive training in schizophrenia patients. Schizophr Res. 2020;215:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tada M, Kirihara K, Mizutani S, et al. Mismatch negativity (MMN) as a tool for translational investigations into early psychosis: a review. Int J Psychophysiol. 2019;145:5–14. [DOI] [PubMed] [Google Scholar]
- 23. Light GA, Braff DL. Stability of mismatch negativity deficits and their relationship to functional impairments in chronic schizophrenia. Am J Psychiatry. 2005;162(9):1741–1743. [DOI] [PubMed] [Google Scholar]
- 24. Light GA, Swerdlow NR, Rissling AJ, et al. Characterization of neurophysiologic and neurocognitive biomarkers for use in genomic and clinical outcome studies of schizophrenia. PLoS One. 2012;7(7):e39434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koshiyama D, Kirihara K, Tada M, et al. Reduced auditory mismatch negativity reflects impaired deviance detection in schizophrenia. Schizophr Bull. 2020;46(4):937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knight R. Contribution of human hippocampal region to novelty detection. Nature. 1996;383(6597):256–259. [DOI] [PubMed] [Google Scholar]
- 27. Javitt DC, Doneshka P, Grochowski S, Ritter W. Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Arch Gen Psychiatry. 1995;52(7):550–558. [DOI] [PubMed] [Google Scholar]
- 28. Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry. 2005;62(2):127–136. [DOI] [PubMed] [Google Scholar]
- 29. Koshiyama D, Kirihara K, Tada M, et al. Duration and frequency mismatch negativity shows no progressive reduction in early stages of psychosis. Schizophr Res. 2017;190:32–38. [DOI] [PubMed] [Google Scholar]
- 30. Salisbury DF, Polizzotto NR, Nestor PG, Haigh SM, Koehler J, McCarley RW. Pitch and Duration Mismatch Negativity and Premorbid Intellect in the First Hospitalized Schizophrenia Spectrum. Schizophr Bull. 2017;43(2):407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koshiyama D, Kirihara K, Tada M, et al. Electrophysiological evidence for abnormal glutamate-GABA association following psychosis onset. Transl Psychiatry. 2018;8(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koshiyama D, Kirihara K, Tada M, et al. Association between mismatch negativity and global functioning is specific to duration deviance in early stages of psychosis. Schizophr Res. 2018;195:378–384. [DOI] [PubMed] [Google Scholar]
- 33. Hermens DF, Ward PB, Hodge MA, Kaur M, Naismith SL, Hickie IB. Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):822–829. [DOI] [PubMed] [Google Scholar]
- 34. Wynn JK, Sugar C, Horan WP, Kern R, Green MF. Mismatch negativity, social cognition, and functioning in schizophrenia patients. Biol Psychiatry. 2010;67(10):940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rasser PE, Schall U, Todd J, et al. Gray matter deficits, mismatch negativity, and outcomes in schizophrenia. Schizophr Bull. 2011;37(1):131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamasue H, Yamada H, Yumoto M, et al. Abnormal association between reduced magnetic mismatch field to speech sounds and smaller left planum temporale volume in schizophrenia. Neuroimage. 2004;22(2):720–727. [DOI] [PubMed] [Google Scholar]
- 37. Braeutigam S, Dima D, Frangou S, James A. Dissociable auditory mismatch response and connectivity patterns in adolescents with schizophrenia and adolescents with bipolar disorder with psychosis: a magnetoencephalography study. Schizophr Res. 2018;193:313–318. [DOI] [PubMed] [Google Scholar]
- 38. Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76(1):1–23. [DOI] [PubMed] [Google Scholar]
- 39. Erickson MA, Ruffle A, Gold JM. A meta-analysis of mismatch negativity in schizophrenia: from clinical risk to disease specificity and progression. Biol Psychiatry. 2016;79(12):980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rissling AJ, Miyakoshi M, Sugar CA, Braff DL, Makeig S, Light GA. Cortical substrates and functional correlates of auditory deviance processing deficits in schizophrenia. Neuroimage Clin. 2014;6:424–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Toyomaki A, Kusumi I, Matsuyama T, Kako Y, Ito K, Koyama T. Tone duration mismatch negativity deficits predict impairment of executive function in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(1):95–99. [DOI] [PubMed] [Google Scholar]
- 42. Bodatsch M, Ruhrmann S, Wagner M, et al. Prediction of psychosis by mismatch negativity. Biol Psychiatry. 2011;69(10):959–966. [DOI] [PubMed] [Google Scholar]
- 43. Bodatsch M, Brockhaus-Dumke A, Klosterkötter J, Ruhrmann S. Forecasting psychosis by event-related potentials-systematic review and specific meta-analysis. Biol Psychiatry. 2015;77(11):951–958. [DOI] [PubMed] [Google Scholar]
- 44. Lepock JR, Ahmed S, Mizrahi R, et al. Relationships between cognitive event-related brain potential measures in patients at clinical high risk for psychosis. Schizophr Res. In press. [DOI] [PubMed] [Google Scholar]
- 45. Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11(2):100–113. [DOI] [PubMed] [Google Scholar]
- 46. Tada M, Kirihara K, Koshiyama D, et al. Gamma-Band Auditory Steady-State Response as a neurophysiological marker for excitation and inhibition balance: a review for understanding schizophrenia and other neuropsychiatric disorders. Clin EEG Neurosci. 2020;51(4):234–243. [DOI] [PubMed] [Google Scholar]
- 47. Uhlhaas PJ, Pipa G, Neuenschwander S, Wibral M, Singer W. A new look at gamma? High- (>60 Hz) γ-band activity in cortical networks: function, mechanisms and impairment. Prog Biophys Mol Biol. 2011;105(1–2):14–28. [DOI] [PubMed] [Google Scholar]
- 48. Uhlhaas PJ, Pipa G, Lima B, et al. Neural synchrony in cortical networks: history, concept and current status. Front Integr Neurosci. 2009;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bosman CA, Lansink CS, Pennartz CM. Functions of gamma-band synchronization in cognition: from single circuits to functional diversity across cortical and subcortical systems. Eur J Neurosci. 2014;39(11):1982–1999. [DOI] [PubMed] [Google Scholar]
- 50. Kwon JS, O’Donnell BF, Wallenstein GV, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56(11):1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brenner CA, Sporns O, Lysaker PH, O’Donnell BF. EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am J Psychiatry. 2003;160(12):2238–2240. [DOI] [PubMed] [Google Scholar]
- 52. Hamm JP, Bobilev AM, Hayrynen LK, et al. Stimulus train duration but not attention moderates γ-band entrainment abnormalities in schizophrenia. Schizophr Res. 2015;165(1):97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hirano Y, Oribe N, Kanba S, Onitsuka T, Nestor PG, Spencer KM. Spontaneous gamma activity in schizophrenia. JAMA Psychiatry. 2015;72(8):813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kirihara K, Rissling AJ, Swerdlow NR, Braff DL, Light GA. Hierarchical organization of gamma and theta oscillatory dynamics in schizophrenia. Biol Psychiatry. 2012;71(10):873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Light GA, Hsu JL, Hsieh MH, et al. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60(11):1231–1240. [DOI] [PubMed] [Google Scholar]
- 56. Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64(5):369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tada M, Nagai T, Kirihara K, et al. Differential alterations of auditory gamma oscillatory responses between pre-onset high-risk individuals and first-episode schizophrenia. Cereb Cortex. 2016;26(3):1027–1035. [DOI] [PubMed] [Google Scholar]
- 58. Koshiyama D, Kirihara K, Tada M, et al. Auditory gamma oscillations predict global symptomatic outcome in the early stages of psychosis: a longitudinal investigation. Clin Neurophysiol. 2018;129(11):2268–2275. [DOI] [PubMed] [Google Scholar]
- 59. Koshiyama D, Kirihara K, Tada M, et al. Gamma-band auditory steady-state response is associated with plasma levels of d-serine in schizophrenia: an exploratory study. Schizophr Res. 2019;208:467–469. [DOI] [PubMed] [Google Scholar]
- 60. Edgar JC, Chen YH, Lanza M, et al. Cortical thickness as a contributor to abnormal oscillations in schizophrenia? Neuroimage Clin. 2014;4:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Teale P, Collins D, Maharajh K, Rojas DC, Kronberg E, Reite M. Cortical source estimates of gamma band amplitude and phase are different in schizophrenia. Neuroimage. 2008;42(4):1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tsuchimoto R, Kanba S, Hirano S, et al. Reduced high and low frequency gamma synchronization in patients with chronic schizophrenia. Schizophr Res. 2011;133(1–3):99–105. [DOI] [PubMed] [Google Scholar]
- 63. Vierling-Claassen D, Siekmeier P, Stufflebeam S, Kopell N. Modeling GABA alterations in schizophrenia: a link between impaired inhibition and altered gamma and beta range auditory entrainment. J Neurophysiol. 2008;99(5):2656–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wilson TW, Hernandez OO, Asherin RM, Teale PD, Reite ML, Rojas DC. Cortical gamma generators suggest abnormal auditory circuitry in early-onset psychosis. Cereb Cortex. 2008;18(2):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thuné H, Recasens M, Uhlhaas PJ. The 40-Hz auditory steady-state response in patients with schizophrenia: a meta-analysis. JAMA Psychiatry. 2016;73(11):1145–1153. [DOI] [PubMed] [Google Scholar]
- 66. Koshiyama D, Miyakoshi M, Thomas ML, et al. Unique contributions of sensory discrimination and gamma synchronization deficits to cognitive, clinical, and psychosocial functional impairments in schizophrenia. bioRxiv 2020. doi: 10.1101/2020.07.19.211193 [DOI] [PubMed] [Google Scholar]
- 67. Garrido MI, Friston KJ, Kiebel SJ, Stephan KE, Baldeweg T, Kilner JM. The functional anatomy of the MMN: a DCM study of the roving paradigm. Neuroimage. 2008;42(2):936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Phillips HN, Blenkmann A, Hughes LE, Bekinschtein TA, Rowe JB. Hierarchical organization of frontotemporal networks for the prediction of stimuli across multiple dimensions. J Neurosci. 2015;35(25):9255–9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. MacLean SE, Ward LM. Temporo-frontal phase synchronization supports hierarchical network for mismatch negativity. Clin Neurophysiol. 2014;125(8):1604–1617. [DOI] [PubMed] [Google Scholar]
- 70. Randeniya R, Oestreich LKL, Garrido MI. Sensory prediction errors in the continuum of psychosis. Schizophr Res. 2018;191:109–122. [DOI] [PubMed] [Google Scholar]
- 71. Corlett PR, Honey GD, Krystal JH, Fletcher PC. Glutamatergic model psychoses: prediction error, learning, and inference. Neuropsychopharmacology. 2011;36(1): 294–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sterzer P, Adams RA, Fletcher P, et al. The predictive coding account of psychosis. Biol Psychiatry. 2018;84(9):634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ishishita Y, Kunii N, Shimada S, et al. Deviance detection is the dominant component of auditory contextual processing in the lateral superior temporal gyrus: a human ECoG study. Hum Brain Mapp. 2019;40(4):1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Farahani ED, Goossens T, Wouters J, van Wieringen A. Spatiotemporal reconstruction of auditory steady-state responses to acoustic amplitude modulations: potential sources beyond the auditory pathway. Neuroimage. 2017;148:240–253. [DOI] [PubMed] [Google Scholar]
- 75. Tada M, Kirihara K, Ishishita Y, et al. Global and parallel cortical processing of auditory gamma oscillatory responses in humans. 2019; Cell-Reports-D-19-02197. Available at SSRN: https://ssrn.com/abstract=3417938, 10.2139/ssrn.3417938 [DOI] [PMC free article] [PubMed]
- 76. Kirihara K, Tada M, Koshiyama D, et al. A predictive coding perspective on mismatch negativity impairment in schizophrenia. Front Psychiatry. 2020;11:660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hagoort P, Hald L, Bastiaansen M, Petersson KM. Integration of word meaning and world knowledge in language comprehension. Science. 2004;304(5669):438–441. [DOI] [PubMed] [Google Scholar]
- 78. Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, Gordon JA. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature. 2015;522(7556):309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Galuske RAW, Munk MHJ, Singer W. Relation between gamma oscillations and neuronal plasticity in the visual cortex. Proc Natl Acad Sci U S A. 2019;116(46):23317–23325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Senkowski D, Gallinat J. Dysfunctional prefrontal gamma-band oscillations reflect working memory and other cognitive deficits in schizophrenia. Biol Psychiatry. 2015;77(12):1010–1019. [DOI] [PubMed] [Google Scholar]
- 81. Youn T, Park HJ, Kim JJ, Kim MS, Kwon JS. Altered hemispheric asymmetry and positive symptoms in schizophrenia: equivalent current dipole of auditory mismatch negativity. Schizophr Res. 2003;59(2–3):253–260. [DOI] [PubMed] [Google Scholar]
- 82. Opitz B, Rinne T, Mecklinger A, von Cramon DY, Schröger E. Differential contribution of frontal and temporal cortices to auditory change detection: fMRI and ERP results. Neuroimage. 2002;15(1):167–174. [DOI] [PubMed] [Google Scholar]
- 83. Takahashi H, Rissling AJ, Pascual-Marqui R, et al. Neural substrates of normal and impaired preattentive sensory discrimination in large cohorts of nonpsychiatric subjects and schizophrenia patients as indexed by MMN and P3a change detection responses. Neuroimage. 2013;66:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Oknina LB, Wild-Wall N, Oades RD, et al. Frontal and temporal sources of mismatch negativity in healthy controls, patients at onset of schizophrenia in adolescence and others at 15 years after onset. Schizophr Res. 2005;76(1):25–41. [DOI] [PubMed] [Google Scholar]
- 85. Miyanishi T, Sumiyoshi T, Higuchi Y, Seo T, Suzuki M. LORETA current source density for duration mismatch negativity and neuropsychological assessment in early schizophrenia. PLoS One. 2013;8(4):e61152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Molholm S, Martinez A, Ritter W, Javitt DC, Foxe JJ. The neural circuitry of pre-attentive auditory change-detection: an fMRI study of pitch and duration mismatch negativity generators. Cereb Cortex. 2005;15(5):545–551. [DOI] [PubMed] [Google Scholar]
- 87. Gaebler AJ, Mathiak K, Koten JW Jr, et al. Auditory mismatch impairments are characterized by core neural dysfunctions in schizophrenia. Brain. 2015;138(Pt 5):1410–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mathiak K, Rapp A, Kircher TT, et al. Mismatch responses to randomized gradient switching noise as reflected by fMRI and whole-head magnetoencephalography. Hum Brain Mapp. 2002;16(3):190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schall U, Johnston P, Todd J, Ward PB, Michie PT. Functional neuroanatomy of auditory mismatch processing: an event-related fMRI study of duration-deviant oddballs. Neuroimage. 2003;20(2):729–736. [DOI] [PubMed] [Google Scholar]
- 90. Dima D, Frangou S, Burge L, Braeutigam S, James AC. Abnormal intrinsic and extrinsic connectivity within the magnetic mismatch negativity brain network in schizophrenia: a preliminary study. Schizophr Res. 2012;135(1–3):23–27. [DOI] [PubMed] [Google Scholar]
- 91. Koshiyama D, Miyakoshi M, Joshi YB, et al. Abnormal effective connectivity underlying auditory mismatch negativity impairments in schizophrenia. Biol Psychiary Cogn Neurosci Neuroimaging. In press. [DOI] [PubMed] [Google Scholar]
- 92. Koshiyama D, Miyakoshi M, Joshi YB, et al. A distributed frontotemporal network underlies gamma-band synchronization impairments in schizophrenia patients. Neuropsychopharmacology In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rissling AJ, Braff DL, Swerdlow NR, et al. Disentangling early sensory information processing deficits in schizophrenia. Clin Neurophysiol. 2012;123(10):1942–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nagai T, Tada M, Kirihara K, et al. Mismatch negativity as a “translatable” brain marker toward early intervention for psychosis: a review. Front Psychiatry 2013;4:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Delis DC, Kramer JH, Kaplan E, Ober BA California Verbal Learning Test. 2nd ed. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- 96. Crowe SF. Does the letter number sequencing task measure anything more than digit span? Assessment. 2000;7(2):113–117. [DOI] [PubMed] [Google Scholar]
- 97. Andreasen NC The Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 98. Rapaport MH, Bazzetta J, McAdams LA, Patterson T, Jeste DV. Validation of the scale of functioning in older outpatients with schizophrenia. Am J Geriatr Psychiatry. 1996;4(3):218–228. [DOI] [PubMed] [Google Scholar]
- 99. Kline R Principles and Practice of Structural Equation Modeling. New York, NY: Guilford Press; 1998. [Google Scholar]
- 100. Bollen KA Structural Equations With Latent Variables. New York, NY: Wiley; 1989. [Google Scholar]
- 101. Rosseel Y. lavaan: An R Package for Structural Equation Modeling. J Stat Softw. 2012;48:1–36. [Google Scholar]
- 102. Hu L, Bentler P. Cutoffcriteriaforfitindexes in covariance structure analysis: conventional criteria vs new alternatives. Struct Equ Modeling 1999;6:1–55. [Google Scholar]
- 103. Vauth R, Rüsch N, Wirtz M, Corrigan PW. Does social cognition influence the relation between neurocognitive deficits and vocational functioning in schizophrenia? Psychiatry Res. 2004;128(2):155–165. [DOI] [PubMed] [Google Scholar]
- 104. Bell M, Tsang HW, Greig TC, Bryson GJ. Neurocognition, social cognition, perceived social discomfort, and vocational outcomes in schizophrenia. Schizophr Bull. 2009;35(4):738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Schmidt SJ, Mueller DR, Roder V. Social cognition as a mediator variable between neurocognition and functional outcome in schizophrenia: empirical review and new results by structural equation modeling. Schizophr Bull. 2011;37Suppl 2:S41–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lin CH, Huang CL, Chang YC, et al. Clinical symptoms, mainly negative symptoms, mediate the influence of neurocognition and social cognition on functional outcome of schizophrenia. Schizophr Res. 2013;146(1–3):231–237. [DOI] [PubMed] [Google Scholar]
- 107. Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci U S A. 1996;93(21):11962–11967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57(12):1139–1147. [DOI] [PubMed] [Google Scholar]
- 109. Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. [DOI] [PubMed] [Google Scholar]
- 110. de la Salle S, Shah D, Choueiry J, et al. NMDA Receptor Antagonist Effects on Speech-Related Mismatch Negativity and Its Underlying Oscillatory and Source Activity in Healthy Humans. Front Pharmacol 2019;10:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Medalia A, Saperstein AM, Qian M, Javitt DC. Impact of baseline early auditory processing on response to cognitive remediation for schizophrenia. Schizophr Res. 2019;208:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Swerdlow NR, Bhakta SG, Light GA. Room to move: plasticity in early auditory information processing and auditory learning in schizophrenia revealed by acute pharmacological challenge. Schizophr Res. 2018;199:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Joshi YB, Light GA. Using EEG-Guided Basket and Umbrella Trials in Psychiatry: a Precision Medicine Approach for Cognitive Impairment in Schizophrenia. Front Psychiatry. 2018;9:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.