Abstract
BACKGROUND AND PURPOSE:
Cerebral sparganosis is a rare parasitic infection caused by sparganum, which can migrate in the brain. The purpose of this study was to demonstrate the migration of cerebral sparganosis and describe its patterns on MR imaging.
MATERIALS AND METHODS:
MR images of 14 patients with cerebral sparganosis treated from 2005 to 2011 were retrospectively reviewed. Diagnosis was made on the basis of a constellation of clinical history, laboratory tests, imaging findings, and histopathology. At least 3 MR imaging studies were performed for each patient during the follow-up period ranging from 12 to 38 months. Time interval, sites, enhanced pattern, and presumed routes of migration were evaluated.
RESULTS:
Both the initial lesions and migrated ones exhibited the “tunnel” sign and multiloculated rim enhancement. Migration was detected between 4 and 18 months after the baseline MR imaging in 14 lesions (in 14 patients), while 3 of 14 lesions showed a second migration between 22 and 38 months. Nearly all migrations were limited to the same hemisphere except for 2 contralateral migrations through the thalamus. Most of the migrations were in close proximity (within the same lobe, to the adjacent lobe, from the basal ganglia to the cortex, from the cerebellum to the pons and interthalamus) except 1 from the basal ganglia to the cerebellum. A signal change along the presumed route of migration was seen in 3 patients.
CONCLUSIONS:
Migration is a notable feature of cerebral sparganosis. Demonstration of migration on MR imaging could be a key diagnostic clue and beneficial for the treatment policy.
Human sparganosis is a rare parasitic disease caused by infection of sparganum, a second-stage larva of Spirometra mansoni.1 It is endemic in East and Southeast Asia and occurs sporadically in the rest of the world. Most infections of human sparganosis involve the subcutaneous tissue, muscle, and eye2,3; nonetheless, the parasite can also be found in many other parts of the human body, such as the breast,4 peritoneum, pleura, spinal cord,5 and brain. It usually manifests as a migrating subcutaneous nodule in the chest wall, abdominal wall, scrotum, lower extremities,6 and breast.7 The postulated route of sparganum infestation to the brain is that first it passes through the alimentary canal to the abdominal cavity; then it penetrates the diaphragm and mediastinum upwards to reach the neck, where it further migrates up through the foramen magnum or jugular foramen; and finally, it reaches the brain.8 A definite diagnosis of cerebral sparganosis is challenging due to its nonspecific clinical manifestation. Previously, it was often misdiagnosed as a neoplasm,9 infarct,10 tuberculoma,11 or other diseases. With the availability of high-resolution CT and MR imaging, the ELISA test for parasitic antibodies, and stereotactic biopsy, more cases of sparganosis are being diagnosed currently.8,12 Characteristic imaging features like the “tunnel” sign and “beadlike” or conglomerated ringlike enhancement on MR imaging were described by Song et al.12 Other features described in the literature that could be useful for diagnosis include coexistence of active and degenerated lesions on the same image and a change in the location or shape of the lesions on follow-up scans.13,14 Until now, there were only a few cases15–17 of cerebral sparganosis migration reported in the literature. In this study, we demonstrate the migration of cerebral sparganosis and describe its patterns on follow-up MR imaging studies.
Materials and Methods
Fourteen patients (9 male, 5 female; mean age, 25.1 years; age range, 12–36 years) with cerebral sparganosis who underwent treatment from 2005 to 2011 were included in our study. Diagnosis was based on a constellation of findings, including clinical history, manifestation, positive ELISA results of sparganosis antibody in serum and CSF, imaging features, and histopathology. For each patient, at least 3 MR imaging studies were performed during the follow-up period, ranging from 12 to 38 months. The first MR imaging study was the baseline examination performed before antiparasitic treatment. The follow-up MRI should be performed at 3–6 months and 12 months after medical treatment or the appearance of new symptoms. The patients avoided the parasite-contaminated risk factors after they were suspected of sparganum infection; thus, the possibility of reinfection decreased to a minimum. Approval from the local ethics committee and informed consent from each patient were obtained for this retrospective study.
MR imaging studies were performed on both Signa 3T (GE Healthcare, Milwaukee, Wisconsin) and Verio 3T (Siemens, Erlangen, Germany) scanners with 8-channel head coils. All examination sequences and parameters are shown in Table 1. After intravenous injection of gadopentetate dimeglumine (0.1–0.2 mmol/kg), sagittal and axial T1-weighted images were obtained. The section thickness was 6 mm with an acquisition matrix of 256 × 256 and an FOV of 240 mm. Two experienced radiologists reviewed the baseline and follow-up MR images. The migrated lesion was defined as follows: 1) the appearance of a new lesion only on follow-up MR imaging, 2) the location and contour of the new lesion being different from the initial one, and 3) contrast enhancement of the new lesion with regression of the initial one. Time range, sites, enhanced pattern, and presumed routes of migration were evaluated.
Table 1:
MR imaging sequences and parameters
| Sequences | Signa 3T | Verio 3T |
|---|---|---|
| Axial and sagittal T1WI FLAIR | TR/TI/TE = 2025/860/15 ms | TR/TI/TE = 2000/860/17 ms |
| Axial T2WI | TR/TE = 3600/115 ms | TR/TE = 6450/203 ms |
| Axial T2WI FLAIR | TR/TI/TE = 8500/2250/120 ms | TR/TI/TE = 9000/2500/102 ms |
| Axial DWI (b = 0, 1000 s/mm2) | TR/TE = 4800/74 ms | TR/TE = 5000/104 ms |
Results
Clinical Findings
In the study group, 12 patients were rural inhabitants. Eleven patients had a history of eating raw/undercooked contaminated food. Three patients were living in the countryside and used to drink unpurified water due to poor sanitary conditions. Seizure was the most common clinical manifestation. Other symptoms included hemiparesis, headache, and aphasia. The disease duration ranged from 1 to 20 years. The ELISA test was positive for sparganosis in serum in 12 patients and in CSF in 7 patients. Sparganosis granulomas were found histopathologically in 8 patients, 3 from surgical resection and 5 from stereotactic biopsy. The living larva was taken out in 1 patient through resection. Nine patients underwent 3–10 courses of praziquantel treatment (10 days per course; total dosage per course = 15 mg/kg × body weight × 10 days; low dose initially with gradual increase). The detailed clinical findings in patients with cerebral sparganosis are shown in Table 2.
Table 2:
Clinical findings of the patients with cerebral sparganosis
| Case/Sex/Age (yr) | Clinical History | Clinical Manifestations | Results of ELISA |
Medical Intervention | |
|---|---|---|---|---|---|
| Serum | CSF | ||||
| 1/M/35 | Eating raw snake gallbladder | Seizures, right hemiparesis | (−) | (−) | Resection |
| 2/F/27 | Drinking contaminated water | Seizures | (+) | (−) | Biopsy and 3 courses of praziquantel |
| 3/M/12 | Eating raw frog flesh | Seizures | (+) | (+) | 3 courses of praziquantel |
| 4/M/18 | Drinking contaminated water | Seizures, left hemiparesis | (+) | (−) | Resection |
| 5/M/36 | Eating raw frog and snake | Seizures, left hemiparesis | (+) | (−) | 6 courses of praziquantel |
| 6/F/33 | Eating undercooked winkles | Seizures | (+) | (−) | Resection |
| 7/M/19 | Eating raw snake gallbladder | Seizures | (+) | (+) | 3 courses of praziquantel |
| 8/F/33 | Eating raw frog flesh | Headache, left hemiparesis | (+) | (+) | Biopsy and 3 courses of praziquantel |
| 9/M/24 | Eating raw snake flesh | Seizures | (+) | (+) | 4 courses of praziquantel |
| 10/M/27 | Eating raw frog flesh | Seizures, headache | (+) | (+) | 10 courses of praziquantel |
| 11/F/18 | Drinking contaminated water | Seizures | (−] | (−) | Biopsy and 6 courses of praziquantel |
| 12/F/28 | Eating raw frog and snake | Aphasia, headache | (+) | (+) | Biopsy and 3 courses of praziquantel |
| 13/M/29 | Eating raw snake gallbladder | Seizures | (+) | (+) | 6 courses of praziquantel |
| 14/M/13 | Drinking contaminated water | Seizures | (+) | (−) | Biopsy and 3 courses of praziquantel |
Note:—(+) indicates positive; (−), negative.
Baseline MR Images
Each patient had an active lesion while undergoing the initial MR imaging examination. Fourteen lesions showed hypointensity on T1WI and hyperintensity with central iso-/hypointensity on T2WI, FLAIR, and DWI. After injection of contrast medium, all active lesions exhibited the tunnel sign and multiloculated rim enhancement (Fig 1). In addition, degenerated lesions, such as white matter degeneration and cortical atrophy, were seen in 10 patients. The active lesion and degenerated lesions coexisted in the same hemisphere in 9 patients, and dilation of the ipsilateral lateral ventricle was seen in 4 patients. These lesions are further described in Table 3.
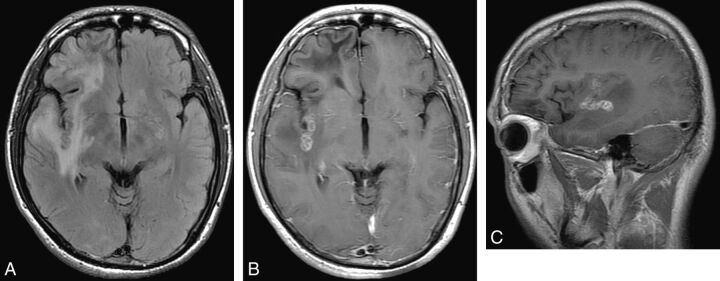
Fig 1.
Case 9. Baseline MR images of a 24-year-old male patient with a 1-year history of seizures. A, Axial FLAIR image shows an abnormal curvilinear iso-/hypointensity in the right insular subcortex area surrounded by edema. B and C, After intravenous contrast injection, the lesion shows multiloculated rim enhancement on the axial image, and the tunnel sign is obvious on the sagittal image. In addition, a degenerated lesion without enhancement can be seen in the right frontal lobe.
Table 3:
Baseline and follow-up MRI of patients with cerebral sparganosis
| Case/Sex/Age (yr) | Baseline MRI |
Follow-Up MRI |
||
|---|---|---|---|---|
| Location of Active Lesion | Degenerated Lesions | Time Interval between the Follow-Up and Baseline MRI (mo) | Location of Migrated Lesions | |
| 1/M/35 | L frontal lobe | No | 1, 2, 5, 8, 12a | L parietal lobe |
| 2/F/27 | R frontal lobe | No | 2, 15, 18a | R parietal lobe |
| 3/M/12 | L thalamus | 1 in L frontal lobe | 4a, 6, 13 | R thalamus |
| 4/M/18 | GM in R frontal lobe | No | 2, 6, 15,a 32 | WM in the same lobe |
| 5/M/36 | R BG | 2 in R temporal lobe and occipital lobe | 11,a 13, 16, 22a | R cerebellar hemisphere; then to pons |
| 6/F/33 | L parietal lobe | 2 in L temporal lobe and frontal lobe | 15,a 22, 25 | L occipital lobe |
| 7/M/19 | L occipital lobe | 2 in L frontal lobe and occipital lobe | 10,a 21 | L temporal lobe |
| 8/F/33 | WM in R frontal lobe | 2 in L frontal lobe and occipital lobe | 2, 5, 6,a 8, 18 | GM in the same lobe |
| 9/M/24 | WM in R temporal lobe | 4 in L parietal lobe, BG, frontal lobe, genu of CC and R frontal lobe, respectively | 2, 4,a 14 | GM in the same lobe |
| 10/M/27 | L parietal lobe | 1 in L frontal lobe | 11,a 38a | L occipital lobe, then to temporal lobe |
| 11/F/18 | GM in L frontal lobe | 2 in L frontal lobe and occipital lobe | 6,a 1, 14 | WM in the same lobe |
| 12/F/28 | L BG | 1 in L occipital lobe | 8, 14,a 22, 24,a 26, 30 | L temporal lobe, then to parietal lobe |
| 13/M/29 | L BG | No | 2, 10, 18,a 23 | L frontal lobe |
| 14/M/13 | R thalamus | 1 in R frontal lobe | 2, 7,a 12 | L thalamus |
Note:—L indicates left; R, right; BG, basal ganglia; CC, corpus callosum; GM, gray matter.
Migration detected.
Follow-Up MR Images
Migration was detected between 4 and 18 months after the baseline MR imaging in 14 lesions (in 14 patients), and 3 of 14 lesions showed a second migration between 22 and 38 months. Hence, a total of 17 migrated lesions were found in 14 patients. Migrations occurred in 9 patients after praziquantel treatment, in 4 patients after surgical resection or biopsy failing to take out the living larvae, and in 1 patient after antiepileptic treatment. The migrated lesions also showed the tunnel sign and multiloculated rim enhancement. Nearly all migrations (15/17) were in the ipsilateral hemisphere. Four lesions moved from white to gray matter or vice versa within the same lobe; 6 moved from 1 lobe to the adjacent one; 3, from the basal ganglia to the cortex; 1, from the basal ganglia to the cerebellum; and 1, from the cerebellum to the pons (Fig 2). Two lesions migrated from one thalamus to the contralateral one (Fig 3). Between the primary lesions and the migrated one, a signal change along the presumed route of migration could be seen in 3 patients. One case showed branchlike hypointensity on T2WI without enhancement, and 2 other cases showed hypointensity on T2WI with curvilinear enhancement (Figs 4 and 5). The initial lesions regressed with mild or scarce enhancement and receding edema on follow-up MRI. The migration time interval and migration sites of each patient are described in Table 3.
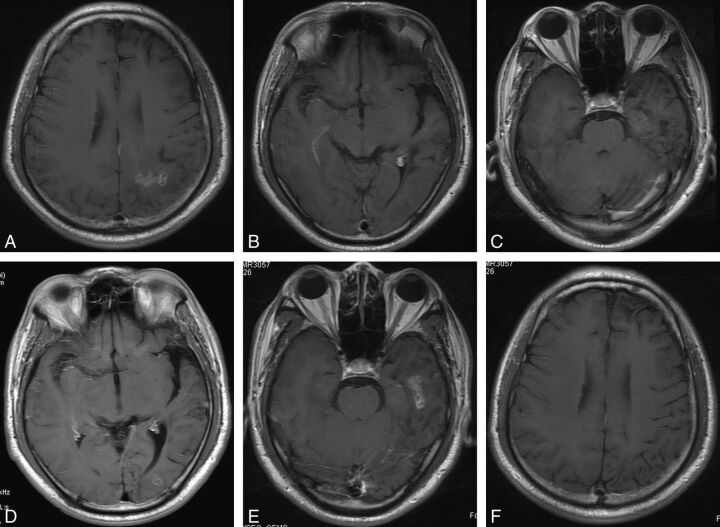
Fig 2.
Case 10. Axial postcontrast T1-weighted images of a 27-year-old male patient with a 2-year history of seizures and headaches. A–C, There is an irregular enhancing lesion with a central tunnel in the left parietal lobe on baseline MR imaging. No active lesion can be seen in the occipital and temporal lobes. D, Eleven months later, a migrated lesion with ringlike enhancement is detected in the left occipital lobe. E and F, The lesion continues migrating to the left temporal lobe, showing typical tunnel-like enhancement 38 months after baseline MR imaging. The initial lesion in the left parietal lobe has regressed with no enhancement.
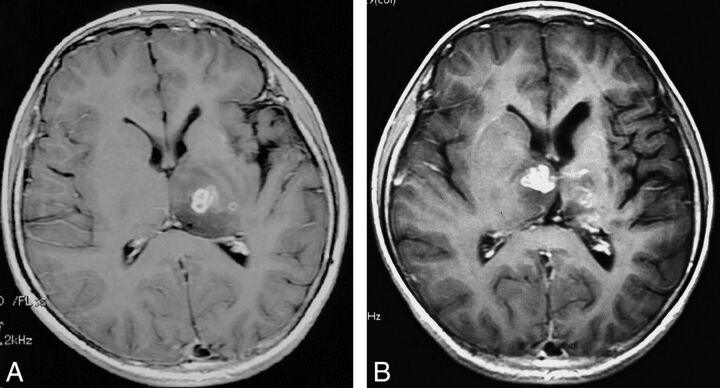
Fig 3.
Case 3. Axial postcontrast T1-weighted images of a 12-year-old male patient with a 4-year history of seizures. A, Baseline MR imaging shows a multiloculated lesion with rim enhancement in the left thalamus. B, Four months later, a migrated lesion is detected in the right thalamus. The initial lesion is regressing, with mild enhancement.
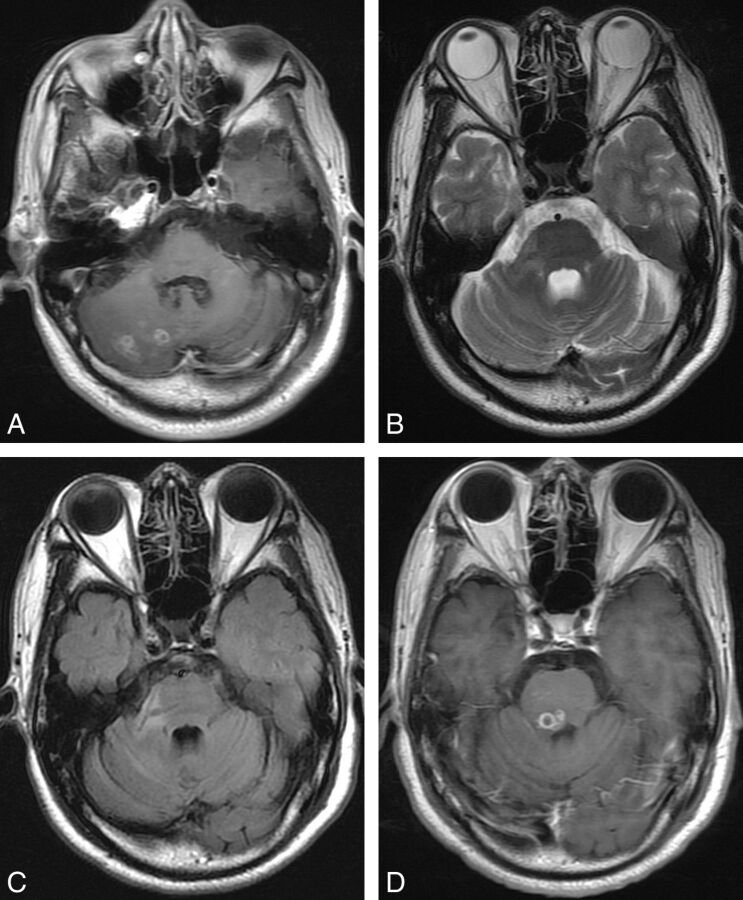
Fig 4.
Case 5. Axial images of a 36-year-old male patient with seizures and left hemiparesis for 17 years. A, Axial postcontrast T1-weighted image shows a lesion with multi-ringlike enhancement in the right cerebral hemisphere. B and C, Four months later, an abnormal branchlike hypointensity is detected in the right cerebellar peduncle on T2-weighted and FLAIR images. D, Eleven months later, a migrated lesion with small ringlike enhancement is detected in the pons. Note that the signal change on B and C indicates the continuity between the primary lesion and the migrated one.
Fig 5.
Case 12. Axial postcontrast T1-weighted images of a 28-year-old female patient with headache and aphasia for 5 years. A and B, Baseline MR imaging shows an irregular enhancing lesion with a central tunnel in the left basal ganglia. There is also a degenerated lesion in the left occipital lobe with dilation of lateral ventricle. C and D, Eight months later, the lesion exhibits long curvilinear enhancement and extends to the insular lobe. The original lesion shows regression, with mild enhancement. E and F, Fourteen months later, a migrated lesion is observed in the left temporal lobe, and the original lesion in the basal ganglia has changed into a degenerated one. Note that the curvilinear enhancement of the lesion on B is presumably the route of migration.
Discussion
Cerebral sparganosis is a rare parasitic infection caused by sparganum. Humans are intermediate hosts for the parasite. Factors resulting in infection mainly included eating raw/undercooked frog or snake, using that meat as a poultice to an open wound, and drinking contaminated water.3 The characteristic host tissue reaction is an elongated tunnel-like cavity, which represents the pathway of the larva. The larva can survive in a tunnel, encapsulated by hyperplastic fibrous tissue for several years,2,18 or it can penetrate the wall of the granuloma and move into the brain. Therefore, some researchers call it the wandering lesion,17 and we concur with this point of view.
In our study, migration was detected between 4 and 18 months after the baseline MR imaging in 14 lesions, while 3 of 14 lesions showed a second migration between 22 and 38 months. The time interval for sparganum to migrate varies greatly, ranging from 1 month to 10 years.8,13–17 Shirakawa et al17 reported the migration of the lesion from the cerebellar hemisphere to the vermis during 7 weeks, which was the shortest reported time interval. In our opinion, the migrated lesion detected in a short time interval was presumably due to the short moving pathway and opportune MR imaging examination. Reasons for the long time interval of migration were the following: first, the sparganum could survive in a tunnel for several years. Second, some patients were asymptomatic while the larva migrated, so no radiographic examination was performed at that time. In our study, the time interval of migration was counted from the baseline MR imaging because the initial lesions showed typical enhancement at this time. However, these might regress and degenerate on the last MR imaging before migration. Moreover, it was difficult to know whether the sparganum was stationary in the initial lesion or had moved elsewhere. Therefore, regular follow-up MR imaging is helpful to know how fast and how often the sparganum has migrated, and the migrated lesions detected on follow-up MR imaging can provide key clues for diagnosis of cerebral sparganosis.
We also noticed that nearly all migrated lesions and the initial one were in the ipsilateral hemisphere. Four lesions moved from white to gray matter or vice versa within the same lobe; 6 lesions moved from one lobe to the adjacent one; 3, from the basal ganglia to the cortex; 1, from the basal ganglia to the cerebellum; and 1, from the cerebellum to the pons. Although variable, most migrations occurred in close proximity. Contralateral migrations were observed in 2 patients, both through the thalamus, but also in close proximity. According to our findings, the main migration pattern seemed to be one limited to the same hemisphere and occurring in proximity. They also indicated that the migrated lesion was a continuity of the previous one rather than the new one appearing from elsewhere in the body or reinfection. Although the migrations in proximity were relative to the short time interval of the follow-up MR imaging, the relationship between the migrating distance and the time interval was still uncertain. In our series, one of the patients (case 5) showed the longest migration distance from the basal ganglia to the ipsilateral cerebellum in 11 months. The longest time interval of migration (case 10) was 27 months from the left occipital lobe to the temporal lobe.
In our study, we found that all initial lesions and migrated ones exhibited the tunnel sign and multiloculated rim enhancement on MR imaging, in agreement with the previous report.12 The wall of the tunnel showed patchy or curvilinear enhancement due to surrounding reactive inflammatory tissue. As for the other feature referred to as “multiloculated rim enhancement,” it presumably was the cross-section of the tortuous tunnel rather than multiple small pyogenic abscesses, due to the absence of restrictive diffusion on DWI.19 However, cerebral sparganosis exhibited amorphous iso-/hypointensity on DWI. The presence of paramagnetic free radicals, ions, and calcification might contribute to the signal change. This finding was consistent with the report of Moon et al.14 The initial lesions regressed with mild or scarce enhancement on follow-up MR imaging and at last changed into degeneration and atrophy. Therefore, the tunnel sign and multiloculated rim enhancement are the characteristic MR imaging features of cerebral sparganosis, and coexistence of active migrated and degenerated lesions on same image is its other feature.
We also observed a signal change between the initial lesion and the migrated one, with the former regressing on follow-up images in 3 patients. One case showed branchlike hypointensity on T2WI and FLAIR images without enhancement, and 2 cases exhibited curvilinear enhancement. This signal change could be the presumed migration route, which indicated that the migrated lesions were actually continuous. Although the migration route is rarely seen, it can be a unique diagnostic MR imaging feature of cerebral sparganosis.
At present, it is generally believed that the effective treatment for cerebral sparganosis is stereotactic surgical removal of the larva.8,13,20 Deng et al8 proposed stereotactic aspiration to minimize the size of the surgical wound. In our study, 9 patients received praziquantel treatment. These patients were inoperable due to the following: First, the lesions were in deep structures or important functional areas. Second, there were multifocal degenerated lesions on baseline MR imaging, and functional deficiency had occurred. Third, the patients did not agree to an invasive treatment because the initial lesions had already decreased on follow-up MR imaging. For patients in medical treatment, follow-up MR imaging is necessary to detect new migrated lesions appearing and to evaluate the therapeutic effect. As for the lesions in deep structures or important functional areas, surgical removal should occur when the larva migrates to the brain superficial area. This migration is another reason for follow-up MR imaging. Surgical resection was performed in 3 patients, and stereotactic biopsy was performed in 5 patients. The living larva was taken out in only 1 patient. Under these circumstances, follow-up MR imaging is of obvious benefit for the neurosurgeon for detecting the position of the migrated lesions.
The limitation of this retrospective study is that the time interval of follow-up MRI was irregular. Most patients underwent MR imaging only when new symptoms occurred. Therefore, we could not identify the exact time and definite route of migrations. The possibility of new infections was minimal because our patients avoided the parasitic environment. Nonetheless, we were not certain that the migrated lesions described in our study were not from elsewhere in the body.
Conclusions
Migration is a notable feature of cerebral sparganosis. It is usually limited to the same hemisphere and occurs in proximity to the original location within 18 months after the initial examination. The features demonstrated on MR imaging can provide key diagnostic clues to this condition. For patients with medical treatment and those with failed surgical removal of living larvae, follow-up MR imaging is necessary to detect this migration.
ABBREVIATION:
- ELISA
enzyme-linked immunosorbant assay
Footnotes
Paper previously presented at: Annual Meeting of the Radiological Society of North America (SSK12-09), November 27–December 2, 2011; Chicago, Illinois.
References
- 1. Mueller JF. The biology of Spirometra. J Parasitol 1974;60:3–14 [PubMed] [Google Scholar]
- 2. Cho JH, Lee KB, Yong TS, et al. Subcutaneous and musculoskeletal sparganosis: imaging characteristics and pathologic correlation. Skeletal Radiol 2000;29:402–08 [DOI] [PubMed] [Google Scholar]
- 3. Wiwanitkit V. A review of human sparganosis in Thailand. Int J Infect Dis 2005;9:312–06 [DOI] [PubMed] [Google Scholar]
- 4. Moon HG, Jung EJ, Park ST. Breast sparganosis presenting as a breast mass with vague migrating pain. J Am Coll Surg 2008;207:292. [DOI] [PubMed] [Google Scholar]
- 5. Bao XY, Ding XH, Lu YC. Sparganosis presenting as radiculalgia at the conus medullaris. Clin Neurol Neurosurg 2008;110:843–46 [DOI] [PubMed] [Google Scholar]
- 6. Lee KJ, Myung NH, Park HW. A case of sparganosis in the leg. Korean J Parasitol 2010;48:309–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koo M, Kim JH, Kim JS. Cases and literature review of breast sparganosis. World J Surg 2011;35:573–79 [DOI] [PubMed] [Google Scholar]
- 8. Deng L, Xiong P, Qian S. Diagnosis and stereotactic aspiration treatment of cerebral sparganosis: summary of 11 cases. J Neurosurg 2011;114:1421–25 [DOI] [PubMed] [Google Scholar]
- 9. Cummings TJ, Madden JF, Gray L, et al. Parasitic lesion of the insula suggesting cerebral sparganosis: case report. Neuroradiology 2000;42:206–08 [DOI] [PubMed] [Google Scholar]
- 10. Murata K, Abe T, Gohda M, et al. Difficulty in diagnosing a case with apparent sequel cerebral sparganosis. Surg Neurol 2007;67:409–11, discussion 412 [DOI] [PubMed] [Google Scholar]
- 11. Rengarajan S, Nanjegowda N, Bhat D, et al. Cerebral sparganosis: a diagnostic challenge. Br J Neurosurg 2008;22:784–86 [DOI] [PubMed] [Google Scholar]
- 12. Song T, Wang WS, Zhou BR, et al. CT and MR characteristics of cerebral sparganosis. AJNR Am J Neuroradiol 2007;28:1700–05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim DG, Paek SH, Chang KH, et al. Cerebral sparganosis: clinical manifestations, treatment, and outcome. J Neurosurg 1996;85:1066–71 [DOI] [PubMed] [Google Scholar]
- 14. Moon WK, Chang KH, Cho SY, et al. Cerebral sparganosis: MR imaging versus CT features. Radiology 1993;188:751–57 [DOI] [PubMed] [Google Scholar]
- 15. Eom KS, Kim TY. Migration of cerebral sparganosis to the ipsilateral cerebellar hemisphere. Acta Parasitologica 2009;54:276–80 [Google Scholar]
- 16. Kim IY, Jung S, Jung TY. Contralateral migration of cerebral sparganosis through the splenium. Clin Neurol Neurosurg 2007;109:720–24 [DOI] [PubMed] [Google Scholar]
- 17. Shirakawa K, Yamasaki H, Ito A, et al. Cerebral sparganosis: the wandering lesion. Neurology 2010;74:180. [DOI] [PubMed] [Google Scholar]
- 18. Qiu MH, Qiu MD. Human plerocercoidosis and sparganosis. II. A historical review on pathology, clinics, epidemiology and control [in Chinese]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2009;27:54–60 [PubMed] [Google Scholar]
- 19. Luthra G, Parihar A, Nath K. Comparative evaluation of fungal, tubercular, and pyogenic brain abscesses with conventional and diffusion MR imaging and proton MR spectroscopy. AJNR Am J Neuroradiol 2007;28:1332–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen H, Wu JS, Zhou LF, et al. The diagnosis and treatment of cerebral sparganosis. Chin J Clin Neurosci 2003;11:166–69 [Google Scholar]