Abstract
BACKGROUND AND PURPOSE:
Parameters other than luminal narrowing are needed to predict the risk of stroke more reliably, particularly in patients with <70% stenosis. The goal of our study was to identify clinical risk factors and CT features of carotid atherosclerotic plaques, in a retrospective cohort of patients free of stroke at baseline, that are independent predictors of incident stroke on follow-up.
MATERIALS AND METHODS:
We identified a retrospective cohort of patients admitted to our emergency department with suspected stroke between 2001–2007 who underwent a stroke work-up including a CTA of the carotid arteries that was subsequently negative for acute stroke. All patients also had to receive a follow-up brain study at least 2 weeks later. From a random sample, we reviewed charts and imaging studies of patients with subsequent new stroke on follow-up as well as those who remained stroke-free. All patients were classified either as “new carotid infarct patients” or “no-new carotid infarct patients” based on the Causative Classification for Stroke. Independently, the baseline CTA studies were processed using a custom, CT-based automated computer classifier algorithm that quantitatively assesses a set of carotid CT features (wall thickness, plaque ulcerations, fibrous cap thickness, lipid-rich necrotic core, and calcifications). Univariate and multivariate statistical analyses were used to identify any significant differences in CT features between the patient groups in the sample. Subsequent ROC analysis allowed comparison to the classic NASCET stenosis rule in identifying patients with incident stroke on follow-up.
RESULTS:
We identified a total of 315 patients without a new carotid stroke between baseline and follow-up, and 14 with a new carotid stroke between baseline and follow-up, creating the main comparison groups for the study. Statistical analysis showed age and use of antihypertensive drugs to be the most significant clinical variables, and maximal carotid wall thickness was the most relevant imaging variable. The use of age ≥75 years, antihypertensive medication use, and a maximal carotid wall thickness of at least 4 mm was able to successfully identify 10 of the 14 patients who developed a new incident infarct on follow-up. ROC analysis showed an area under the ROC curve of 0.706 for prediction of new stroke with this new model.
CONCLUSIONS:
Our new paradigm of using age ≥75 years, history of hypertension, and carotid maximal wall thickness of >4 mm identified most of the patients with subsequent new carotid stroke in our study. It is simple and may help clinicians choose the patients at greatest risk of developing a carotid infarct, warranting validation with a prospective observational study.
Carotid atherosclerotic disease is considered to be responsible for 30% of all ischemic strokes. Luminal narrowing is the standard parameter used to report the severity of carotid atherosclerosis. Indeed, several randomized clinical trials have shown a reduced risk of ischemic stroke in patients with luminal stenosis of ≥70% (assessed by conventional angiograms) after carotid endarterectomy (compared with medical treatment alone.)1–4 However, even if they have a higher individual risk of developing a stroke, patients with ≥70% carotid stenosis represent fewer than 5% of patients. Most strokes occur in patients with <70% carotid stenosis, which represents a large proportion of the general population (70% in men and 60% in women over 64 years of age).5,6 In patients with <70% carotid stenosis, the benefit of surgery is less clear because of the small individual risk of stroke (1.3%–3.3% annually).1,7–11 The high prevalence of <70% stenosis, however, translates into a large number of ischemic strokes on a population level and represents a major public health issue. Better characterization of the risk of stroke in patients with <70% carotid stenosis could impact the criteria used to make treatment decisions in these patients, notably to refer them for stent placement or endarterectomy, and could ultimately result in a decrease in the number of disabling strokes and related deaths in this group.
Luminal narrowing on conventional angiography is only an indirect measure of the carotid atherosclerosis process, as it occurs in the vessel wall, not the lumen. Parameters other than luminal narrowing are needed to predict the risk of stroke more reliably, particularly in patients with <70% stenosis. A number of carotid plaque morphologic features have been suggested as potential markers of the “vulnerable plaque” and are possibly associated with an increased risk of stroke, the most studied of which being the CCA intima-media thickness.5,6,12–19 In addition, embolic phenomena have been reported as being associated with thinning and subsequent ulceration of the fibrous cap on the surface of the atherosclerotic plaque,20–24 resulting in release into the parent vessel of necrotic lipoid debris from the plaque substance, especially in the case of a high plaque lipid content.25,26 On the contrary, carotid plaque calcifications appear to be protective.27,28 Carotid wall features have typically been studied using sonography 5,6,12–14,26 and MR imaging.29–38 Recently, a 3D computerized interpretation of multidetector row, isotropic resolution CTA studies was reported to assess, in a quantitatively accurate and standardized fashion, the histologic composition (including noncalcified components) and characteristics of carotid artery atherosclerotic plaques. In this study, there was 72.6% agreement between CTA and histology for carotid plaque classification, perfect concordance for calcifications, and good correlation with histology for large lipid cores. CTA was also accurate in the detection of ulcerations and in the measurement of fibrous cap thickness.23 Using the standardized, computerized assessment of CTA studies just described, Wintermark et al39 performed a retrospective cross-sectional study to identify the CT features of carotid atherosclerotic plaques that were significantly associated with the occurrence of ischemic stroke. This study revealed that a small number of carotid wall CT features were significantly associated with acute carotid stroke. Specifically, increased risk of acute carotid stroke was associated with an increased wall volume, a thinner fibrous cap, a higher number of lipid clusters, and lipid clusters closer to the lumen.39 The number of calcium clusters was a protective factor.39 Unfortunately, there were several limitations to the pilot study. The design of the study was cross-sectional and it involved only a small number of patients. The authors concluded that their results needed to be confirmed in a large cohort study.
The goal of our study was to identify clinical risk factors and CT features of carotid atherosclerotic plaques, in a large cohort of patients free of stroke at baseline, that are independent predictors of new incident carotid infarct on follow-up.
Materials and Methods
Study Design
Clinical and imaging data, obtained as part of standard clinical stroke care at our institution, were retrospectively reviewed with the approval of the institutional review board. At our institution, patients with suspicion of acute stroke and no history of significant renal insufficiency or contrast allergy routinely undergo a stroke CT survey including the following imaging protocol: noncontrast CT, perfusion CT at 2 cross-sectional positions, CTA of the cervical and intracranial vessels, and a postcontrast cerebral CT.
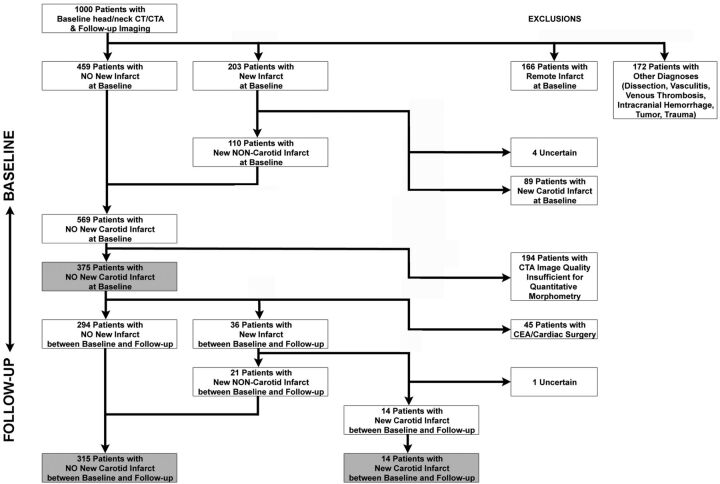
We retrospectively identified 1000 consecutive patients admitted to our emergency department from January 2001 to January 2007 who had undergone a CT/CTA to evaluate their brain and carotid arteries. Only patients who also had a follow-up brain imaging study at least 14 days after baseline CTA were included. Our focus of interest was the carotid artery plaque CT features at baseline on the initial CTA, and whether or not the patient developed a brain infarct on the follow-up brain imaging that was not present on the baseline brain CT (Fig 1).
Fig 1.
Graphic depiction of our study population and how it was selected and distributed into different groups for purposes of statistical analysis. CEA indicates carotid endarterectomy.
Patients with remote or old infarcts in a carotid distribution were excluded from our analysis because carotid atherosclerotic disease is an evolving process, and the carotid artery condition may have evolved in the time interval between when the remote infarct occurred and the time of our CTA study, which was used to characterize the carotid artery plaque features. This could have interfered with our identification of the carotid wall features predictive of future infarct.
Patients who had a carotid endarterectomy were also excluded. Their inclusion would have dramatically affected our results, as the risk of subsequent infarct would be confounded by the procedure. Further, infarct is itself a risk inherent to endarterectomies and invasive cardiac surgeries.
Finally, we excluded patients who had presented with diagnoses that may predispose them to infarcts, independent of carotid atherosclerosis, including carotid dissection, intracranial hemorrhage, and vasculitides. Although we used a comprehensive protocol to differentiate infarcts (ie, between carotid and noncarotid), the source of some infarcts could not be identified. These were labeled “uncertain” infarcts and were subsequently excluded from the study.
CTA Imaging Protocol
CTA studies of the carotid arteries were obtained on 16-section and 64-section CT scanners (GE Healthcare, Milwaukee, Wisconsin). The image acquisition protocol was as follows: spiral mode, 0.6- to 0.8-second gantry rotation; collimation: 16 or 64 × 0.625 mm or 1.25 mm; pitch: 1.375:1 or 0.982:1; section thickness: 0.625 mm; reconstruction interval: 0.5 mm; acquisition parameters: 120 kVp/240 mA. A caudocranial scanning direction was selected, covering the mid-chest to the vertex of the brain. Seventy mL of iohexol (Omnipaque; Amersham Health, Princeton, New Jersey; 300 mg/mL of iodine) was injected into an antecubital vein with a power injector at a rate of 4 mL per second. Optimal timing of the CTA acquisition was achieved by using a test bolus technique. Effective dose associated with this CTA protocol was approximately 7–10 mSv.
Image Postprocessing
CTA studies of the carotid arteries were processed automatically using a custom CT-based automated classifier computer algorithm that was validated by using histology derived from carotid endarterectomy specimens as a criterion standard. This algorithm automatically segments the inner and outer contours of the carotid artery wall and distinguishes between the histologic components of the wall (lipids, calcium) by using appropriate thresholds of CT attenuation.23,29 The algorithm creates a color overlay affording a visual display of the composition of the carotid wall for each CTA image (On-line Fig 1).23 It then automatically analyzes several CT features of the carotid arteries and quantifies them 3-dimensionally (not in a plane, as with B-mode sonography), independent of any subjective human interpretation.32 The location of the largest lipid and calcium clusters was described as a percent of the carotid wall thickness, with 0% indicating the center of the cluster immediately adjacent to the inner contour and 100% indicating the center of the cluster immediately adjacent to the outer contour. Measurements were recorded for the 2.5 cm of the CCA immediately proximal to the carotid bifurcation and the 2.5 cm of the ICA immediately distal to the carotid bifurcation.41 Maximal carotid wall thickness was measured as the greatest distance from the lumen-intima interface to the outer edge of the adventitia. The physician processing the CTA datasets was blinded to the clinical findings of the imaged patients and to the group to which they belonged. Further, the physician was not aware of the reason for imaging or the patients' medical histories.
Baseline Image Review
The CT studies of the brain parenchyma obtained at baseline and the brain imaging studies obtained within the first 14 days after the baseline CT were reviewed by a neuroradiologist for the presence or absence of an acute infarct and its distribution (unilateral or bilateral, single or multiple vascular territories, and location of vascular territory). The 14-day delay was selected because an acute infarct may not show up conspicuously on the day 0 brain parenchymal imaging, especially if the initial imaging is a CT. However, in such a setting, the patient is likely to get a repeat CT or a MR imaging of the brain within the first 2 weeks that would show the acute infarct. The neuroradiologist reviewed the same studies of the brain parenchyma for old remote infarcts. The neuroradiologist also reviewed the intracranial portion of the baseline CTA of the carotid arteries for the degree of completeness of the circle of Willis.
The neuroradiologist assessed the degree of carotid stenosis on the cervical portion of the baseline CTA but did not record any information regarding the carotid wall. During the review, the neuroradiologist was blinded to both the results of the automatic analysis of the carotid wall produced by the computer algorithm, as well as any clinical variables or characteristics.
Follow-Up Image Review
The neuroradiologist evaluated all follow-up studies of the brain parenchyma obtained at least 14 days after the baseline CTA for the presence or absence of an acute infarct and its distribution (unilateral or bilateral, single or multiple vascular territories, and location of vascular territory). The assumption was that any brain parenchymal imaging study obtained more than 14 days after an initial stroke study would probably be triggered by a new clinical event. This comparison to the baseline study allowed verification that any infarcts observed were in fact new.
Medical Chart Review
The medical records of the patients were reviewed, independently of any imaging findings, for age, sex, ethnicity, smoking history, history of diabetes, hypertension, hyperlipidemia, drug treatment, and clinical diagnosis at the time of the follow-up imaging.
Patient Classification
The medical records of the patients were reviewed to determine the probable etiology of the stroke. Using a system based on the Causative Classification System for Ischemic Stroke42 developed by the A.A. Martinos Center, and on the review of the imaging studies of the brain parenchyma by the neuroradiologist,43,44 as well as the degree of carotid stenosis, cardiac disease, and cardiac risk factors (independent of any carotid wall CT features), infarcts were categorized as “carotid infarcts” if they were in a carotid distribution, with the probable mechanism being large artery atherosclerosis. Nonacute strokes and acute strokes in a distribution not consistent with a carotid origin were categorized as “noncarotid infarcts.” Our classification method and the criteria used for classification are summarized in On-line Fig 2 and On-line Tables 1–4.
Statistical Analysis
Our statistical analysis consisted of 2 parts: univariate analyses of the clinical and imaging variables, followed by a multivariate analysis to determine which combination of clinical variables and carotid wall characteristics indicated an increased risk of subsequent new carotid infarct. For our univariate analyses, different types of statistical tests were used for different clinical or imaging variables to verify any association with new incident carotid stroke. Simple t tests were used for continuous clinical variables, while Mann-Whitney tests were performed for the dichotomous clinical variables. For the imaging variables, we used a mixed effect logistic model (with fixed effect for patient).
Variables that showed P values <.2 in the univariate analyses were considered for use in the multivariate analysis, which used .05 as a threshold for statistical significance. However, many of the variables (especially the carotid imaging characteristics) were collinear, indicating overlap (ie, NASCET degree of narrowing and minimal cross-sectional lumen area). In these cases of collinear variables, we only used the one variable that was the most statistically significant (from the univariate analyses) in the final multivariate analysis. This multivariate model allowed us to discover which combination of clinical and/or imaging variables was associated with the greatest risk for subsequent new carotid infarct. Using ROC analysis, we compared different thresholds of the relevant variables to understand which helped provide the most optimal model. This resultant model was then compared with the conventional NASCET rule (ie, luminal stenosis) to find which was more effective in predicting strokes on follow-up in our sample.
Results
Patient Population
One thousand consecutive patients admitted to our emergency department from January 2001 to January 2007 were considered for this study. They all received a brain/carotid CT/CTA at baseline and subsequent follow-up brain imaging at least 2 weeks later. Of this initial group, 89 patients were excluded because they had an acute carotid stroke observed on the baseline CTA (110 with a noncarotid stroke were allowed to continue to the follow-up portion of the study). Further, remote or old carotid infarcts were observed at baseline in 166 patients. The infarct source for 4 patients was not clear (“uncertain”), and 172 patients were excluded due to other diagnoses that may have predisposed them to infarcts independent of any carotid atherosclerosis. One hundred ninety-four patients were excluded because the image quality of the CTA was insufficient to allow the morphometric analysis of the carotid plaque (152 patients with no thin—0.625 or 0.1.25 mm—CTA sections available on PACS, 42 patients with failed contrast bolus and insufficient enhancement of the carotid lumen). Three hundred seventy-five patients were retained in the study.
The most frequent indications for follow-up imaging were altered mental status (27%), suspicion of stroke (15%), suspected or known malignancy (13%), and seizure (10%). Less frequent were suspicion of infection (7%), trauma (6%), complicated migraine (4%), and suspicion of vasculitis (1%); other various indications were noted in 18% of the patients. Two hundred and ninety-four had no new infarcts (ie, compared with the baseline CT imaging), while 36 had a new incident infarct. Fourteen of these 36 patients were deemed to have a new carotid infarct, and 21 had a new noncarotid infarct (the source of 1 patient's infarct could not be properly identified and was labeled “uncertain”). Forty-five of the 375 patients considered in the second portion of the study had a carotid endarterectomy or cardiac surgery during the follow-up period (between the baseline CT/CTA and their follow-up brain imaging study) and were excluded from the study. Consequently, we identified a total of 315 patients without a new carotid infarct between baseline and follow-up, and 14 patients with a new carotid stroke between baseline and follow-up, creating the main comparison groups for the study (Fig 1).
Clinical Variables
In terms of clinical characteristics (Table 1) and on univariate analyses, patients with new carotid infarcts were older and more likely to have arterial hypertension and to be treated with aspirin, antihypertensive drugs, and lipid-lowering drugs. The use of antihypertensive medication and a diagnosis of arterial hypertension were collinear, so the latter was subsequently dropped from the following multivariate analysis. The time between the baseline CT/CTA and the subsequent follow-up imaging was similar across both groups (mean of 390.2 days to follow-up for the new carotid infarct group, and mean of 447.5 days to follow-up for the no-new carotid infarct group). In terms of the technique for follow-up imaging, 64.3% of patients in the new carotid infarct group had CT, and 35.7% had MR imaging, while in the no-new carotid infarct group, 52.7% had CT and 47.3% had MR imaging. This conveys that there was no significant difference in terms of type of follow-up imaging between the new carotid infarct group and the no-new carotid infarct group.
Table 1:
Clinical characteristics of the study patients
| All Patients | Patients with New Carotid Infarcts | Patients with No New Carotid Infarct | P Value | |
|---|---|---|---|---|
| Agea | 64.9 ± 16.2 | 77.2 ± 11.9 | 64.3 ± 16.2 | .14 |
| Gendera | Male 117 (36%) | Male 1 (7.1%) | Male 116 (36.8%) | .02 |
| Female 212 (64%) | Female 13 (92.9%) | Female 199 (63.2%) | ||
| Race/Ethnicity | White 166 (50.5%) | White 7 (50%) | White 159 (50.5%) | .21 |
| Hispanic 33 (10%) | Hispanic 33 (10.5%) | |||
| Asian 90 (27.4%) | Asian 4 (28.6%) | Asian 86 (27.3%) | ||
| African American 30 (9.1%) | African American 3 (21.4%) | African American 27 (8.6%) | ||
| Pacific Islander 1 (0.3%) | Pacific Islander 1 (0.3%) | |||
| Unknown 9 (2.7%) | Unknown 9 (2.8%) | |||
| Smoking history | 52 (15.8%) | 4 (28.6%) | 48 (15.2%) | .29 |
| Diabetes | 105 (31.9%) | 5 (35.7%) | 100 (31.7%) | .58 |
| Hypertensiona | 233 (70.8%) | 13 (92.9%) | 220 (69.8%) | .10 |
| Hyperlipidemia | 186 (56.5%) | 10 (71.4%) | 176 (55.9%) | .26 |
| ASAa | 191 (58.1%) | 11 (78.6%) | 180 (57.1%) | .17 |
| NSAIDs | 78 (23.7%) | 3 (21.4%) | 75 (23.8%) | 1.00 |
| Anticoagulants | 93 (28.3%) | 5 (35.7%) | 88 (27.9%) | .55 |
| Antihypertensive Rxa | 213 (64.7%) | 13 (92.9%) | 200 (63.5%) | .02 |
| Diabetes Rx | 59 (17.9%) | 4 (28.6%) | 55 (17.5%) | .29 |
| Lipid-lowering Rxa | 163 (49.5%) | 10 (71.4%) | 153 (48.6%) | .11 |
| Time elapsed between baseline brain/carotid CT/CTA and follow-up imaging (days) | 445.1 ± 442 | 390.2 ± 288 | 447.5 ± 447.7 | .48 |
| Follow-up imaging modality | CT 175 (53.2%), MRI 154 (46.8%) | CT 9 (64.3%), MRI 5 (35.7%) | CT 166 (52.7%), MRI 149 (47.3%) | .39 |
Note:—ASA indicates acetylsalicylic acid; NSAIDs, nonsteroidal anti-inflammatory drugs; Rx, treatment.
Indicates variables that were different (P < .2) in the patients with new carotid infarcts and in the patients with no new carotid infarct and retained for the multivariate analysis.
Imaging Variables
In terms of carotid artery wall CT features (Table 2), and on univariate analyses, the minimal cross-sectional lumen area and the NASCET percent of lumen narrowing were significantly different between the patient group with carotid infarct and the patient group with no-new carotid infarct. Because of colinearity, only the NASCET percent of lumen narrowing was retained for the multivariate analysis. The other imaging variables retained for the multivariate analysis were wall maximal thickness, fibrous cap thickness, presence of ulcerations, percent lipid in plaque, and percent calcification in plaque. Interestingly, calcium in the carotid wall was larger in patients with stroke on the infarct side compared with the noninfarct side and the patients with no stroke, and represented a risk factor rather than a protective factor. In addition, the computer classifier algorithm identified blood products within the carotid wall, but these were not found to be statistically significant.
Table 2:
Carotid artery wall CT features of the study patients
| Carotid Artery Wall Descriptor | Carotid Infarct Patients |
No New Carotid Infarct Patients |
P Value | ||||
|---|---|---|---|---|---|---|---|
| Infarct Side |
Contralateral Side |
||||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Lumen volume (mm3) | 1519.07 | 255.05 | 1589.81 | 247.86 | 1603.93 | 40.14 | .543 |
| Lumen minimal cross-sectional area (mm2)a | 20.82 | 4.59 | 24.91 | 3.99 | 24.75 | 0.72 | .109 |
| Lumen minimal diameter (mm) | 3.77 | 0.38 | 3.90 | 0.48 | 3.87 | 0.08 | .693 |
| NASCET % lumen stenosis (%)a | 0.29 | 0.07 | 0.26 | 0.10 | 0.21 | 0.02 | .082 |
| Wall volume (mm3) | 1689.72 | 109.08 | 1717.05 | 117.38 | 1623.03 | 20.04 | .353 |
| Wall maximal thickness (mm)a | 5.69 | 1.30 | 4.80 | 1.13 | 3.97 | 0.10 | .000 |
| Fibrous cap thickness (mm)a | 0.49 | 0.14 | 0.49 | 0.20 | 0.30 | 0.03 | .054 |
| Ulcerationsa | 0.43 | 0.27 | 0.43 | 0.27 | 0.27 | 0.03 | .194 |
| Volume of lipids (mm3) | 15.79 | 13.36 | 13.43 | 11.60 | 8.19 | 1.61 | .203 |
| Percent of lipids (compared with the total number of voxels in the carotid wall)a | 0.94 | 0.76 | 0.86 | 0.81 | 0.49 | 0.09 | .197 |
| Number of lipid clusters (20 or more “lipid” voxels adjacent to each other) | 7.29 | 6.61 | 6.29 | 4.03 | 4.51 | 0.62 | .221 |
| Lipid cluster maximal size (mm3)a | 3.11 | 2.44 | 2.30 | 1.36 | 1.36 | 0.17 | .018 |
| Location of the largest lipid cluster (% from wall lumen) | 7.71 | 0.82 | 8.04 | 1.47 | 7.81 | 0.37 | .584 |
| Volume of calcium (mm3)a | 89.57 | 64.17 | 79.37 | 64.28 | 28.67 | 5.74 | .012 |
| Percent of calcium (compared to the total number of voxels in the carotid wall)a | 4.77 | 3.27 | 4.16 | 3.28 | 1.48 | 0.27 | .004 |
| Number of calcium clusters (20 or more “calcium” voxels adjacent to each other)a | 2.50 | 1.49 | 2.79 | 1.87 | 1.37 | 0.18 | .098 |
| Calcium cluster maximal size (mm3)a | 15.69 | 9.30 | 11.53 | 7.74 | 5.62 | 0.80 | .002 |
| Location of the largest calcium cluster (% from wall lumen) | 11.94 | 1.68 | 13.75 | 2.55 | 13.45 | 0.36 | .301 |
Note:—NASCET indicates North American Symptomatic Carotid Endarterectomy Trial.
Indicates features that were different (P < .2) in the patients with new carotid infarcts and in the patients with no new carotid infarct and retained for the multivariate analysis.
Multivariate Statistical Analyses
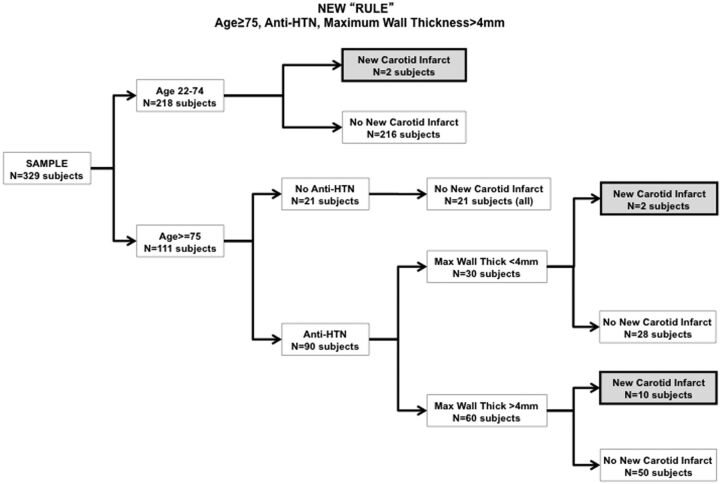
The multivariate analysis assessed the clinical and imaging variables found significant in the univariate analyses, and found the following variables to be associated with P values <.05: age, use of antihypertensive medication, and maximal carotid wall thickness. Of the 14 patients with new stroke, 12 were ≥75 years old. Only 2 of the patients with new carotid stroke were between 22 and 74 years old; both were African American patients with very uncontrolled hypertension and history of heavy drug abuse. All of the older patients were being treated for hypertension. In terms of the maximal carotid wall thickness, different thresholds led to different negative and positive predictive values (Table 3). We retained 4 mm as a threshold because of its optimal negative predictive value.
Table 3:
Number of true and false positive and negative cases, as well as sensitivity, specificity, and negative and positive predictive values using different thresholds of maximal carotid wall thickness
| >4 mm | >5 mm | >6 mm | |
|---|---|---|---|
| True-positive cases | 10 | 7 | 5 |
| False-negative cases | 2 | 5 | 7 |
| True-negative cases | 28 | 39 | 53 |
| False-positive cases | 50 | 39 | 25 |
| Sensitivity | 83.3% | 58.3% | 41.7% |
| Specificity | 35.9% | 50.0% | 67.9% |
| Negative predictive value | 93.3% | 88.6% | 88.3% |
| Positive predictive value | 16.7% | 15.2% | 16.7% |
Our final statistical model is represented as a tree with our patient population in Fig 2. By narrowing the patients based on age ≥75 years, use of antihypertensive medication, and a maximal carotid wall thickness >4 mm, we identified 10 of the 14 patients with new carotid stroke. In terms of ROC analysis, our new “rule” had an area under the ROC curve of 0.706. Interestingly, only 1 of the 14 patients with new carotid stroke had a NASCET stenosis >70%.
Fig 2.
Final statistical model and patient tree distribution in our study population; Anti-HTN indicates anti-hypertensive treatment.
Discussion
In this cohort study of a large sample of patients, we were able to identify 14 patients who developed a new carotid ischemic stroke between the time when they underwent their baseline CTA of the neck and a follow-up imaging study of the brain. We identified 3 risk factors that would predict 10 of the 14 carotid ischemic strokes, including 2 clinical risk factors (age ≥75 years and antihypertensive treatment) and 1 CT feature of carotid atherosclerotic disease—maximal carotid wall thickness >4 mm. This new rule is straightforward to remember and apply clinically.
In our study population, the variables “hypertensive” and “antihypertensive” treatment were collinear, which means that a large amount of patients with a history of hypertension were also taking antihypertensive medications. Our retrospective approach did not allow us to assess the compliance of our patients with respect to their antihypertensive medications, or to assess their blood pressure control. However, it is reasonable to think that compliance and hypertension control were not perfect, and that arterial hypertension is actually the risk factor of importance in our new rule, along with age ≥75 years and maximal carotid wall thickness >4 mm, to predict which patient is going to develop a new carotid ischemic stroke.
In our study, only 1 of the patients who developed a new carotid ischemic stroke had a NASCET stenosis >70%. One of the contributing elements to this observation may be that the large amount of patients diagnosed with a NASCET stenosis >70% on the baseline CTA of the neck likely underwent a prophylactic carotid endarterectomy or stent placement (45 of 375 patients) and were thus excluded from the present study. The justification for this exclusion was that the risk of carotid ischemic stroke after carotid endarterectomy or stent placement is significantly altered and, therefore, no longer representative of the carotid wall CT features before endarterectomy or stent placement.
The originality of our research lies in the use of an imaging technique, CT, that has been demonstrated as accurate compared with conventional angiography in characterizing the degree of carotid luminal stenosis, but that has not historically been used to investigate the carotid wall, except for its calcium content. For the present study, we used an automated classifier computer algorithm to quantify the CT carotid imaging features. However, the simplicity of the most significant imaging feature, maximal carotid wall thickness, likely makes this elaborate approach unnecessary. The importance of this specific imaging feature corresponds well with prior studies that have shown that carotid wall thickness (more specifically intima-media thickness) is an independent predictor of stroke and cardiovascular events, as it is presumably a surrogate measure of atherosclerosis. A simple manual measurement by the observer on the CTA image is likely sufficient to assess the risk of new carotid stroke and can probably be extended to other imaging techniques such as MR imaging or sonography. The advantage of CT is that it is fast and widely available in most hospitals. In addition, CT angiography is often the first study ordered in patients admitted in the emergency department with symptoms suggesting stroke or transient ischemic attack. MR imaging of the carotid plaques requires dedicated coils, is a time-consuming test, and is typically not part of the standard-of-care MR imaging work-up of patients with stroke or transient ischemic attacks. Last, although sonography can be performed at bedside, it has known pitfalls in patients with carotid atherosclerotic disease, and carotid wall measurements are operator-dependent.
Our study has several limitations. Our study population included patients who presented to our emergency department and underwent a baseline CTA of their carotid arteries. This is a selected sample with a probable greater risk of stroke than the general population, which somewhat limits our ability to generalize our results to the entire population. The internal validity of our study should, however, not be affected.
Our classification of patients as “carotid stroke patients” and “noncarotid stroke patients” was based on published criteria43,44 and the Causative Classification System for Ischemic Stroke.42 This classification is probably not perfect, even if it has a reported interexaminer reliability of .90 in characterizing the probable cause of a stroke, presenting the advantage of a very low rate (4%) of indeterminate-unclassified results.42
We assessed 2 imaging time points for each patient (the baseline CTA and a follow-up brain imaging study), which allowed us to evaluate the interval development of any new stroke. Inherent in our study is variability in the time of the follow-up imaging. Some would argue that we did not allow enough time for the patients with noncarotid stroke to develop an infarct. However, our follow-up time was similar across both the patients with new carotid stroke and those with no-new carotid stroke (a mean of 390.2 days to follow-up for the new carotid stroke group and 447.5 days to follow-up for the no-new carotid stroke group), allowing us to conclude that the variability of the follow-up time did not introduce a significant bias in our results. Similarly, the nature of the follow-up imaging was similar in the no-new stroke and new-stroke groups (52.7% and 64.3%, respectively, had a CT at the second time point).
Conclusions
Our study has identified a simple rule that may help stratify stroke risk. Our rule comprises 3 items–age of 75 years or more, presence of hypertension, and a maximal carotid wall thickness of 4 mm or more. It was able to successfully predict 10 of the 14 patients who developed a new carotid ischemic stroke in our retrospective cohort study. These findings warrant validation in a prospective observational study.
Supplementary Material
ABBREVIATIONS:
- CCA
common carotid artery
- ROC
receiver operating characteristic
Footnotes
Disclosures: Rajiv Magge—RELATED: Grant: Doris Duke Clinical Research Fellowship, Comments: Received financial support as clinical research fellow (during medical school; Support for Travel to Meetings for the Study or Other Purposes: Doris Duke Clinical Research Fellowship, Comments: Received travel support as clinical research fellow (during medical school). W. Scott Fischette—RELATED: Grant: UCSF Dean's Research Grant, Comments: Research grant provided by UCSF to medical students for summer research. Max Wintermark—RELATED: Grant: Dana,* NIH,* Doris Duke,* Comments: This research project was conducted with the support of a grant from the Dana Foundation (http://www.dana.org/). Max Wintermark was supported by Grant KL2 RR024130 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and the NIH Roadmap for Medical Research, and by Grant NS045085 from the National Institute of Neurological Disorders and Stroke, at the NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the Dana Foundation, Doris Duke Charitable Foundation, NCRR or NIH; UNRELATED: Grant: GE Healthcare.* (* Money paid to institution)
References
- 1. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1991;325:445–53 [DOI] [PubMed] [Google Scholar]
- 2. Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998;339:1415–25 [DOI] [PubMed] [Google Scholar]
- 3. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998;351:1379–87 [PubMed] [Google Scholar]
- 4. Mayberg MR, Wilson SE, Yatsu F, et al. Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. Veterans Affairs Cooperative Studies Program 309 Trialist Group. JAMA 1991;266:3289–94 [PubMed] [Google Scholar]
- 5. Ebrahim S, Papacosta O, Whincup P, et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke 1999;30:841–50 [DOI] [PubMed] [Google Scholar]
- 6. O'Leary DH, Polak JF, Kronmal RA, et al. Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. The CHS Collaborative Research Group. Stroke 1992;23:1752–60 [DOI] [PubMed] [Google Scholar]
- 7. Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995;273:1421–28 [PubMed] [Google Scholar]
- 8. Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 2000;342:1693–700 [DOI] [PubMed] [Google Scholar]
- 9. Longstreth WT, Jr, Shemanski L, Lefkowitz D, et al. Asymptomatic internal carotid artery stenosis defined by ultrasound and the risk of subsequent stroke in the elderly. The Cardiovascular Health Study. Stroke 1998;29:2371–76 [DOI] [PubMed] [Google Scholar]
- 10. Norris JW, Zhu CZ, Bornstein NM, et al. Vascular risks of asymptomatic carotid stenosis. Stroke 1991;22:1485–90 [DOI] [PubMed] [Google Scholar]
- 11. Wilterdink JL, Easton JD. Vascular event rates in patients with atherosclerotic cerebrovascular disease. Arch Neurol 1992;49:857–63 [DOI] [PubMed] [Google Scholar]
- 12. Bonithon-Kopp C, Scarabin PY, Taquet A, et al. Risk factors for early carotid atherosclerosis in middle-aged French women. Arterioscler Thromb 1991;11:966–72 [DOI] [PubMed] [Google Scholar]
- 13. Bonithon-Kopp C, Touboul PJ, Berr C, et al. Relation of intima-media thickness to atherosclerotic plaques in carotid arteries. The Vascular Aging (EVA) Study. Arterioscler Thromb Vasc Biol 1996;16:310–16 [DOI] [PubMed] [Google Scholar]
- 14. Lorenz MW, von Kegler S, Steinmetz H, et al. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke 2006;37:87–92 [DOI] [PubMed] [Google Scholar]
- 15. O'Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 1999;340:14–22 [DOI] [PubMed] [Google Scholar]
- 16. Zureik M, Touboul PJ, Bonithon-Kopp C, et al. Cross-sectional and 4-year longitudinal associations between brachial pulse pressure and common carotid intima-media thickness in a general population. The EVA study. Stroke 1999;30:550–55 [DOI] [PubMed] [Google Scholar]
- 17. Polak JF, Pencina MJ, Pencina KM, et al. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med 2011;365:213–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Weert TT, Cretier S, Groen HC, et al. Atherosclerotic plaque surface morphology in the carotid bifurcation assessed with multidetector computed tomography angiography. Stroke 2009;40:1334–40 [DOI] [PubMed] [Google Scholar]
- 19. Saba L, Mallarini G. Fissured fibrous cap of vulnerable carotid plaques and symptomaticity: are they correlated? Preliminary results by using multi-detector-row CT angiography. Cerebrovasc Dis 2009;27:322–27 [DOI] [PubMed] [Google Scholar]
- 20. Ballotta E, Da Giau G, Renon L. Carotid plaque gross morphology and clinical presentation: a prospective study of 457 carotid artery specimens. J Surg Res 2000;89:78–84 [DOI] [PubMed] [Google Scholar]
- 21. Rothwell PM, Gibson R, Warlow CP. Interrelation between plaque surface morphology and degree of stenosis on carotid angiograms and the risk of ischemic stroke in patients with symptomatic carotid stenosis. On behalf of the European Carotid Surgery Trialists' Collaborative Group. Stroke 2000;31:615–21 [DOI] [PubMed] [Google Scholar]
- 22. Prabhakaran S, Rundek T, Ramas R, et al. Carotid plaque surface irregularity predicts ischemic stroke: the northern Manhattan study. Stroke 2006;37:2696–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wintermark M, Jawadi SS, Rapp JH, et al. High-resolution CT imaging of carotid artery atherosclerotic plaques. AJNR Am J Neuroradiol 2008;29:875–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Troyer A, Saloner D, Pan XM, et al. Major carotid plaque surface irregularities correlate with neurologic symptoms. J Vasc Surg 2002;35:741–47 [DOI] [PubMed] [Google Scholar]
- 25. Bassiouny HS, Sakaguchi Y, Mikucki SA, et al. Juxtalumenal location of plaque necrosis and neoformation in symptomatic carotid stenosis. J Vasc Surg 1997;26:585–94 [DOI] [PubMed] [Google Scholar]
- 26. Biasi GM, Froio A, Diethrich EB, et al. Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation 2004;110:756–62 [DOI] [PubMed] [Google Scholar]
- 27. McKinney AM, Casey SO, Teksam M, et al. Carotid bifurcation calcium and correlation with percent stenosis of the internal carotid artery on CT angiography. Neuroradiology 2005;47:1–9 [DOI] [PubMed] [Google Scholar]
- 28. Nandalur KR, Hardie AD, Raghavan P, et al. Composition of the stable carotid plaque: insights from a multidetector computed tomography study of plaque volume. Stroke 2007;38:935–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Worthley SG, Helft G, Fuster V, et al. Serial in vivo MRI documents arterial remodeling in experimental atherosclerosis. Circulation 2000;101:586–89 [DOI] [PubMed] [Google Scholar]
- 30. Adame IM, van der Geest RJ, Wasserman BA, et al. Automatic segmentation and plaque characterization in atherosclerotic carotid artery MR images. MAGMA 2004;16:227–34 [DOI] [PubMed] [Google Scholar]
- 31. Clarke SE, Hammond RR, Mitchell JR, et al. Quantitative assessment of carotid plaque composition using multicontrast MRI and registered histology. Magn Reson Med 2003;50:1199–208 [DOI] [PubMed] [Google Scholar]
- 32. Coombs BD, Rapp JH, Ursell PC, et al. Structure of plaque at carotid bifurcation: high-resolution MRI with histological correlation. Stroke 2001;32:2516–21 [DOI] [PubMed] [Google Scholar]
- 33. Shinnar M, Fallon JT, Wehrli S, et al. The diagnostic accuracy of ex vivo MRI for human atherosclerotic plaque characterization. Arterioscler Thromb Vasc Biol 1999;19:2756–61 [DOI] [PubMed] [Google Scholar]
- 34. Yuan C, Mitsumori LM, Ferguson MS, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation 2001;104:2051–56 [DOI] [PubMed] [Google Scholar]
- 35. Saam T, Hatsukami TS, Takaya N, et al. The vulnerable, or high-risk, atherosclerotic plaque: noninvasive MR imaging for characterization and assessment. Radiology 2007;244:64–77 [DOI] [PubMed] [Google Scholar]
- 36. Moody AR, Murphy RE, Morgan PS, et al. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation 2003;107:3047–52 [DOI] [PubMed] [Google Scholar]
- 37. Yuan C, Kerwin WS, Ferguson MS, et al. Contrast-enhanced high resolution MRI for atherosclerotic carotid artery tissue characterization. J Magn Reson Imaging 2002;15:62–67 [DOI] [PubMed] [Google Scholar]
- 38. Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI–initial results. Stroke 2006;37:818–23 [DOI] [PubMed] [Google Scholar]
- 39. Wintermark M, Arora S, Tong E, et al. Carotid plaque computed tomography imaging in stroke and nonstroke patients. Ann Neurol 2008;64:149–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wintermark M, Jawadi SS, Rapp JH, et al. High-resolution CT imaging of carotid artery atherosclerotic plaques. AJNR Am J Neuroradiol 2008;29:875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arora S, Chien JD, Cheng SC, et al. Optimal carotid artery coverage for carotid plaque CT—imaging in predicting ischemic stroke. J Neuroradiol 2010;37:98–103 [DOI] [PubMed] [Google Scholar]
- 42. Ay H, Furie KL, Singhal A, et al. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol 2005;58:688–97 [DOI] [PubMed] [Google Scholar]
- 43. Kang DW, Chalela JA, Ezzeddine MA, et al. Association of ischemic lesion patterns on early diffusion-weighted imaging with TOAST stroke subtypes. Arch Neurol 2003;60:1730–34 [DOI] [PubMed] [Google Scholar]
- 44. Rovira A, Grive E, Alvarez-Sabin J. Distribution territories and causative mechanisms of ischemic stroke. Eur Radiol 2005;15:416–26 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.