SUMMARY:
18F-FDG-PET has been widely used in patients with brain tumors. However, the reported sensitivity and specificity of 18F-FDG-PET for brain tumor differentiation varied greatly. We performed this meta-analysis to systematically assess the diagnostic performance of 18F-FDG-PET in differentiating brain tumors. The diagnostic performance of 11C-methionine PET was assessed for comparison. Relevant studies were searched in PubMed/MEDLINE, Scopus, and China National Knowledge Infrastructure (until February 2013). The methodologic quality of eligible studies was evaluated, and a meta-analysis was performed to obtain the combined diagnostic performance of 18F-FDG and 11C-methionine PET with a bivariate model. Thirty eligible studies, including 5 studies with both 18F-FDG and 11C-methionine PET data were enrolled. Pooled sensitivity, pooled specificity, and area under the receiver operating characteristic curve of 18F-FDG-PET (n = 24) for differentiating brain tumors were 0.71 (95% CI, 0.63–0.78), 0.77 (95% CI, 0.67–0.85), and 0.80. Heterogeneity was found among 18F-FDG studies. Subsequent subgroup analysis revealed that the disease status was a statistically significant source of the heterogeneity and that the sensitivity in the patients with recurrent brain tumor was markedly higher than those with suspected primary brain tumors. Pooled sensitivity, pooled specificity, and area under the receiver operating characteristic of 11C-methionine PET (n = 11) were 0.91 (95% CI, 0.85–0.94), 0.86 (95% CI, 0.78–0.92), and 0.94. No significant statistical heterogeneity was found among 11C-methionine studies. This meta-analysis suggested that 18F-FDG-PET has limited diagnostic performance in brain tumor differentiation, though its performance may vary according to the status of brain tumor, whereas 11C-methionine PET has excellent diagnostic accuracy in brain tumor differentiation.
18F-FDG-PET has been widely used in brain tumor differentiation. Commonly the glucose use in malignant brain tumors is increased, and 18F-FDG-PET performs well in identifying high-grade gliomas.1 However, because of the high physiologic glucose metabolism in normal brain tissue, the diagnostic accuracy of 18F-FDG-PET in brain tumors is reduced, especially in low-grade brain tumors, which typically have lower levels of glucose metabolism.2,3 Moreover, 18F-FDG uptake in brain tumors demonstrates great variety and might not be closely associated with the malignant grade,3 making 18F-FDG-PET unreliable in differentiating brain tumors.4
A number of 18F-FDG-PET studies for differentiating brain tumors, including suspected primary brain tumor (SPBT) and/or suspected recurrence of brain tumors after treatment (SRBT), have shown a wide range of sensitivity and specificity, from 0.25 to 1.00 and 0.22 to 1.00, respectively.1,2,5–27 This great disparity of diagnostic values causes confusion on the application of 18F-FDG-PET for brain tumor differentiation. Therefore, although 18F-FDG-PET remains the dominant approach for brain tumor imaging, a systematic assessment of the diagnostic performance of 18F-FDG-PET for brain tumor differentiation is imperative.
In view of the limitation of 18F-FDG, amino acid and amino acid analog PET tracers have been developed. PET imaging with these tracers improved the ability to differentiate brain tumors due to high tumor uptake and low uptake in normal brain tissue.28 Among these tracers, 11C-methionine (MET) is one of the most extensively investigated. 11C-MET accumulates extensively in proliferating tumors by the mechanism of increased amino acid transport and protein synthesis. Several reports confirmed that 11C-MET PET differentiated brain tumors with high sensitivity and specificity.29,30
A recent meta-analysis concluded that 18F-FDG and 11C-MET PET have moderately good overall accuracy for diagnosing recurrent glioma.31 However, other types of brain tumors with high clinical incidence, such as SPBTs and nongliomas, have not been well studied so far. A thorough understanding of the diagnostic effectiveness of 18F-FDG and 11C-MET PET for differentiating brain tumors could be highly referential in clinical practice. Therefore, we performed a meta-analysis to comprehensively investigate the diagnostic performance of 18F-FDG and 11C-MET PET for differentiating brain tumors with various statuses and histologic types.
Materials and Methods
Identification and Eligibility of Studies
A systematic search was performed in MEDLINE, Scopus, and the China National Knowledge Infrastructure data bases from January 1991 to February 2013, restricted to human studies in English and Chinese. The detailed search strategies are presented in the On-line Appendix. To search for more potential studies, we also screened references of the retrieved studies.
Studies using 18F-FDG or/and 11C-MET for the assessment of SPBT or SRBT were enrolled. Inclusion criteria were the following: 1) the purpose of the study was to differentiate SPBT or SRBT, 2) the study population consisted of a minimum of 10 patients, 3) histology or clinical follow-up was used as a reference standard, and 4) the reported primary data were sufficient to calculate both sensitivity and specificity. Exclusion criteria were the following: 1) the PET tracer not being 18F-FDG or 11C-MET; 2) animal or in vitro studies; 3) abstracts, systematic reviews, editorials, letters, comments, and case reports; and 4) studies for staging, searching for metastasis, and evaluating the therapeutic response of definitely diagnosed brain tumors.
Data Extraction and Study Quality
Two experienced nuclear medicine physicians (C.Z., Y.Z.) screened titles, abstracts, and full texts of eligible studies independently. Characteristics of eligible studies were extracted to 2 predefined forms, including the first author's name, year of publication, study country of origin, study design, sample size, mean or median age of patients, male to female ratio, type of brain tumor, disease status, PET tracers, prior imaging tests, reference standard, clinical or radiologic follow-up after PET, PET scanner type, injected dose, time of scanning after injection, scan time, analysis method for diagnostic performance, positive criteria of visual assessment, and cutoff value of quantitative parameters. After data extraction, discrepancies were resolved by consensus and discussion.
The methodologic quality of eligible studies was assessed by using 14 items of the Quality Assessment of Diagnostic Accuracy Studies.32 Each item of the Quality Assessment of Diagnostic Accuracy Studies was described as yes (score = 2), unclear (score = 1), and no (score = 0). The total score was summarized from all the items with a range of 0–28. Two experienced reviewers (C.Z., Y.Z.) independently evaluated the quality of selected studies, and disagreements were resolved by discussion.
True-positive, false-positive, true-negative, and false-negative values for 18F-FDG or 11C-MET PET in differentiating brain tumors were extracted for each eligible study to construct a contingency table.
Data Analysis
Pooled estimates for sensitivity, specificity, likelihood ratios (LRs) with corresponding 95% confidence intervals, and area under receiver operating characteristic curve (AUC) for 18F-FDG and 11C-MET PET were analyzed for the primary meta-analysis on the basis of the bivariate mixed-effects regression model.33 The bivariate model uses a random effects approach for both sensitivity and specificity, which allows heterogeneity beyond chance as a result of clinical and methodologic differences between studies, and the bivariate model is a more valid statistical model for a diagnostic meta-analysis.34 The LRs indicate by how much a given test would raise or lower the probability of having a disease. Generally, a good diagnostic test may have LR+ above 5 and LR− below 0.2. The AUC is the average true-positive rate over the entire range of false-positive rate values and serves as a global measure of test performance. The guidelines for the interpretation of intermediate AUC values are the following: low accuracy, 0.5 ≤ AUC ≤ 0.7; moderate accuracy, 0.7 < AUC ≤ 0.9; or high accuracy, 0.9 < AUC ≤ 1.35
To graphically describe the results, we plotted the hierarchic summary receiver operating characteristic (HSROC) curves. The HSROC curve is a recommended standard method for diagnostic meta-analysis.36 Heterogeneity among the studies was checked by using the χ2-based Q-test and the I2 statistic.37,38 The existence of significant heterogeneity was assumed with a P value < .05 for the Q-test and/or an I2 statistic ≥ 50%. If significant heterogeneity was observed, subgroup analysis by using meta-regression was adopted to explore a potential source of heterogeneity by calculating the I2 statistics. The covariates investigated included study design, imaging method, analysis method for diagnostic performance, malignant grade of brain tumor, disease status, and histology. The malignant grades of brain tumors in the studies were assigned according to the classification of World Health Organization.39 The stability of our analysis model was tested by 1-way sensitivity analysis if heterogeneity existed. We excluded each study in turn and checked how the new summary diagnostic values could be influenced by the removed one.
We also performed direct comparison of the diagnostic values of 18F-FDG and 11C-MET PET from the 5 studies with the same population of the patients22–26 to diminish the potential bias induced by pooling data from all the studies, though the data subset was smaller.
Publication bias was tested by using the linear regression method and funnel plot of Deeks et al.40 A P value < .05 in this linear regression indicates potential publication bias.
Statistical analyses were performed with STATA 12.0 (StataCorp, College Station, Texas). The commands used are presented in the On-line Appendix. A P value < .05 was considered statistically significant. All P values were 2-sided.
Results
Study Characteristics, Quality, and Publication Bias
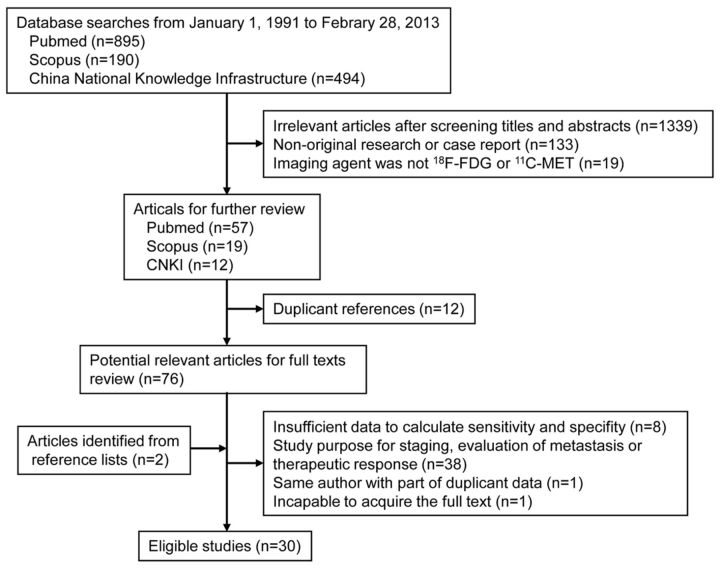
According to the search strategies, the electronic search yielded 1579 articles: 895 from PubMed, 190 from Scopus, and 494 from the China National Knowledge Infrastructure. After we screened article types, titles, and abstracts, 76 studies remained and the full-text versions were reviewed. After we reviewed full texts, 48 studies were excluded and 2 studies identified from the reference lists of other eligible studies were included (Fig 1). Finally 30 eligible studies were enrolled, including 19 for 18F-FDG-PET,1,2,5–21 6 for 11C-MET PET,29,30,41–44 and 5 for both.22–26 The characteristics of the studies are summarized in On-line Tables 1 and 2.
Fig 1.
Flow chart of identification of eligible studies.
The quality of included studies was assessed on the basis of the Quality Assessment of Diagnostic Accuracy Studies (On-line Table 3). The overall quality of the included studies was considered acceptable for most of the items. The total score varied from 13 to 24 in 18F-FDG studies and from 18 to 22 in 11C-MET studies. The proportion of studies with a total score of >20 in 18F-FDG studies (11/24) was apparently lower than that in 11C-MET studies (9/11); this difference indicated the overall higher quality of 11C-MET studies. A common poor-quality item (item 6) in most studies was the failure to use the same reference standard.
We found no significant evidence of publication bias in both 18F-FDG (P = .07, On-line Fig 1) and 11C-MET (P = .26, On-line Fig 2) studies by using the linear regression method of Deeks et al.40
Heterogeneity of 18F-FDG-PET Studies and Sensitivity Analysis
The sensitivity and specificity of 18F-FDG-PET for brain tumor differentiating across 24 eligible studies ranged from 0.25 to 1.00 and 0.22 to 1.00, respectively. The test of heterogeneity revealed significant statistical heterogeneity (Q-value for sensitivity = 83.23, P = .00, I2 = 72.37%; Q-value for specificity = 57.11, P = .00, I2 = 59.73%).
We excluded 1 study from the overall pooled analysis each time to check the influence of the removed dataset on the summary estimates. When a single study was excluded, the new pooled sensitivity and specificity remained close to those obtained with all eligible studies (On-line Table 4).
Diagnostic Values and HSROC Curve of 18F-FDG-PET Studies
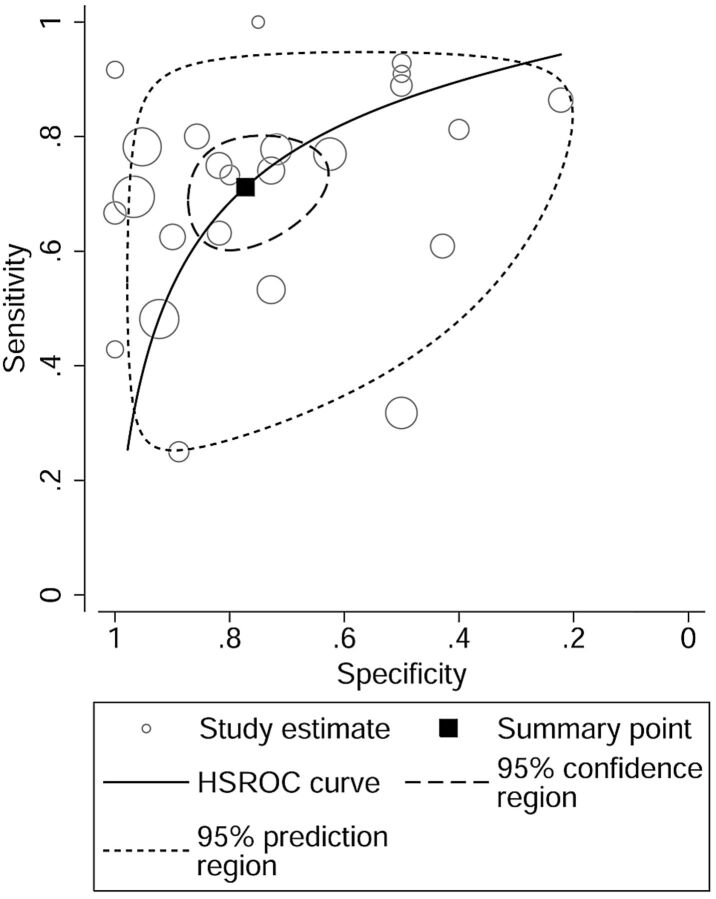
When all twenty-four 18F-FDG studies were pooled, the sensitivity, specificity, and AUC for differentiating brain tumors were 0.71 (95% CI, 0.63–0.78), 0.77 (95% CI, 0.67–0.85), and 0.8. The overall LR+ and LR− were 3.13 (95% CI, 2.11–4.64) and 0.37 (95% CI, 0.29–0.48). The HSROC curve is shown in Fig 2.
Fig 2.
HSROC curve of 18F-FDG-PET for differentiating brain tumors.
In 5 studies with the same population of the patients for both 18F-FDG and 11C-MET PET, the pooled sensitivity, pooled specificity, and AUC for 18F-FDG-PET were 0.70 (95% CI, 0.50–0.85), 0.78 (95% CI, 0.59–0.90), and 0.81. The overall LR+ and LR− of 18F-FDG-PET were 3.17 (95% CI, 1.73–5.82) and 0.38 (95% CI, 0.22–0.65).
Heterogeneity of 11C-MET PET Studies
The sensitivity and specificity of 11C-MET PET for brain tumor differentiation across 11 eligible studies ranged from 0.75 to 1.00 and 0.6 to 1.00, respectively. The test of heterogeneity indicated no significant statistical heterogeneity (Q-value for sensitivity = 12.81, P = .23, I2 = 21.92%; Q-value for specificity = 10.98, P = .36, I2 = 8.89%).
Diagnostic Values and HSROC Curve of 11C-MET PET Studies
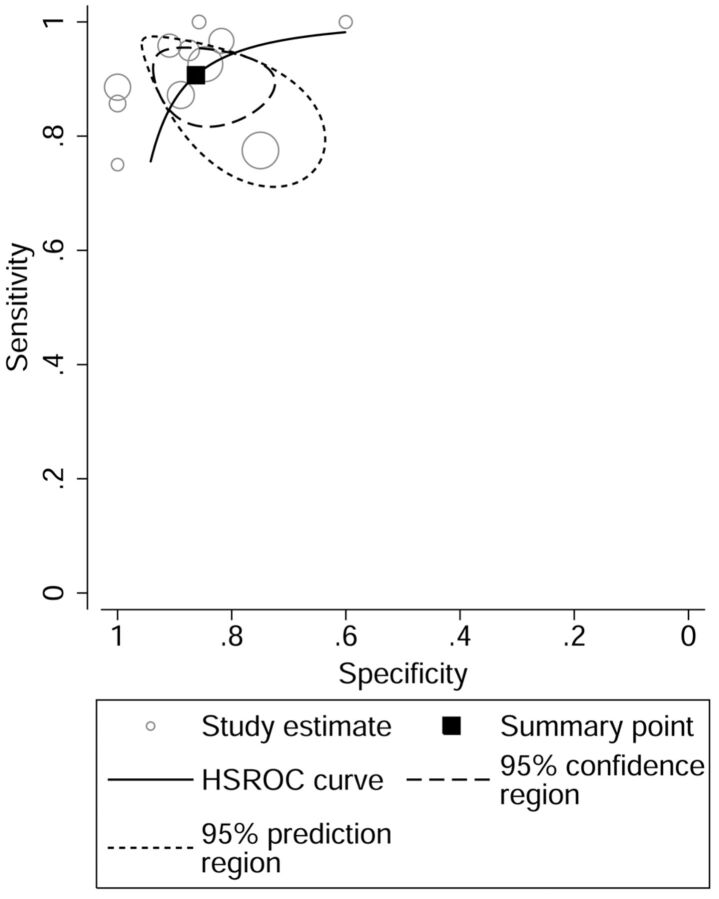
When all eleven 11C-MET studies were pooled, the sensitivity, specificity, and AUC for differentiating brain tumors were 0.91 (95% CI, 0.85–0.94), 0.86 (95% CI, 0.78–0.92), and 0.94. The overall LR+ and LR− were 6.60 (95% CI, 3.93–11.07) and 0.11 (95% CI, 0.07–0.18). The HSROC curve is shown in Fig 3.
Fig 3.
HSROC curve of 11C-MET PET for differentiating brain tumors.
In 5 studies with the same population of the patients for both 18F-FDG and 11C-MET PET, the pooled sensitivity, pooled specificity, and AUC for 11C-MET PET were 0.94 (95% CI, 0.88–0.97), 0.87 (95% CI, 0.76–0.93), and 0.96. The overall LR+ and LR− of 11C-MET PET were 7.28 (95% CI, 3.81–13.92) and 0.07 (95% CI, 0.04–0.14).
Subgroup Analyses of 18F-FDG and 11C-MET PET Studies
Metaregression was performed for 18F-FDG-PET studies to explore the potential source of heterogeneity. The results of subgroup meta-analyses are shown in Table 1. The source of the heterogeneity among 18F-FDG-PET studies was not observed with respect to study design, imaging method, malignant grade of brain tumor, and histology (P > .05). However, the disease status had a statistically significant influence on the heterogeneity (I2 = 77.72; P = .01). The sensitivity in SPBT (0.43; 95% CI, 0.28–0.59) was markedly lower than that in SRBT (0.75; 95% CI, 0.67–0.81).
Table 1:
Metaregression analyses and diagnostic performance of 18F-FDG-PET in subgroups of brain tumors
| Analysis | No. of Studies (pts) | I2 of Metaregression (95% CI, P Value) | Independent Estimates (95% CI) |
LR (95% CI) |
||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | LR+ | LR− | |||
| Design | ||||||
| Retrospective | 9 (333) | 0.00 (0–100, .86) | 0.71 (0.58–0.81) | 0.73 (0.57–0.85) | 2.63 (1.68–4.13) | 0.40 (0.29–0.55) |
| Prospective | 15 (524) | 0.71 (0.60–0.79) | 0.80 (0.66–0.89) | 3.45 (1.96–6.07) | 0.37 (0.26–0.52) | |
| PET or PET/CT | ||||||
| PET | 17 (511) | 0.00 (0–100, .38) | 0.74 (0.64–0.81) | 0.74 (0.60–0.84) | 2.82 (1.74–4.55) | 0.36 (0.25–0.51) |
| PET/CT | 7 (346) | 0.65 (0.50–0.78) | 0.84 (0.68–0.92) | 3.97 (2.09–7.54) | 0.42 (0.29–0.59) | |
| PET measurement | ||||||
| Qualitative | 19 (757) | 57.87 (4.96–100, .09) | 0.69 (0.60–0.76) | 0.79 (0.67–0.87) | 3.24 (2.00–5.24) | 0.40 (0.30–0.52) |
| Quantitative | 4 (85) | 0.86 (0.71–0.94) | 0.67 (0.43–0.84) | 2.59 (1.40–4.81) | 0.21 (0.09–0.45) | |
| Malignant grade | ||||||
| Low | 14 (330)a | 0.00 (0–100, .46) | 0.60 (0.35–0.81) | 0.79 (0.64–0.89) | 2.87 (1.52–5.41) | 0.50 (0.27–0.92) |
| High | 17 (473)a | 0.74 (0.68–0.80) | 0.78 (0.65–0.87) | 3.35 (2.09–5.38) | 0.33 (0.26–0.42) | |
| NS | 7 (192) | 0.70 (0.50–0.84) | 0.70 (0.54–0.82) | 2.33 (1.47–3.67) | 0.44 (0.26–0.74) | |
| Disease status | ||||||
| SPBT | 3 (127)b | 77.72 (51.54–100, .01) | 0.43 (0.28–0.59) | 0.74 (0.49–0.90) | 1.67 (0.59–4.76) | 0.77 (0.48–1.24) |
| SRBT | 20 (643)b | 0.75 (0.67–0.81) | 0.79 (0.66–0.88) | 3.51 (2.17–5.66) | 0.32 (0.25–0.41) | |
| Histology | ||||||
| Glioma | 17 (609)c,d | 0.00 (0–100, .47) | 0.75 (0.64–0.83) | 0.78 (0.64–0.87) | 3.36 (2.02–5.59) | 0.33 (0.23–0.47) |
| Nonglioma or NS | 8 (250)c | 0.64 (0.52–0.75) | 0.75 (0.63–0.84) | 2.58 (1.69–3.94) | 0.48 (0.35–0.65) | |
Metaregression was not performed for 11C-MET PET studies because no statistically significant heterogeneity was found. However, the sensitivity, specificity, and LRs in subgroups by study design, PET measurement, imaging method, malignant grade, disease status, and histologic finding were also calculated and are listed in Table 2. No apparent difference was observed among the subgroups.
Table 2:
Diagnostic performance of 11C-MET PET in subgroups of brain tumors
| Analysis | No. of Studies (pts) | Independent Estimates (95% CI) |
LR (95% CI) |
||
|---|---|---|---|---|---|
| Sensitivity | Specificity | LR+ | LR− | ||
| Design | |||||
| Prospective | 4 (156) | 0.91 (0.84–0.95) | 0.92 (0.78–0.97) | 11.49 (3.87–34.08) | 0.10 (0.06–0.18) |
| Retrospective | 7 (260) | 0.91 (0.81–0.96) | 0.84 (0.73–0.90) | 5.50 (3.18–9.51) | 0.11 (0.05–0.24) |
| PET measurement | |||||
| Qualitative | 5 (189) | 0.93 (0.87–0.96) | 0.85 (0.73–0.92) | 6.10 (3.34–11.15) | 0.08 (0.04–0.16) |
| Quantitative | 6 (227) | 0.89 (0.79–0.94) | 0.89 (0.74–0.96) | 8.38 (3.07–22.88) | 0.13 (0.07–0.25) |
| PET or PET/CT | |||||
| PET | 7 (232) | 0.86 (0.78–0.91) | 0.89 (0.72–0.96) | 7.96 (2.75–23.00) | 0.16 (0.10–0.26) |
| PET/CT | 4 (184) | 0.95 (0.89–0.97) | 0.86 (0.74–0.93) | 6.62 (3.48–12.59) | 0.06 (0.03–0.13) |
| Malignant grade | |||||
| Low | 5 (97)a | 0.90 (0.76–0.96) | 0.88 (0.77–0.94) | 7.44 (3.68–15.02) | 0.12 (0.05–0.30) |
| High | 7 (132)a | 0.98 (0.75–1.00) | 0.88 (0.75–0.95) | 8.51 (3.78–19.17) | 0.02 (0.00–0.34) |
| NS | 4 (153) | 0.87 (0.79–0.93) | 0.87 (0.69–0.96) | 6.93 (2.52–19.04) | 0.15 (0.08–0.26) |
| Disease status | |||||
| SPBT | 2 (85) | 0.95 (0.85–0.98) | 0.83 (0.65–0.93) | 5.49 (2.47–12.21) | 0.07 (0.02–0.20) |
| SRBT | 8 (238) | 0.92 (0.83–0.97) | 0.87 (0.75–0.93) | 6.81 (3.39–13.69) | 0.09 (0.04–0.21) |
| NS | 2 (93) | 0.88 (0.78–0.94) | 0.95 (0.71–0.99) | 16.69 (2.47–112.66) | 0.13 (0.07–0.24) |
| Histology | |||||
| Glioma | 9 (292)b | 0.92 (0.85–0.95) | 0.87 (0.76–0.93) | 7.01 (3.67–13.38) | 0.10 (0.05–0.18) |
| Nonglioma and NS | 4 (182)b | 0.88 (0.79–0.93) | 0.83 (0.66–0.92) | 5.07 (2.39–10.79) | 0.14 (0.08–0.28) |
Discussion
We analyzed 24 18F-FDG-PET studies with an accumulated population of 857 patients for differentiating brain tumors, including SPBT and/or SRBT. The meta-analysis showed that 18F-FDG-PET has moderately good pooled sensitivity (0.71; 95% CI, 0.63–0.78) and specificity (0.77; 95% CI, 0.67–0.85) for differentiating brain tumors. In the assessment of intracranial masses and the recurrence of brain tumors with 18F-FDG-PET, a positive 18F-FDG lesion without the presence of tumor (false-positive) often indicates inflammatory tissue or other nontumor tissues and thus limits the specificity.3,45 On the other hand, absent or decreased 18F-FDG uptake in pathologically identified brain tumors (false-negative) reflects the lower levels of glucose metabolism and is usually highly influenced by the high physiologic glucose metabolism in surrounding normal brain tissue, leading to a decrease of sensitivity.11 The relatively low pooled sensitivity and specificity of 18F-FDG-PET for differentiating brain tumors demonstrated in our meta-analysis indicates a considerably high incidence of both false-positives and false-negatives.
In the subgroup analyses of 18F-FDG studies, the disease status was identified as the only possible source of heterogeneity. We found that the sensitivity of 18F-FDG-PET was the worst (0.43; 95% CI, 0.3–0.58) when applied to the patients with SPBT. However, because no study on only SPBT was found, the SPBT data were extracted from 3 eligible studies on brain tumors with various statuses. As a result, the number of patients with SPBTs in subgroup analysis was limited, and the reliability of the subgroup analysis might be impaired to some extent. In subgroup analysis by malignant grade, low-grade brain tumors showed slightly less sensitivity (0.60; 95% CI, 0.35–0.81) compared with high-grade ones (0.74; 95% CI, 0.68–0.80). These results were consistent with those in previous reports by other investigators indicating that 18F-FDG-PET was less effective in low-grade brain tumors,2,29 though this difference was not a statistically significant source of heterogeneity (P = .46) in our analysis. In subgroup analysis by histology, the patients with glioma showed sensitivity and specificity similar to that of pooled data, suggesting that the type of brain tumor has no apparent influence on the diagnostic performance of 18F-FDG-PET in brain tumor differentiation, regardless of the grading of glioma.
Qualitative assessment was used for image interpretation in most of the eligible 18F-FDG studies, but there were various criteria for visual assessment as shown in On-line Table 2. These different criteria for visual assessment in 18F-FDG studies may inevitably bring bias to our pooled data. Moreover, Tripathi et al24 reported that even within the same 18F-FDG study, interobserver agreement for visual interpretation was not good. The difficulty in discriminating the lesion and surrounding normal brain tissue in some patients and the subjectivity of visual assessment of the interpreter may together contribute to the low diagnostic accuracy and require us to explain the pooled results prudently when using qualitative evaluation in 18F-FDG-PET. Quantitative assessments were adopted in four 18F-FDG-PET studies20,22,24,26 that used a lesion-to–normal tissue ratio or standard uptake value as the criterion and showed better sensitivity but worse specificity than qualitative assessment. However, this difference was not a statistically significant source of heterogeneity (P = .09) in our analysis. The setting of the cutoff value in quantitative assessment may greatly influence the results of diagnostic estimates and consequently affect the reliability of direct comparison of the diagnostic values by quantitative and qualitative assessment. The limited number of patients analyzed with quantitative methods may also bring bias to the subgroup analysis by the method of assessment. In addition, due to the lack of the ability to distinguish lesion and normal brain tissue in 18F-FDG-PET, the veracity of quantitative or semiquantitative methods for the interpretation of 18F-FDG-PET images for evaluating brain tumor was also unreliable and could not provide additional information compared with visual assessment.1,10,27 On the basis of the results of our meta-analysis, 18F-FDG-PET does not appear to be an ideal approach for differentiating brain tumors, and we do not recommend its routine use for this purpose because a rather large number of diseases would be missed.
On the other hand, the meta-analysis in 11 11C-MET PET studies demonstrated excellent pooled sensitivity (0.91; 95% CI, 0.85–0.94) and specificity (0.86; 95% CI, 0.78–0.92) for differentiating brain tumors. The overall high diagnostic accuracy of 11C-MET PET (AUC = 0.94) over 18F-FDG-PET (AUC = 0.80) for brain tumor differentiation is likely due to the high uptake in tumor and low accumulation in normal brain tissue.25,41 The sensitivity and specificity of 11C-MET PET for differentiating brain tumors in the subgroups by various conditions showed higher values than those in pooled and subgroup 18F-FDG-PET analyses, indicating the superiority of 11C-MET PET over 18F-FDG-PET and the stability of the diagnostic effectiveness of 11C-MET PET in patients with various tumor types. This result was further verified by the direct comparison of the diagnostic values of 18F-FDG and 11C-MET PET from the 5 studies.22–26 After removing the potential bias caused by pooling all the data, the overall diagnostic accuracy of 11C-MET PET (AUC = 0.96) in the pooled data of the 5 studies was much higher than that of 18F-FDG-PET (AUC = 0.81). Although there were 4 eligible 11C-MET PET studies that included patients with different types of brain tumors,23,29,41,42 no heterogeneity was observed. Therefore, the results of meta-analysis in 11C-MET PET studies are more convincing, though there were fewer patients in 11C-MET PET studies than in 18F-FDG-PET studies. On the basis of our meta-analysis, it is always preferable to use 11C-MET PET instead of 18F-FDG-PET for differentiating brain tumors if possible.
However, although 11C-MET PET appears to be a promising tracer for brain tumor differentiation, the use of 11C-MET PET is restricted to the PET centers with a cyclotron due to the short half-life of 11C (20 minutes) and the rapid catabolism of 11C-MET.25 As substitutes for 11C-MET and 18F-FDG, 18F (half-life = 110 minutes) labeled PET tracers such as O-(2-[18F]fluoroethyl)-L-tyrosine (18F-FET),8 3,4-dihydroxy-6-18F-fluoro-L-phenylalanine (18F-FDOPA),46 and 3′-deoxy-3′-18F-fluorothymidine (18F-FLT)18,20 have been developed and applied in brain tumor imaging. Among these 18F-labeled tracers, 18F-FET and 18F-FDOPA show superiority over 18F-FDG in brain tumor differentiation, especially in low-grade brain tumors,1,47 on the basis of the advantage of their high uptake in tumor tissue and low uptake in normal brain tissue. A meta-analysis with 18F-FET PET studies in patients with SPBT has demonstrated the high performance of 18F-FET PET with a pooled sensitivity and specificity of 0.82 and 0.76, respectively.48 However, these values were not as good as our results for 11C-MET PET. As for the 18F-FLT PET, Choi et al7 reported that although 18F-FLT PET is useful for evaluating tumor grade and cellular proliferation in brain tumors, it is not useful enough for differentiating tumors from nontumorous lesions. Because the PET studies using 18F-FDOPA for brain tumor differentiation are still insufficient, further systematic evaluation using this tracer is expected in the future.
The recent meta-analysis by Nihashi et al31 on the diagnostic accuracy of PET for recurrent glioma reported that 18F-FDG-PET had moderately good sensitivity and specificity in either pooled glioma with different grades (sensitivity = 0.77, specificity = 0.78) or high-grade glioma (sensitivity = 0.79, specificity = 0.70). The subgroup analysis in our study showed similar sensitivity and specificity of 18F-FDG-PET in the glioma group. However, Nihashi et al reported a pooled sensitivity as low as 0.7 for 11C-MET PET for high-grade gliomas, which is considerably different from our results. We believe our results are more reliable because we enrolled more eligible studies with more abundant data and investigated more brain tumor types.
However, a few limitations should be addressed in this study. First, although the overall sample size was as much as 857 in 18F-FDG-PET and 416 in 11C-MET PET settings, there was still incomplete data collection as reflected by the failure to access the full text of an eligible study.49 In addition, the insufficient data in certain subtypes of brain tumors may impair the reliability of the analysis. We searched potential sources for heterogeneity in 18F-FDG-PET studies and found that disease status was a significant source. Although the use of the bivariate model could at least correct this issue to some extent, the results should be interpreted cautiously. Second, methodologic quality might be a source of heterogeneity in 18F-FDG-PET studies. We found that some included studies had low methodologic quality with few Y scores (≤6 items) in the Quality Assessment of Diagnostic Accuracy Studies.5,10,13 In addition, a number of eligible studies included patients with various types of brain tumors.1,7,9,10,14,16,17,23 Because the characteristics of different brain tumors may vary greatly (eg, brain lymphoma usually presents with higher 18F-FDG uptake than normal brain tissue),50 the composition of different brain tumors might bring bias by a nonrepresentative patient spectrum in 18F-FDG-PET studies. Third, some characteristics of the eligible studies were not in good agreement. For example, the starting time of PET imaging after tracer injection differed widely, especially in the 18F-FDG-PET studies. The differences of study characteristics may also contribute to the heterogeneity of our meta-analysis.
Conclusions
Our meta-analysis shows that 18F-FDG-PET has limited diagnostic performance in brain tumor differentiation. However, 11C-MET PET has excellent diagnostic performance in brain tumor differentiation and should be considered as a preferential approach for this purpose if available. Due to the inconvenience of the supply of 11C, other 18F-labeled tracers such as 18F-FDOPA could be considered as potential alternatives for brain tumor differentiation and deserve future systematic evaluation after accumulating relevant studies in the future.
Supplementary Material
Acknowledgments
We thank Jing Guo (Department of Epidemiology and Health Statistics, School of Public Health, Zhejiang University) for statistical support.
ABBREVIATIONS:
- AUC
area under receiver operating characteristic curve
- 18F-FDOPA
3,4-dihydroxy-6-18F-fluoro-L-phenylalanine
- 18F-FET
O-(2-[18F]fluoroethyl)-L-tyrosine
- 18F-FLT
3′-deoxy-3′-18F-fluorothymidine
- HSROC
hierarchic summary receiver operating characteristics
- LR
likelihood ratio
- MET
methionine
- SPBT
suspected primary brain tumor
- SRBT
suspected recurrence of brain tumor after treatment
References
- 1. Chen W, Silverman DH, Delaloye S, et al. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG-PET and evaluation of diagnostic accuracy. J Nucl Med 2006;47:904–11 [PubMed] [Google Scholar]
- 2. Ricci PE, Karis JP, Heiserman JE, et al. Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? AJNR Am J Neuroradiol 1998;19:407–13 [PMC free article] [PubMed] [Google Scholar]
- 3. Wong TZ, van der Westhuizen GJ, Coleman RE. Positron emission tomography imaging of brain tumors. Neuroimaging Clin N Am 2002;12:615–26 [DOI] [PubMed] [Google Scholar]
- 4. Sasaki M, Kuwabara Y, Yoshida T, et al. A comparative study of thallium-201 SPET, carbon-11 methionine PET and fluorine-18 fluorodeoxyglucose PET for the differentiation of astrocytic tumours. Eur J Nucl Med 1998;25:1261–69 [DOI] [PubMed] [Google Scholar]
- 5. Zuo C, Liu Y, Guan Y, et al. Clinical application of FDG-PET for the diagnosis of recurrent glioma. [in Chinese] Nuclear Techniques 2001;24:899–902 [Google Scholar]
- 6. Gómez-Río M, Rodriguez-Fernandez A, Ramos-Font C, et al. Diagnostic accuracy of 201Thallium-SPECT and 18F-FDG-PET in the clinical assessment of glioma recurrence. Eur J Nucl Med Mol Imaging 2008;35:966–75 [DOI] [PubMed] [Google Scholar]
- 7. Choi SJ, Kim JS, Kim JH, et al. [18F]3′-deoxy-3′-fluorothymidine PET for the diagnosis and grading of brain tumors. Eur J Nucl Med Mol Imaging 2005;32:653–59 [DOI] [PubMed] [Google Scholar]
- 8. Pauleit D, Stoffels G, Bachofner A, et al. Comparison of (18)F-FET and (18)F-FDG-PET in brain tumors. Nucl Med Biol 2009;36:779–87 [DOI] [PubMed] [Google Scholar]
- 9. Lau EW, Drummond KJ, Ware RE, et al. Comparative PET study using F-18 FET and F-18 FDG for the evaluation of patients with suspected brain tumour. J Clin Neurosci 2010;17:43–49 [DOI] [PubMed] [Google Scholar]
- 10. Estrada G, Gonzalez-Maya L, Celis-Lopez MA, et al. Diagnostic approach in suspected recurrent primary brain tumors using (18)FDG-PET/MRI, perfusion MRI, visual and quantitative analysis, and three dimensional stereotactic surface projections: first experience in Mexico. Rev Esp Med Nucl 2008;27:329–39 [DOI] [PubMed] [Google Scholar]
- 11. McCarthy M, Yuan JB, Campbell A, et al. 18F-fluorodeoxyglucose positron emission tomography imaging in brain tumours: the Western Australia positron emission tomography/cyclotron service experience. J Med Imaging Radiat Oncol 2008;52:564–69 [DOI] [PubMed] [Google Scholar]
- 12. Kahn D, Follett KA, Bushnell DL, et al. Diagnosis of recurrent brain tumor: value of 201Tl SPECT vs 18F-fluorodeoxyglucose PET. AJR Am J Roentgenol 1994;163:1459–65 [DOI] [PubMed] [Google Scholar]
- 13. Thompson TP, Lunsford LD, Kondziolka D. Distinguishing recurrent tumor and radiation necrosis with positron emission tomography versus stereotactic biopsy. Stereotact Funct Neurosurg 1999;73:9–14 [DOI] [PubMed] [Google Scholar]
- 14. Sun A, Sun L, Wang R, et al. Value of 18F-FDG-PET in differentiation of brain tumor recurrence from radiation necrosis after radiotherapy. [in Chinese] Chin J Med Imaging Technol 2004;20:1484–86 [Google Scholar]
- 15. Santra A, Kumar R, Sharma P, et al. 18F-FDG-PET-CT in patients with recurrent glioma: comparison with contrast enhanced MRI. Eur J Radiol 2012;81:508–13 [DOI] [PubMed] [Google Scholar]
- 16. Hong IK, Kim JH, Ra YS, et al. Diagnostic usefulness of 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography in recurrent brain tumor. J Comput Assist Tomogr 2011;35:679–84 [DOI] [PubMed] [Google Scholar]
- 17. Tan H, Chen L, Guan Y, et al. Comparison of MRI, 18F-FDG, and 11C-choline PET/CT for their potentials in differentiating brain tumor recurrence from brain tumor necrosis following radiotherapy. Clin Nucl Med 2011;36:978–81 [DOI] [PubMed] [Google Scholar]
- 18. Enslow MS, Zollinger LV, Morton KA, et al. Comparison of 18F-fluorodeoxyglucose and 18F-fluorothymidine PET in differentiating radiation necrosis from recurrent glioma. Clin Nucl Med 2012;37:854–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Belohlávek O, Klener J, Vymazal J, et al. The diagnostics of recurrent gliomas using FDG-PET: still questionable? Nucl Med Rev Cent East Eur 2002;5:127–30 [PubMed] [Google Scholar]
- 20. Spence AM, Muzi M, Link JM, et al. NCI-sponsored trial for the evaluation of safety and preliminary efficacy of 3′-deoxy-3′-[18F]fluorothymidine (FLT) as a marker of proliferation in patients with recurrent gliomas: preliminary efficacy studies. Mol Imaging Biol 2009;11:343–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Janus TJ, Kim EE, Tilbury R, et al. Use of [18F]fluorodeoxyglucose positron emission tomography in patients with primary malignant brain tumors. Ann Neurol 1993;33:540–48 [DOI] [PubMed] [Google Scholar]
- 22. Kim YH, Oh SW, Lim YJ, et al. Differentiating radiation necrosis from tumor recurrence in high-grade gliomas: assessing the efficacy of 18F-FDG-PET, 11C-methionine PET and perfusion MRI. Clin Neurol Neurosurg 2010;112:758–65 [DOI] [PubMed] [Google Scholar]
- 23. Cai L, Gao S, Li DC, et al. Value of 18F-FDG and 11C-MET PET-CT in differentiation of brain ringlike-enhanced neoplastic and non-neoplastic lesions on MRI imaging [in Chinese]. Zhonghua Zhong Liu Za Zhi 2009;31:134–38 [PubMed] [Google Scholar]
- 24. Tripathi M, Sharma R, Varshney R, et al. Comparison of F-18 FDG and C-11 methionine PET/CT for the evaluation of recurrent primary brain tumors. Clin Nucl Med 2012;37:158–63 [DOI] [PubMed] [Google Scholar]
- 25. Li DL, Xu YK, Wang QS, et al. 11C-methionine and 18F-fluorodeoxyglucose positron emission tomography/CT in the evaluation of patients with suspected primary and residual/recurrent gliomas. Chin Med J (Engl) 2012;125:91–96 [PubMed] [Google Scholar]
- 26. Ye C, Pan M, Li Y, et al. 11C-MET PET/CT in the detection of recurrent cerebral glioma. [in Chinese] Chin J Stereotact Funct Neurosurg 2009;22:229–31 [Google Scholar]
- 27. Hustinx R, Smith RJ, Benard F, et al. Can the standardized uptake value characterize primary brain tumors on FDG-PET? Eur J Nucl Med 1999;26:1501–09 [DOI] [PubMed] [Google Scholar]
- 28. Jager PL, Vaalburg W, Pruim J, et al. Radiolabeled amino acids: basic aspects and clinical applications in oncology. J Nucl Med 2001;42:432–45 [PubMed] [Google Scholar]
- 29. Chung JK, Kim YK, Kim SK, et al. Usefulness of 11C-methionine PET in the evaluation of brain lesions that are hypo- or isometabolic on 18F-FDG-PET. Eur J Nucl Med Mol Imaging 2002;29:176–82 [DOI] [PubMed] [Google Scholar]
- 30. Nakajima T, Kumabe T, Kanamori M, et al. Differential diagnosis between radiation necrosis and glioma progression using sequential proton magnetic resonance spectroscopy and methionine positron emission tomography. Neurol Med Chir (Tokyo) 2009;49:394–401 [DOI] [PubMed] [Google Scholar]
- 31. Nihashi T, Dahabreh IJ, Terasawa T. Diagnostic accuracy of PET for recurrent glioma diagnosis: a meta-analysis. AJNR Am J Neuroradiol 2013;34:944–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arends LR, Hamza TH, van Houwelingen JC, et al. Bivariate random effects meta-analysis of ROC curves. Med Decis Making 2008;28:621–38 [DOI] [PubMed] [Google Scholar]
- 34. Reitsma JB, Glas AS, Rutjes AW, et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982–90 [DOI] [PubMed] [Google Scholar]
- 35. Swets JA. Measuring the accuracy of diagnostic systems. Science 1988;240:1285–93 [DOI] [PubMed] [Google Scholar]
- 36. Harbord RM, Whiting P, Sterne JA, et al. An empirical comparison of methods for meta-analysis of diagnostic accuracy showed hierarchical models are necessary. J Clin Epidemiol 2008;61:1095–103 [DOI] [PubMed] [Google Scholar]
- 37. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58 [DOI] [PubMed] [Google Scholar]
- 39. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882–93 [DOI] [PubMed] [Google Scholar]
- 41. Terakawa Y, Tsuyuguchi N, Iwai Y, et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med 2008;49:694–99 [DOI] [PubMed] [Google Scholar]
- 42. Galldiks N, Kracht LW, Berthold F, et al. [11C]-L-methionine positron emission tomography in the management of children and young adults with brain tumors. J Neurooncol 2010;96:231–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sonoda Y, Kumabe T, Takahashi T, et al. Clinical usefulness of 11C-MET PET and 201T1 SPECT for differentiation of recurrent glioma from radiation necrosis. Neurol Med Chir (Tokyo) 1998;38:342–47, discussion 347–48 [DOI] [PubMed] [Google Scholar]
- 44. Tsuyuguchi N, Takami T, Sunada I, et al. Methionine positron emission tomography for differentiation of recurrent brain tumor and radiation necrosis after stereotactic radiosurgery–in malignant glioma. Ann Nucl Med 2004;18:291–96 [DOI] [PubMed] [Google Scholar]
- 45. Chen W. Clinical applications of PET in brain tumors. J Nucl Med 2007;48:1468–81 [DOI] [PubMed] [Google Scholar]
- 46. Schiepers C, Chen W, Cloughesy T, et al. 18F-FDOPA kinetics in brain tumors. J Nucl Med 2007;48:1651–61 [DOI] [PubMed] [Google Scholar]
- 47. Floeth FW, Pauleit D, Sabel M, et al. Prognostic value of O-(2–18F-fluoroethyl)-L-tyrosine PET and MRI in low-grade glioma. J Nucl Med 2007;48:519–27 [DOI] [PubMed] [Google Scholar]
- 48. Dunet V, Rossier C, Buck A, et al. Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: a systematic review and Metaanalysis. J Nucl Med 2012;53:207–14 [DOI] [PubMed] [Google Scholar]
- 49. Ozsunar Y, Mullins ME, Kwong K, et al. Glioma recurrence versus radiation necrosis? A pilot comparison of arterial spin-labeled, dynamic susceptibility contrast enhanced MRI, and FDG-PET imaging. Acad Radiol 2010;17:282–90 [DOI] [PubMed] [Google Scholar]
- 50. Kosaka N, Tsuchida T, Uematsu H, et al. 18F-FDG-PET of common enhancing malignant brain tumors. AJR Am J Roentgenol 2008;190:W365–69 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.