Abstract
Kidney stones frequently develop as an overgrowth on Randall’s plaque (RP) which is formed in the papillary interstitium. The organic composition of RP is distinct from stone matrix in that RP contains fibrillar collagen; RP in tissue has also been shown to have two proteins that are also found in stones, but otherwise the molecular constituents of RP are unstudied. We hypothesized that RP contains unique organic molecules that can be differentiated from the stone overgrowth by fluorescence. To test this, we used micro CT-guided polishing to expose the interior of kidney stones for multimodal imaging with multiphoton, confocal and infrared microscopy. We detected a blue autofluorescence signature unique to RP, the specificity of which was also confirmed in papillary tissue from patients with stone disease. High-resolution mineral mapping of the stone also showed a transition from the apatite within RP to the calcium oxalate in the overgrowth, demonstrating the molecular and spatial transition from the tissue to the urine. This work provides a systematic and practical approach to uncover specific fluorescence signatures which correlate with mineral type, verifies previous observations regarding mineral overgrowth onto RP and identifies a novel autofluorescence signature of RP demonstrating RP’s unique molecular composition.
Keywords: kidney stones, nephrolithiasis, calcium oxalate, micro CT, fluorescence microscopy, infrared spectroscopy
Introduction
Kidney stones afflict a significant proportion of people in the western world: Approximately one-in-eleven persons will have at least one symptomatic stone episode during their lifetime, and about half of those persons will have more than one episode [1]. The root causes of upper urinary tract stones appear to be diverse [2], but the formation of calcium oxalate stones on Randall’s (interstitial) plaque is an important mechanism for many stone formers. Estimates based on stones turned in for analysis suggest that the proportion of stones with visible signs of having grown on Randall’s plaque increased in France during recent decades, reaching 22% and 32% respectively of all stones analyzed for women and men in their thirties [3].
These stones can be easily identified by the presence of a remnant of Randall’s plaque that is adherent to the stone [3, 4]. The identification of Randall’s plaque origin can also be done with great confidence using micro computed tomographic imaging (micro CT) [5]. Micro CT can also confirm the presence of ‘fossilized’ tubules or vessels from the renal papilla that remained attached to the stones as additional confirmation of the papillary origin of the calculus [5, 6].
A number of imaging modalities have been used to evaluate kidney stones. Non-destructive techniques are useful for delineating mineral composition but lack the cellular resolution that will be important in understanding the contribution of cellular processes in driving stone formation. This is especially relevant in stones that form in contact with tissue such as with Randall’s plaque stones. Randall’s plaque stones present a further unique challenge as the mineral growing from urine (the overgrowth) often surrounds most of the papillary attachment site, with the attached Randall’s plaque being concealed within a concavity of the overgrowth. Furthermore, in high resolution microscopy, which is often limited by objective working distance and narrow optical sectioning, a planar surface is required for proper imaging [7], and stone surfaces are remarkably irregular.
In the present study, we exposed the overgrowth region of stones growing on Randall’s plaque using micro-CT guided polishing which allows high-resolution imaging of the exposed stone interior. We used infrared microscopy to analyze for mineral composition [8] and intrinsic fluorescence to gain inference about organic content. Intrinsic fluorescence (autofluorescence) originates from proteins and metabolites [9], providing an overview of the organic molecules present in Randall’s plaque or deposited from the urine during stone growth. Additional methods, such as detection of second harmonic generation (SHG) also allowed the identification of collagen fibers [9–11].
Here we propose a methodical approach to expose stone overgrowth and Randall’s plaque and enable the interrogation of stone growth by high resolution microscopy to help unlock the underlying pathophysiology of nephrolithiasis. Our findings with infrared microscopy verified that the mineral content of Randall’s plaque was apatite and that the overgrowth mineral was calcium oxalate (CaOx). Fluorescence microscopy revealed that Randall’s plaque consistently showed autofluorescence in the blue range in both stones and papillary tissue from biopsy, defining a unique signature for Randall’s plaque and further evidence that its molecular composition is distinct from both normal tissue and stone matrix.
Methods
Source of the studied kidney stones
Patients were identified from a cohort consented for study during percutaneous nephrolithotomy or ureteroscopic removal of stones (Indiana University Institutional Review Board protocol #1010002261). Beginning in 2005, the stones from these patients were analyzed using a protocol that included micro CT [5] for non-destructive, microscopic viewing of each stone before other analyses were done. Patients described in the present paper include only those for which calcium oxalate was the majority mineral (using overall percentage in each patient as calculated by volume using micro CT [12]), and patients with any brushite or patients with medullary sponge kidney were excluded. In each patient, stones were typically collected one-by-one using a nitinol basket, minimizing damage to the stone during collection as much as possible.
Papillary tissue preparation
A papillary biopsy (taken as previously described [13]) was frozen on dry ice in optimal cutting temperature compound immediately after extraction. The patient from which this biopsy was taken underwent percutaneous nephrolithotomy, and had a large, pelvic stone plus a smaller stone that by micro CT showed Randall’s plaque adherent to a concave surface on the stone. The stone on Randall’s plaque was found loose; that is, it was not observed to have been attached to a papilla before removal (but could have been dislodged from its attachment site prior to its discovery). The biopsied papilla was observed to be rich in Randall’s plaque by endoscopic examination. The papillary biopsy was cryosectioned into consecutive 5 μm sections followed by a 50 μm section. The 50 μm section was immediately fixed overnight with 4% paraformaldehyde and used for label free multiphoton and confocal imaging. The same section underwent fluorescence staining with DAPI and was re-imaged under fluorescence confocal microscopy. 5 μm sections contiguous to the 50 μm section underwent staining for hematoxylin and eosin and Yasue to evaluate the histology and confirm the mineral calcium deposition observed in the confocal and multiphoton imaging.
Micro CT imaging and analysis
Stones were photographed, dried in air, and then imaged by micro CT (Skyscan 1172 Micro CT System, Kontich, Belgium) typically using 60 kVp and a 0.4° rotation step, for a final cubic voxel size of 2–6 μm. Micro CT image stacks were routinely examined using ImageJ (http://imagej.net) [14]. Some image stacks were also studied using Vaa3D. (http://vaa3d.org) [15]. Mineral types were identified in micro CT images using relative X-ray attenuation values and crystal shapes, methods that have been verified over the years by a process of careful dissection of stone regions and confirmatory analyses by traditional infrared spectroscopic methods [16].
Stones on Randall’s plaque were identified using the following criteria: The stone was composed mainly of CaOx and showed by micro CT a region of apatite visible at one surface of the stone. This apatite region showed X-ray attenuation brightest next to the CaOx, usually diminishing gradually from there toward the portion of the plaque that would have been deepest in the tissue. This apatite region also did not show layering (alternating X-ray bright and dark layers, as is commonly seen in apatite formed in urine [12]). Finally, in most cases the apatite region showed luminal spaces, consistent with the presence of calcified tubules and/or vessels [6]. We note that the criteria used here to specify stones grown on plaque are quite strict. It is almost certainly possible for a stone to grow on Randall’s plaque, but not to show adherent plaque on its concave surface, because the stone broke away from the plaque at the plaque-overgrowth interface [6]. In the present study, we did not count stones simply having a concavity on the surface as having grown on Randall’s plaque.
Planar dissection of the stones
Initial experiments demonstrated the ability of micro CT to show the mineral types revealed on internal portions of stones that were exposed by polishing with emery paper, as well as the subsequent imaging of the planar surface by Fourier-infrared spectroscopic methods (FT-IR, described further below). Figure 1 shows the example of a CaOx stone that had veins of apatite running through it. The stone was mounted on polystyrene and polished with emery paper (30-μm, followed by 3-μm and then 0.3-μm) to produce a flat surface (Figure 1B). This was a Randall’s plaque stone, but the plane of polishing did not pass through the plaque region. The mineral composition in these regions were identified by micro CT, confirmed by FT-IR microscopy with characteristic calcium oxalate monohydrate and apatite absorbance peaks (Figure 1C). Note that the visual patterns of X-ray attenuation on the polished surface (Figure 1C, left) match perfectly with the pseudo color image generated using FT-IR identification of the two mineral types (Figure 1C, middle and right). This example also illustrates that the polishing was able to yield a planar surface suitable for high-resolution imaging.
Figure 1. Polishing a stone to create a flat surface (planar dissection) and confirmation of micro CT mineral identification using infrared microscopy.
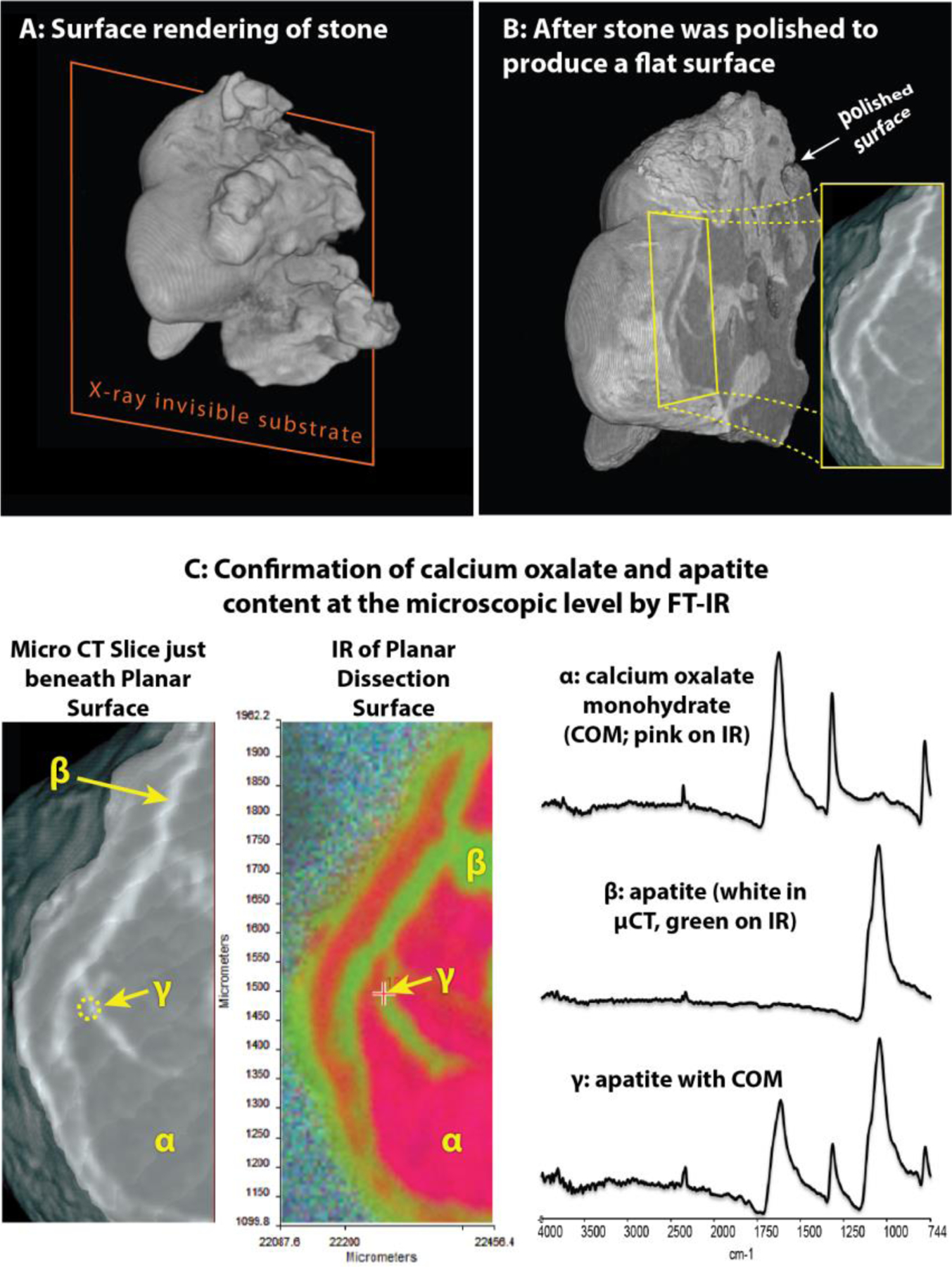
A: 3D rendering of calcium oxalate stone (which was about 2.5 mm long), showing how stone was attached to polystyrene substrate. B: Same stone after being polished with emery paper to expose stone interior. Inset shows region to be examined in C. C: 3D rendering of edge of exposed interior region of stone. Left panel shows micro CT image, with the layers of apatite appearing white. Color panel shows false-color map obtained from infrared spectral shapes. Right side shows infrared spectra for spots marked in other panels.
Stones, less than 2–3 mm in the longest dimension, were mounted on polystyrene rods. These hollow polystyrene rods were modified by adhesion of a small piece of L-channel polystyrene at the end to facilitate mounting of the stone. The stone was secured to the rod by softening the polystyrene with methylene chloride. The best orientation for exposing Randall’s plaque and the overgrowth was decided using initial micro CT scans. The mounted stones were reimaged by micro CT to generate a reference stack to guide the depth of polishing. The mounted stones were affixed into a polishing rig that limited the depth of polishing (Figure 2). Polishing proceeded with 2–3 successive passes on 30-micron emery paper followed by 2–3 successive passes on 0.3-micron emery paper. The surface was assessed by dissecting scope and rescanned by micro CT to verify the interior surface exposed.
Figure 2. Generating planar surface through Randall’s Plaque with iterative polishing.
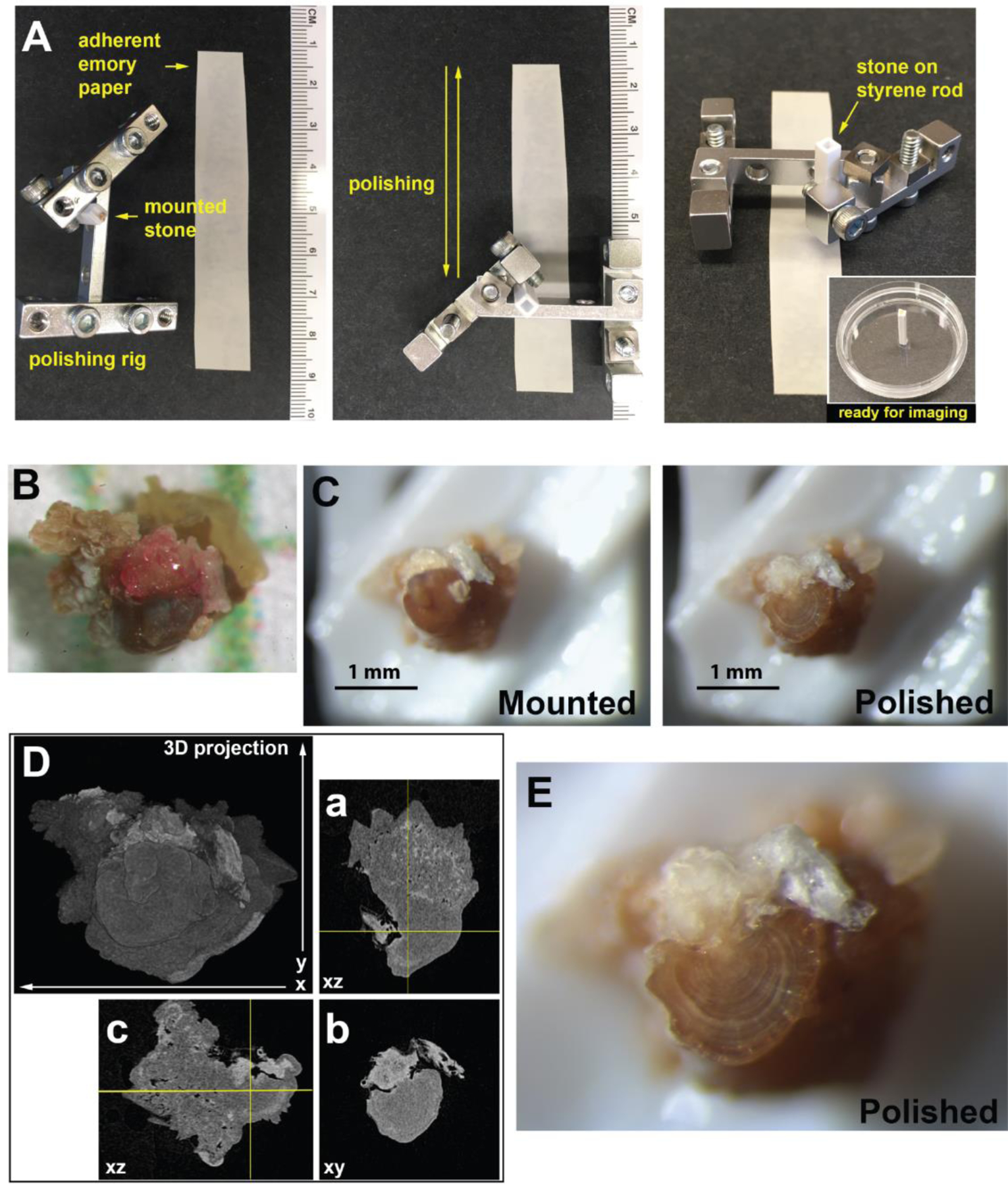
A. A jig was constructed to hold small kidney stones mounted on polystyrene rods to facilitate polishing. The stone was polished by passes up and down emery paper adhered to paperboard surface (left and middle). After polishing the stone, the stone mounted to the styrene rod was attached to a 35 mm dish for imaging (at right). Ruler provided for scale. B. Image of stone following extraction for the patient on 1 mm grid paper. C. Image of mounted and polished stone following iterative polishing and imaging to reach desired depth. D. 3D projection of micro CT volume collected from the kidney stone mounted to a polystyrene rod. Orthogonal sections are given to indicate the cross-sectioning of Randall’s plaque and overgrowth. E. Higher magnification image of polished kidney stone surface given in C, right.
Multiphoton and confocal fluorescence imaging
Intrinsic and autofluorescence imaging were performed on a Leica SP8 confocal and multiphoton microscope equipped with a SpectraPhysics MaiTai DeepSee laser. For imaging of kidney stones, single photon confocal imaging settings were: 405 nm excitation with 410–480 nm emission (blue), 488 nm excitation with 495–550 nm emission (green), 552 nm excitation with 560–640 nm emission (yellow) and 635 nm excitation with 650–700 nm emission (red). For imaging tissue sections, single photon excitation confocal imaging excitation and emission was collected for: 405 nm excitation with 448–470 nm emission (blue), 488 nm excitation with 506–561 nm emission (green), 552 nm excitation with 583–640 nm emission (yellow) and 635 nm excitation with 640–774 nm emission (red). Multiphoton excitation of tissue sections was performed with 910 nm excitation to collect SHG (448–470 collection) and autofluorescence with 400–450 nm emission. In either confocal or multiphoton imaging multiple optical sections were taken. Imaging data was processed, including z-projections, in FIJI [14].
Infrared microscopy
Ground stones were imaged for spectroscopic mapping using a Perkin-Elmer Spectrum Spotlight 400 infrared imaging microscope equipped with a liquid nitrogen-cooled HgCdTe array detector. Low resolution spectroscopic mapping was done using the reflectance imaging mode. High resolution mapping was done using a germanium hemisphere placed in direct contact with the planar surface for attenuated total internal reflection spectroscopy [17]. The resulting infrared maps contained thousands of spectra and principal component analysis was employed to indicate spectral differences across the mapped surface.
Results
Stones growing on Randall’s plaque.
There was a variety of morphologies seen in CaOx stones growing on Randall’s plaque. Figure 3 shows 6 representative stones out of 350 that were identified as having grown on Randall’s plaque. These stones came from 24 different patients (with 6 patients represented in Figure 3), with a male/female ratio of 15/9, and in general the stones in a single patient all showed very similar morphology. 280 of these Randall’s plaque stones (80%) showed lumens of calcified tubules or vessels, a characteristic consistent with the tissue origin of plaque [6, 18]. The stone in Figure 3A displayed one of the largest anchoring sites onto Randall’s plaque that was observed, about 0.7 mm wide and almost 2 mm long. Multiple lumens (representing calcified tubules or vessels, arrowheads in Figure 3) were seen along the part of the plaque that would have pulled away from unmineralized papillary tissue when this attached stone was removed. The stone in Figure 3A showed a fair bit of apatite in the overgrowth along with calcium oxalate, as did the stone in Figure 3F, but this was not typical of the Randall’s plaque stones, in general. The stones shown in Figure 3B, 3C, and 3D illustrate the most typical morphologies seen among Randall’s plaque stones, though the relatively pure dihydrate crystals in 1D were rarer to see than were pure monohydrate or mixed calcium oxalate stones. The stone shown in Figure 3E was growing on a relatively well-mineralized piece of Randall’s plaque, which even showed a prominent passageway for what presumably was a collecting duct lumen (an oval opening measuring 190×370 μm). Note in 3A, 3C, and 3F that the apatite in the stone overgrowth typically showed a higher X-ray density (greater brightness) than did the apatite in Randall’s plaque, which presumably reflects crystal density in these sites. That is, the density of apatite crystals in Randall’s plaque—crystals which would have deposited within the connective tissue of the papillary interstitium—was generally lower than the regions of apatite found in stone overgrowth, where the apatite would have deposited from urine.
Figure 3. Micro CT appearance of stones attached to Randall’s (interstitial) plaque.
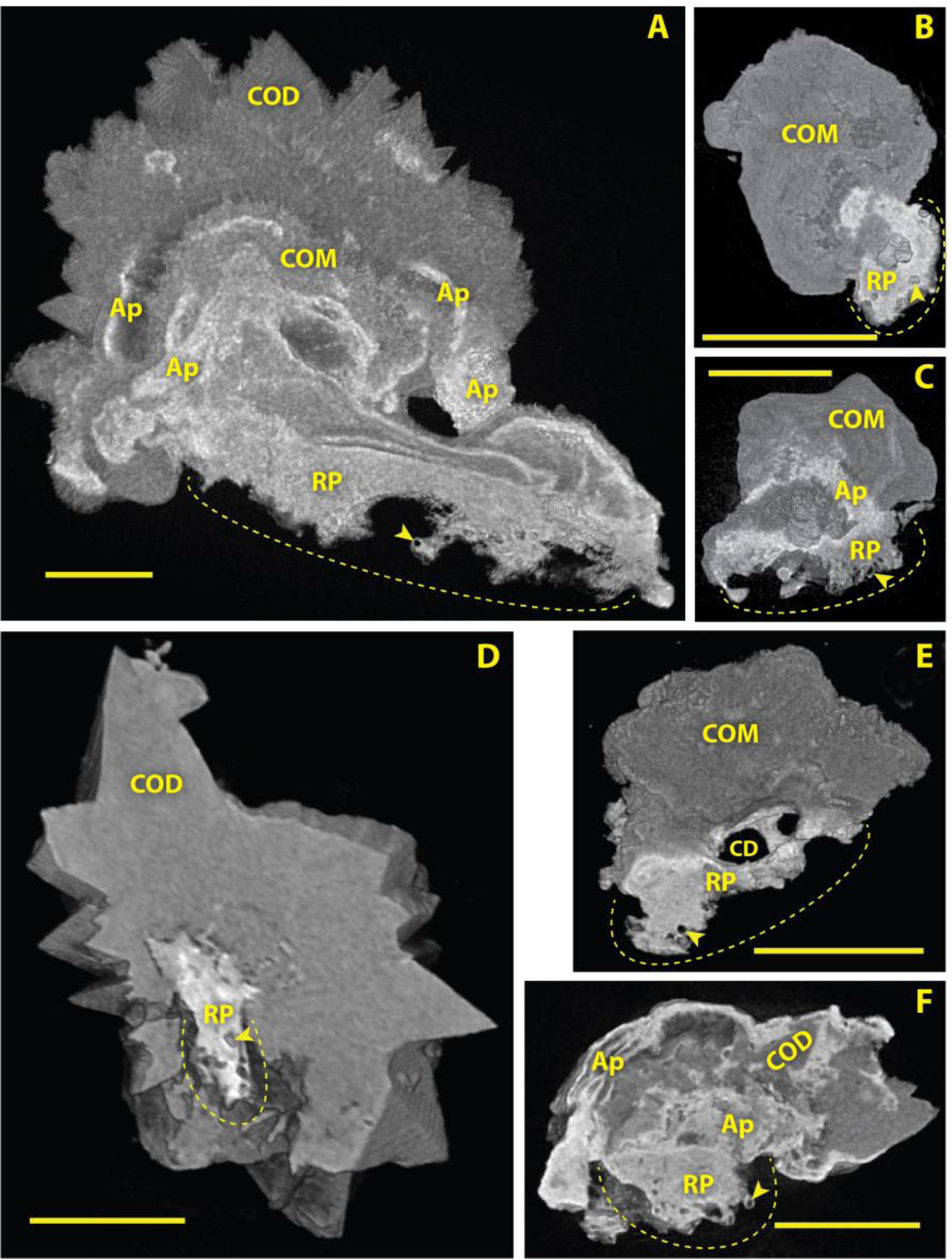
Shown are three-dimensional (3D) renderings of micro CT image stacks, each one cut away (virtually) to show the interior of the stone and the Randall’s plaque attachment. Scale bars = 500 μm. Dotted lines indicate Randall’s plaque attachment site; in vivo, these dotted lines would have lain within the tissue of the renal papilla. Arrowheads indicate lumens of calcified tubules or vessels. RP: Randall’s plaque. COD: calcium oxalate dihydrate. COM: calcium oxalate monohydrate. A: apatite within the stone overgrowth region. CD: Space marking lumen of collecting duct that apparently ran through the Randall’s plaque.
Mineral identification of Randall’s plaque and kidney stone overgrowth.
Micro CT of the same stone shown in Figure 2 clearly showed regions of apatite-containing Randall’s plaque and CaOx overgrowth (Figure 4A). Panel B shows a photomicrograph of the stone surface when mounted in the FT-IR microscope. Panel C shows reflectance-mode FT-IR microscopy results, confirming that Randall’s plaque had a distinct apatite signature while the overgrowth was calcium oxalate monohydrate. Even with this lower resolution mode of FT-IR imaging, we observed a mixture of oxalate and apatite at the interface between Randall’s plaque and the stone overgrowth (Figure 4C). Imaging using the attenuated total internal reflection mode showed considerable interleaving of apatite and oxalate between the apatite-rich Randall’s plaque and the calcium oxalate overgrowth (Figure 4D, bracket). Thus, the planar dissection approach enabled high resolution characterization of mineral composition in this important region, where the initial growth of the stone occurred on Randall’s plaque.
Figure 4. Infrared microscopy of stone polished down to reveal Randall’s plaque (RP) and stone overgrowth region.

A: Micro CT just below planar surface of polished stone; stone measured only 800 μm from lowest part of Randall’s plaque (left) to top of stone (right). B: Photographic montage of polished surface of stone taken on infrared microscope. C: False color image collected using reflectance mode, demonstrating homogeneity of infrared spectra of Randall’s plaque region (showing the mineral apatite) and the stone overgrowth region (showing the mineral calcium oxalate monohydrate, COM). Dotted line indicates field for D. D: False color image collected using attenuated total reflection (ATR) mode, showing neck region of overgrowth onto RP. Note interleaving of apatite and COM in region indicated by bracket.
Autofluorescence of Randall’s plaque and kidney stone overgrowth correlates with mineral composition and access to the urine space.
To assess autofluorescence of a Randall’s plaque stone, we imaged stones that had been polished to expose Randall’s plaque and overgrowth as described above (Figure 2). The higher resolution of fluorescence imaging, as compared to micro CT, is apparent from the detail revealed in both the areas of the Randall’s plaque and the stone overgrowth (Figure 5, left versus right). When excited with a single-photon violet laser (405 nm) or a multiphoton laser tuned to 800 nm, a blue fluorescence was detected in the apatite-rich Randall’s plaque. (This unique fluorescence pattern was observed in 6 of 6 Randall’s plaque stones that were examined using this method.) Calcium oxalate monohydrate overgrowth had a distinct green and yellow fluorescence with 488 or 552 nm single photon excitation, respectively. Strikingly, some calcium oxalate dihydrate and what was apparently monohydrate formed by conversion from the dihydrate [16], found in the overgrowth part of the stone was poorly fluorescent irrespective of excitation modality or wavelength (Figure 5 right, stones 2 and 3, asterisks). Figure 6 shows the fluorescence of stone 1 in a larger format, along with imaging using two-photon microscopy to show the blue fluorescence with even finer detail. Uniquely, stone 1 contained a distinct blue and yellow band between the calcium oxalate overgrowth and the Randall’s plaque. This band was also observed in one other stone sample from a different patient (data not shown).
Figure 5. Intrinsic fluorescence of interior of stones growing on Randall’s plaque (RP).
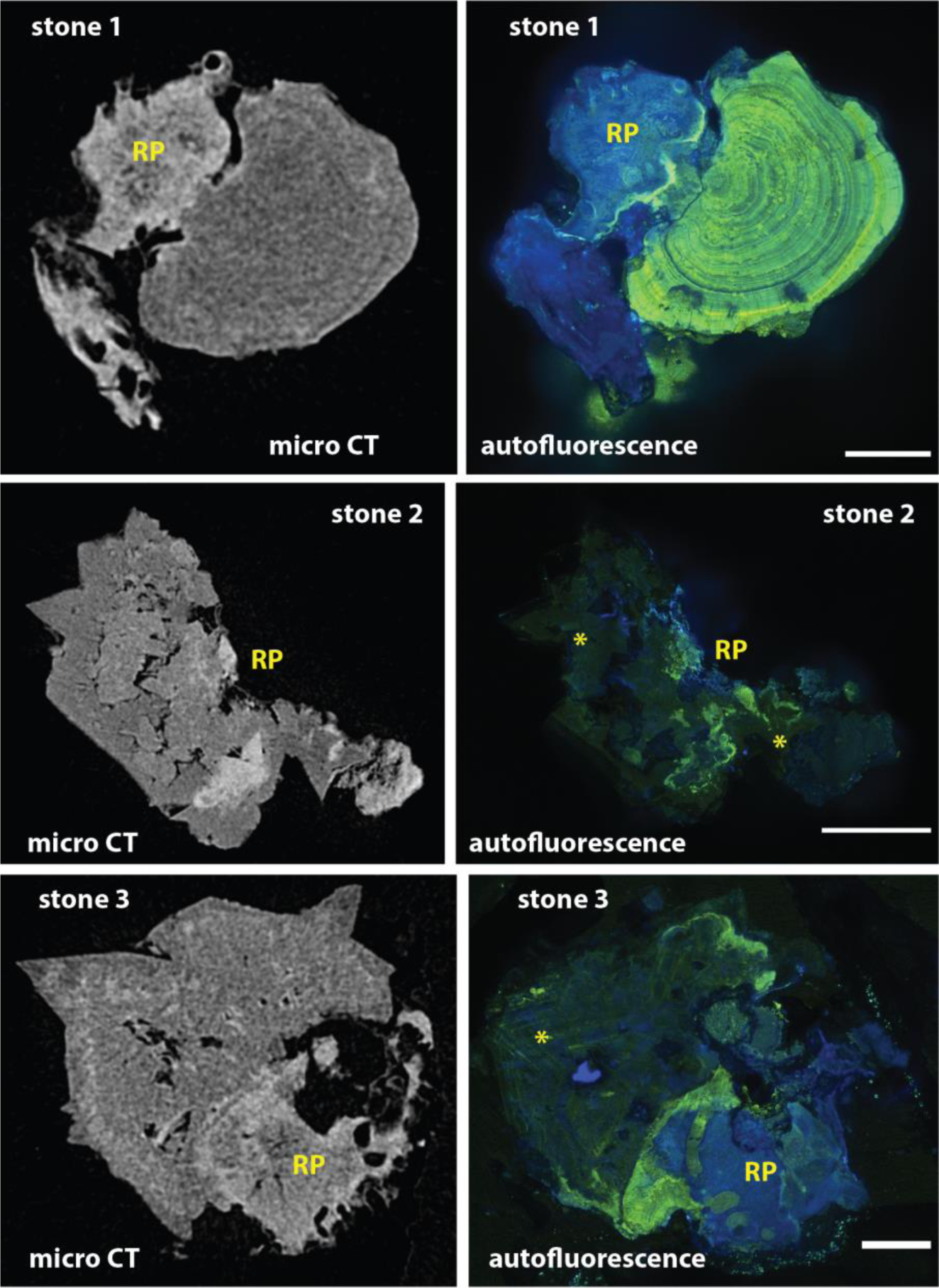
Kidney stones, all from the same patient, polished to expose kidney stone interiors and imaged by confocal fluorescence imaging. Left: Micro CT image slices of approximately the same plane as exposed by polishing. Right: Composite images of three channels of fluorescence imaging (with excitation at 405, 488, and 552 nm). Note that Randall’s plaque fluoresces mainly in the far blue range. Asterisks (*) mark regions of low fluorescence that appear to correlate with calcium oxalate dihydrate or conversion of dihydrate to monohydrate. Scale bars = 200, 500 and 200 μm for stones 1, 2, and 3, respectively.
Figure 6.
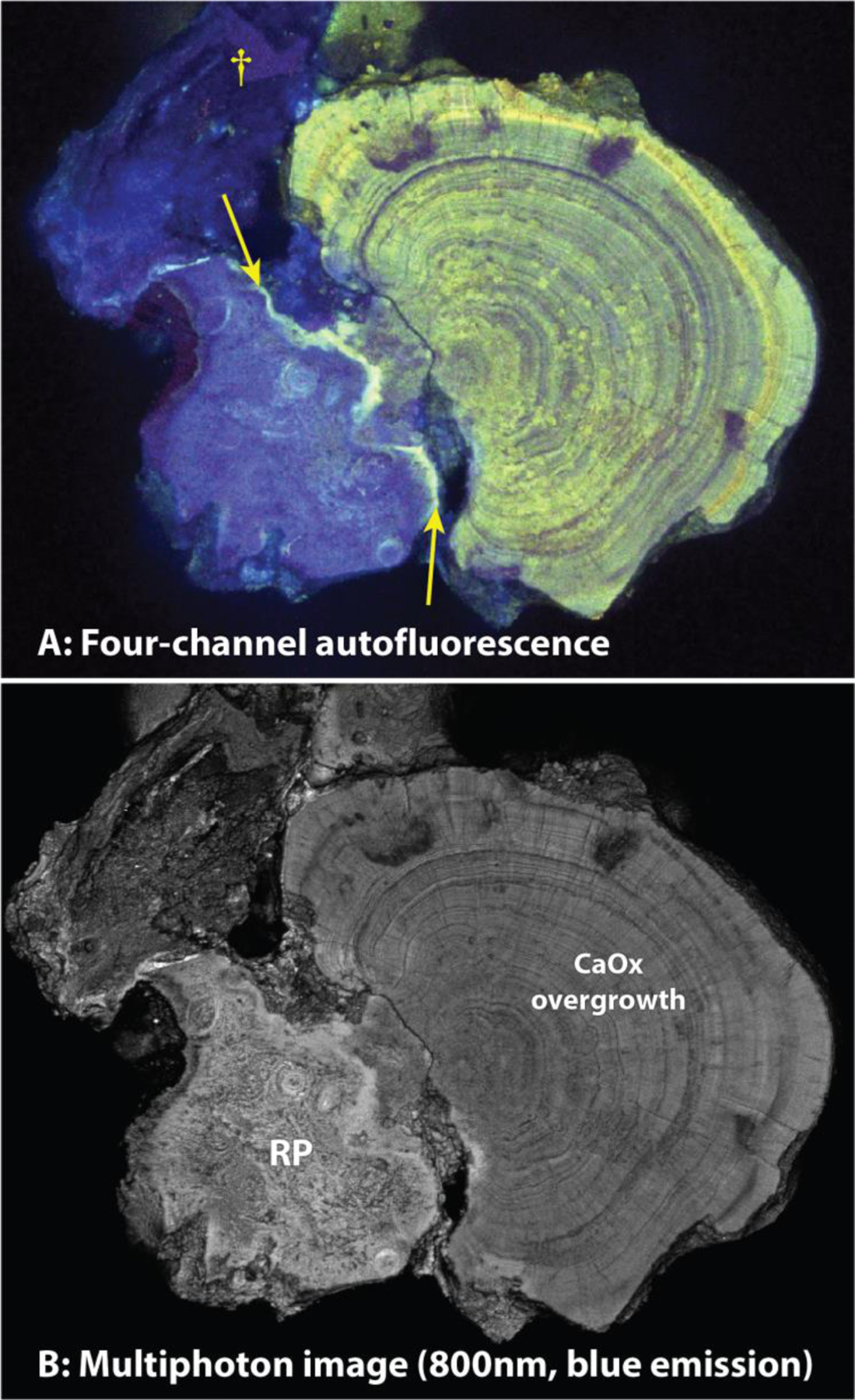
Fluorescence imaging of stone polished to expose the interface between Randall’s plaque (RP) and the calcium oxalate overgrowth (A, four-channel single-photon, and B, multiphoton microscopy in only the blue range). Note fine detail visible in the multiphoton image (B). Arrows point to a layer of bright fluorescence that indicates enrichment in organic material, perhaps where RP was exposed to urine. Dagger (†) marks a piece of additional RP that apparently was torn up from the tissue during the removal of the stone from the renal papilla and which then adhered to the side of the stone.
Label-free imaging of papilla from Randall’s plaque stone formers.
To test whether the blue autofluorescence signal was due to an intrinsic property of Randall’s plaque independent of its presence in the stone or tissue, we imaged Randall’s plaque in a papillary biopsy obtained from a stone forming patient. The presence of Randall’s plaque was confirmed by Yasue staining. Characteristic black silver deposition was observed around tubules in the interstitium in the papilla. Hematoxylin and eosin staining on an adjacent section also showed the characteristic basophilic labeling consistent with apatite deposits (Figure 7A, cyan arrowheads). When an adjacent section was examined by confocal fluorescence microscopy, autofluorescence was detected around tubules in in a pattern similar to the Yasue staining (Figure 7B, arrowheads). The autofluorescence was strongest in the blue emission with near UV excitation (Figure 7B). Blue autofluorescence in similar deposits was detected by label-free imaging with multiphoton excitation (Figure 7C left, arrowhead). Second harmonic generation from fibrillar collagen suggests these deposits were associated in the connective tissue consistent with previously published work [4, 13, 19, 20] (Figure 7C right, arrowhead). (However, note in Figure 7C right that regions of collagen fibrosis did not fluoresce in the blue range.) Following staining for immunofluorescence of the same section with the nuclear dye DAPI, both nuclei and ring structures around putative tubules were observed, matching previous microscopic descriptions of Randall’s plaque [13]. Taken together, these findings support that the blue autofluorescence in Randall’s plaque in papillary tissue (Figure 7D, cyan arrowheads) is the same as that seen in Randall’s plaque on stones (Figures 5 and 6).
Figure 7. Imaging of Randall’s plaque in papillary biopsy.
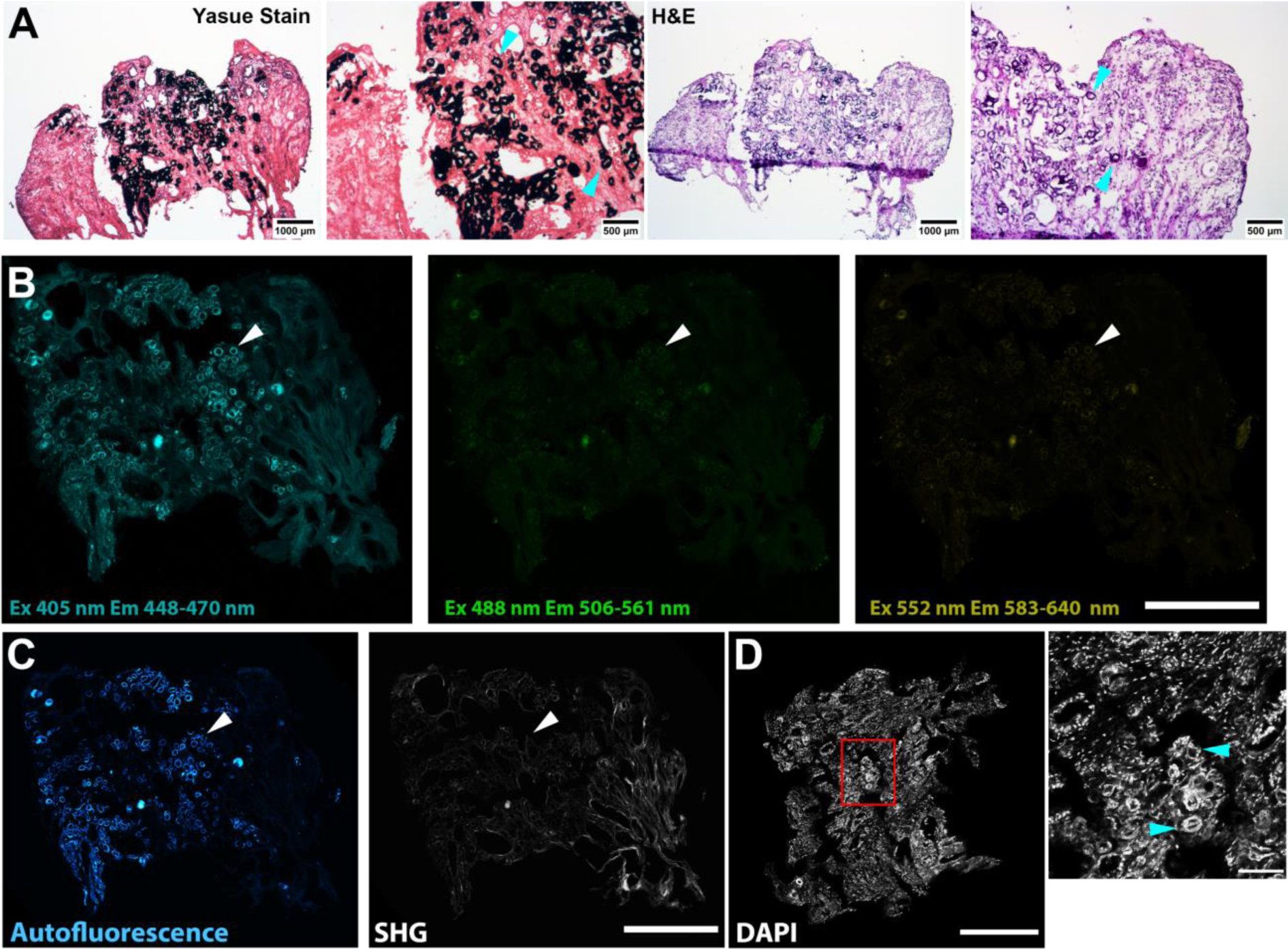
Serial sections were stained with Yasue (for mineral) and H&E, or imaged unstained to detect label-free autofluorescence, followed by reimaging after DAPI staining for nuclei. A: Adjacent sections stained with Yasue (left) and H&E (right). Cyan arrowheads indicate silver precipitation by Yasue staining or basophilic staining by H&E. Scale bars as indicated. B: Label-free autofluorescence imaging of unstained adjacent section with confocal fluorescence microscopy, excitation (Ex) and emission (Em) as indicated. Scale bar = 500 μm. C: Label-free autofluorescence imaging on unstained adjacent section with illumination at 910 nm and emission at 448–470 nm (left) and collection at 400–450 nm (right). Left shows distinct autofluorescence that correlates with Randall’s plaque mineral shown in A (arrowhead). Right shows second harmonic generation (SHG) which indicates the presence of fibrillar collagen. Scale bar = 500 μm. D: Following staining with DAPI, both nuclei and plaque autofluorescence are visible. Randall’s plaque appears as bright rings (arrowheads) fluorescing in the same range as the DAPI-stained nuclei. Left shows low-power with red box indicating field shown on right. Scale bar = 500 (left) and 100 (right) μm.
Discussion
Examination of the structure of Randall’s plaque and the overgrowth onto Randall’s plaque of calcium oxalate from urine using high-resolution methods is technically challenging. This fact is borne out by the relatively small number of stones that have been analyzed in previous studies. For example, in 2007 Evan et al. published extensive examinations of two Randall’s plaque stones taken by biopsy with underlying papillary tissue [21]. One stone was partially decalcified, sectioned, and studied using light microscopy, infrared microscopy, and labeled antibody staining. The other stone was decalcified and sectioned for electron microscopy. In 2017, Sethmann et al. published observations on Randall’s plaque stones using scanning electron microscopy [20]. Only one stone was cut to reveal the region of overgrowth of CaOx onto Randall’s plaque. Thus, these two studies reported analyses of the plaque-overgrowth interface on a total of only 3 stones, with each studied using a different method. Both the Evan [21] and Sethmann [20] studies found apatite (or amorphous calcium phosphate) mixed with CaOx in the earliest deposition from urine, which fits with our data (Figure 4D). Evan [21] also described a rich deposition of organic material at the interface, which seems to match the bright layer of fluorescence covering the plaque in our Figure 6.
Planar polishing uncovers the interface between Randall’s plaque and overgrowth, thereby enabling high resolution imaging methods and novel interrogation of kidney stone disease. We used micro CT to image the entirety of Randall’s plaque containing kidney stones. Unfortunately, micro CT is limited in resolution and spectral information. Furthermore, the irregular shape of kidney stones makes imaging difficult by surface microscopy methods such as confocal and FT-IR microscopy. Polished kidney stone sections have been used previously to study mineral types [22], and for high resolution spectral imaging [23], but this approach yields only a single section of the stone, and it is difficult to section a small stone in a particular plane. Similarly, we have found it extremely challenging to use histologic sectioning of decalcified stones [24] to study Randall’s plaque, as orientation of the demineralized specimen to correctly place the plane of section is technically difficult. In the method described in the present paper, we used micro CT to guide the polishing, allowing us to follow the progress towards uncovering the plaque-overgrowth interface on small kidney stones. The polished planar surface provided a suitable surface for high-resolution confocal and FT-IR imaging of the plaque-overgrowth interface. Although not attempted here, this approach could be done in a repetitive way, successively exposing deeper planes within the stone, to enable high resolution tomographic imaging of stones similar to what has been done in serial multiphoton tomography [25].
The autofluorescence signatures of Randall’s plaque versus stone overgrowth in Randall’s plaque containing stones points to the unique molecular composition of the papillary interstitium versus the urine space. Randall’s plaque apatite is first deposited in the papilla within the basement membrane of the thin limb of Henle’s loop [13]. From this beginning, the plaque apparently grows through additional deposition so that it extends through the interstitium. The plaque may continue to grow without access to the urine space, thereby maintaining a distinct molecular composition and autofluorescence signature. Our data are consistent with this model, whereby Randall’s plaque maintains an autofluorescence signature distinct from the calcium oxalate stone overgrowth that typically occurs in the urinary space. We demonstrate that this autofluorescence signature in a stone is identical to Randall’s plaque apatite depositions around the thin limb of Henle’s loop in tissue. In one example, at the boundary between Randall’s plaque and the overgrowth, we did observe a distinct ribbon of autofluorescence consistent with the overgrowth model proposed previously [21]. Our FT-IR and confocal fluorescence microscopy also suggests that the urine molecules did not penetrate into the matrix of Randall’s plaque during overgrowth and any replacement of apatite with COM apparently occurred superficially [20].
Imaging of autofluorescence is a novel method to contrast in mineralized Randall’s plaque and stone overgrowth. Tissue autofluorescence or intrinsic fluorescence of flavins, such as NADH and FAD, has been used to follow metabolic state in live tissue [9, 26, 27]. Porphyrin rings, lipofuscins and proteins such as elastin and collagen also exhibit intrinsic fluorescence [9]. In the multimodal imaging work of Sivaguru et al., both multiphoton excitation autofluorescence and fluorescence lifetime imaging microscopy (FLIM) were used to image distinct regions of yellow-red autofluorescence in COM growing in the urine space [23]. The authors suggest that these autofluorescence bands are organic material layered within the crystal. We observed a similar signature in calcium oxalate overgrowth (Figures 5 and 6). In our observations, with both confocal and multiphoton excitation microscopy, we further detect a distinct blue fluorescence in mineralized Randall’s plaque. The blue fluorescence is unlikely to be a characteristic of apatite, as apatite in the overgrowth usually contained additional fluorescence. We suppose that this unique signature of Randall’s plaque is either a proteinaceous or metabolite (or adduct) deposition unique to the tissue environment in which Randall’s plaque forms. The use of autofluorescence methods, such as confocal and multiphoton excitation microscopy and FLIM on Randall’s plaque may further elucidate the characteristics of this unique compartment. The further characterization of the regions of blue AF in Randall’s plaque will require a concerted assessment of metabolites and protein constituents with orthogonal approaches like regional proteomics and small-molecule mass spectrometry [28], but the unique fluorescence signature of Randall’s plaque will make this process much easier to accomplish. Up to now, attempts to model Randall’s plaque have relied on educated guesses for matching the molecular environment of these interstitial apatite deposits [29, 30], but the work reported here is a step toward elucidating the organic composition of Randall’s plaque. The discovery of a unique fluorescence of Randall’s plaque will also benefit studies of biopsies from stone patients, enabling localization of plaque in tissue without the need for staining.
Our study was limited in the range of Randall’s plaque studied. We examined plaque found attached to stones, which presumably represents Randall’s plaque in its most mature form, and papillary biopsies that were relatively rich in plaque. We have not yet extended these studies to verify that the unique, blue fluorescence is seen in the early stages of plaque formation [13]. We also note that the planar dissection method to study the interior of stones is intrinsically a destructive process that potentially could alter molecular structure. In the present study, the unique fluorescence of Randall’s plaque was apparently unaffected by the polishing process, and the process of polishing stones has not been thought to affect molecular composition [22, 23], but this assumption has not been rigorously tested for organic constituents.
Conclusion
With a planar polishing approach, we were able to characterize Randall’s plaque and the stone overgrowth with high resolution imaging techniques. With this method, we identified a unique autofluorescence signature of Randall’s plaque that will facilitate identification of this plaque and may correlate with specific organic and possibly proteinaceous constituents. Uncovering these molecular players associated with Randall’s plaque and the overgrowth will allow new molecular hypotheses that could promote novel insights into the pathogenesis of stone disease.
Supplementary Material
Acknowledgements:
We thank Sharon Bledsoe and Tony Gardner for assistance with data collection. This work was funded by the following grants from the National Institutes of Health: P01 DK056788; R01 DK124776, and S10 RR023710
Funding: NIH P01 DK056788; NIH R01 DK124776; NIH S10 RR023710
Footnotes
Conflicts of interest: None
Availability of data and material: not relevant
Code availability: not relevant
Ethics approval: Local Internal Review Board approved stone collection from patients.
Bibliography
- 1.Scales CD Jr, Smith AC, Hanley JM, Saigal CS (2012) Prevalence of Kidney Stones in the United States. European Urology 62: 160–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams JC Jr., McAteer JA (2013) Retention and growth of urinary stones—Insights from imaging. J Nephrol 26: 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Letavernier E, Vandermeersch S, Traxer O, Tligui M, Baud L, Ronco P, Haymann JP, Daudon M (2015) Demographics and characterization of 10,282 Randall plaque-related kidney stones: A new epidemic? Medicine (Baltimore) 94: e566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verrier C, Bazin D, Huguet L, Stéphan O, Gloter A, Verpont M-C, Frochot V, Haymann J-P, Brocheriou I, Traxer O, Daudon M, Letavernier E (2016) Topography, composition and structure of incipient Randall’s plaque at the nanoscale level. J Urol 196: 1566–1574 [DOI] [PubMed] [Google Scholar]
- 5.Williams JC Jr., Lingeman JE, Coe FL, Worcester EM, Evan AP (2015) Micro-CT imaging of Randall’s plaques. Urolithiasis 43: 13–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cifuentes Delatte L, Miñón Cifuentes J, Medina JA (1987) New studies on papillary calculi. J Urol 137: 1024–1029 [DOI] [PubMed] [Google Scholar]
- 7.Murphy DB, Davidson MW (2013) Fundamentals of light microscopy and electronic imaging. In: Editor (ed)^(eds) Book Fundamentals of light microscopy and electronic imaging. Wiley-Blackwell, City, pp. xiii, 538 p. [Google Scholar]
- 8.Anderson J, Dellomo J, Sommer A, Evan A, Bledsoe S (2005) A concerted protocol for the analysis of mineral deposits in biopsied tissue using infrared microanalysis. Urol Res 33: 213–219 [DOI] [PubMed] [Google Scholar]
- 9.Berezin MY, Achilefu S (2010) Fluorescence lifetime measurements and biological imaging. Chem Rev 110: 2641–2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Nadiarynkh O, Plotnikov S, Campagnola PJ (2012) Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat Protoc 7: 654–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campagnola PJ, Millard AC, Terasaki M, Hoppe PE, Malone CJ, Mohler WA (2002) Three-dimensional high-resolution second-harmonic generation imaging of endogenous structural proteins in biological tissues. Biophys J 82: 493–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pramanik R, Asplin JR, Jackson ME, Williams JC Jr. (2008) Protein content of human apatite and brushite kidney stones: significant correlation with morphologic measures. Urol Res 36: 251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao Y, Sommer AJ, Paterson RF, Kuo RL, Grynpas M (2003) Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 111: 607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng H, Bria A, Zhou Z, Iannello G, Long F (2014) Extensible visualization and analysis for multidimensional images using Vaa3D. Nat Protoc 9: 193–208 [DOI] [PubMed] [Google Scholar]
- 16.Daudon M, Williams JC Jr. (2020) Characteristics of Human Kidney Stones. In: Coe F, Worcester EM, Lingeman JE, Evan AP (eds) Kidney Stones. Jaypee Medical Publishers, pp. 77–97 [Google Scholar]
- 17.Gulley-Stahl HJ, Bledsoe SB, Evan AP, Sommer AJ (2010) The advantages of an attenuated total internal reflection infrared microspectroscopic imaging approach for kidney biopsy analysis. Appl Spectrosc 64: 15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams JC Jr., Worcester E, Lingeman JE (2017) What can the microstructure of stones tell us? Urolithiasis 45: 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan SR, Rodriguez DE, Gower LB, Monga M (2012) Association of Randall plaque with collagen fibers and membrane vesicles. J Urol 187: 1094–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sethmann I, Wendt-Nordahl G, Knoll T, Enzmann F, Simon L, Kleebe HJ (2017) Microstructures of Randall’s plaques and their interfaces with calcium oxalate monohydrate kidney stones reflect underlying mineral precipitation mechanisms. Urolithiasis 45: 235–248 [DOI] [PubMed] [Google Scholar]
- 21.Evan AP, Coe FL, Lingeman JE, Shao Y, Sommer AJ, Bledsoe SB, Anderson JC, Worcester EM (2007) Mechanism of formation of human calcium oxalate renal stones on Randall’s plaque. Anat Rec 290: 1315–1323 [DOI] [PubMed] [Google Scholar]
- 22.Cifuentes Delatte L (1977) Etudes sur la structure des calculs à l’aide des coupes minces minéralogiques. J Urol Nephrol (Paris) 83: 592–596 [PubMed] [Google Scholar]
- 23.Sivaguru M, Saw JJ, Williams JC Jr., Lieske JC, Krambeck AE, Romero MF, Chia N, Schwaderer AL, Alcalde RE, Bruce WJ, Wildman DE, Fried GA, Werth CJ, Reeder RJ, Yau PM, Sanford RA, Fouke BW (2018) Geobiology reveals how human kidney stones dissolve in vivo. Sci Rep 8: 13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan SR, Finlayson B, Hackett RL (1983) Agar-embedded urinary stones: a technique useful for studying microscopic architecture. J Urol 130: 992–995 [DOI] [PubMed] [Google Scholar]
- 25.Amato SP, Pan F, Schwartz J, Ragan TM (2016) Whole Brain Imaging with Serial Two-Photon Tomography. Front Neuroanat 10: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winfree S, Hato T, Day RN (2017) Intravital microscopy of biosensor activities and intrinsic metabolic states. Methods 128: 95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hato T, Winfree S, Day R, Sandoval RM, Molitoris BA, Yoder MC, Wiggins RC, Zheng Y, Dunn KW, Dagher PC (2017) Two-Photon Intravital Fluorescence Lifetime Imaging of the Kidney Reveals Cell-Type Specific Metabolic Signatures. Journal of the American Society of Nephrology : JASN 28: 2420–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spraggins JM, Rizzo DG, Moore JL, Noto MJ, Skaar EP, Caprioli RM (2016) Next-generation technologies for spatial proteomics: Integrating ultra-high speed MALDI-TOF and high mass resolution MALDI FTICR imaging mass spectrometry for protein analysis. Proteomics 16: 1678–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovett AC, Khan SR, Gower LB (2019) Development of a two-stage in vitro model system to investigate the mineralization mechanisms involved in idiopathic stone formation: stage 1-biomimetic Randall’s plaque using decellularized porcine kidneys. Urolithiasis 47: 321–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Kell AL, Lovett AC, Canales BK, Gower LB, Khan SR (2019) Development of a two-stage model system to investigate the mineralization mechanisms involved in idiopathic stone formation: stage 2 in vivo studies of stone growth on biomimetic Randall’s plaque. Urolithiasis 47: 335–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


