Abstract
Purpose:
The use of stereotactic body radiotherapy (SBRT) in pediatric patients has been underreported. We reviewed practice patterns, outcomes, and toxicity of SBRT in this population.
Methods and Materials:
In this multi-institutional study, 55 patients with 107 non-CNS lesions treated with SBRT between 2010 and 2016 were reviewed. Treatment response was evaluated by RECIST v1.1 and modified RECIST v1.1 criteria for soft tissue and bone lesions, respectively. Patterns of local failure (LF) were assessed dosimetrically. The cumulative incidence of LF and toxicity were estimated accounting for the competing risk event of death. Predictors of LF were identified through joint frailty models for clustered competing risks.
Results:
The median dose/fraction was 7 Gy (range, 4.5–25 Gy), the total dose/site was 35 Gy (range, 12–45 Gy), and the median number of fractions was 5 (range, 1–9). The radiographic response rates of bone and soft tissue lesions were 90.6% and 76.7%, respectively. Symptom improvement was observed for 62% of symptomatic sites. Twenty-seven LFs were documented, with 14 in-field, 9 marginal, and 4 out-of-field LFs. The 1-year estimated cumulative LF rate, progression-free survival, and overall survival were 25.2% (95% confidence interval (CI), 17.2%−36.1%), 17.5% (95% CI, 9.0%−34.1%), and 61% (95% CI, 48.9%−76.1%), respectively. Lesion type (soft tissue vs. bone) was the only significant predictor of LF on multivariable analysis (P = .04), with increased hazard for soft tissue lesions. No acute or late toxicity of grade 4 or higher was observed; the estimated 1-year cumulative incidence of late toxicity of any grade was 7.5% (95% CI, 3.6%−12.1%).
Conclusion:
SBRT was well tolerated and resulted in radiographic response and symptom palliation in most pediatric patients with advanced disease. The 1-year cumulative LF rate of 25% will serve as a benchmark for further modifications to RT indications, parameters, and combination therapy.
INTRODUCTION
Stereotactic body radiation therapy (SBRT) enables the delivery of ablative doses of highly conformal radiotherapy (RT) over a short treatment course in a noninvasive manner. Multiple studies in adults have demonstrated excellent and durable local tumor control and effective symptom palliation with this technique,1,2 as well as improved survival in patients with oligometastatic disease.3 Furthermore, given the distinctive tumoricidal and immunomodulatory effects of SBRT, this approach might expand the indications for and utility of RT and its integration with systemic agents.4
Reports of SBRT in the pediatric population have been limited primarily to case reports,5–7 small series,8 and practice surveys,9 together with some more recent, larger single-institution reports of palliative hypofractionated RT, stereotactic re-irradiation, and spine radiosurgery.10–12 For this young and developing patient population, concerns remain regarding increased adverse treatment effects in patients treated with hypofractionated regimens, and early reports have documented unique acute toxicities with this approach.5,8 The Children’s Oncology Group recently completed a pediatric clinical trial (NCT02306161) incorporating SBRT as a treatment option for bone metastasis when metastatic site RT was warranted, with results of this approach pending. Another multi-institutional clinical trial of SBRT for bone metastasis in pediatric and young adult patients with sarcoma (NCT01763970) recently completed accrual.
To better define the role of SBRT in pediatric patients and to identify variations in practice patterns, we reviewed disease outcomes, dosimetric patterns of local failure (LF), and treatment toxicity in patients treated with SBRT to non-CNS bone and soft tissue lesions at 2 high-volume cancer centers. We found that the patient demographics and tumor histologies were largely similar for the 2 treating institutions, but the indications for and treatment parameters of SBRT varied. Despite this, treatment responses as indicated by imaging and symptomatology were observed in most patients and the LF rates did not differ by institution. Similarly, SBRT was well tolerated, with limited acute or late toxicity. The LF rates were similar to those observed in larger studies of adults with advanced solid tumors treated with SBRT, and LF occurred predominantly within the high-dose irradiated volume.
METHODS AND MATERIALS
Patient cohort
In this institutional review board-approved (XPD16–118; 18–25067) multi-institutional study, 55 patients (median age, 16.7 years; range, 5.5–25.6 years) from 2 pediatric oncology clinical programs with 107 non-CNS lesions treated with SBRT between 2010 and 2016 and with at least 1 post-SBRT imaging study were reviewed. Based on the ASTRO practice guidelines for SBRT,13 radiotherapy inclusion criteria for this study included i) treatment with ≤10 fractions, ii) use of strict immobilization, and iii) use of stereotactic image guidance with a limited planning target volume (PTV).
SBRT, response assessments, and follow-up
Target volumes were delineated on computed tomography (CT) images, typically assisted by co-registration of magnetic resonance imaging (MRI) and/or positron emission tomography (PET)/CT. The gross tumor volume (GTV) encompassed the gross tumor. Anatomically constrained expansions of the GTV to delineate the clinical target volume (CTV) and the PTV were tailored based on case-specific variables, including tumor location and size, proximity to organs at risk, and prior receipt of same-site RT. For thoracoabdominal tumors, 4D CT or MRI simulation scans were obtained and an internal target volume was delineated to account for respiratory excursion. Daily cone-beam CT was obtained for all treatments at both institutions. The dose and fractionation patterns were individualized but generally consisted of 8 Gy × 5 fractions or 5 Gy × 8 fractions at Institution 1 and 5–7 Gy × 5 fractions at Institution 2. In general, more protracted courses (e.g., 8 Gy x 5) were employed for larger lesions (e.g., over 5 cm), while smaller lesions and/or lesions without close proximity to concerning or sensitive organs at risk were treated with a more aggressive hypofractionated regimen. Dose constraints were generally derived from the TG-101 report.14
Treatment intent was classified as definitive for those sites treated with the intention of providing durable local control through the delivery of an ablative RT dose or as palliative for those sites treated with the intention of improving or delaying symptoms. Treatment response by site was evaluated radiographically by RECIST v1.1 criteria15 for soft tissue lesions and according to the Children’s Oncology Group AEWS1221 clinical trial response criteria (NCT02306161) for bone lesions, and clinically through abstraction of symptom palliation upon review of electronic medical records. Objective radiographic response was defined as complete response (CR) or stable disease (SD) for bone lesions and CR, partial response (PR), or SD for soft tissue lesions, with duration of best radiographic response measured from the start of SBRT until progression or death, taking as reference for time point one the smallest measurements recorded since the start of SBRT. Treatment failure was defined anatomically as LF (within the anatomic primary tumor site) or distant failure (noncontiguous and distinct from the primary site) based on imaging and/or pathology. Local failure was further classified based on the recurrent tumor volume and associated RT dosimetry16 as in-field, when ≥80% of the recurrent tumor volume was within the 95% prescription isodose line (IDL); marginal, when 20%−80% of the recurrent tumor volume was within the 95% IDL; or out-of-field, when <20% of the recurrent tumor volume was within the 95% IDL. Receipt of concomitant chemotherapy by site was defined as administration of systemic therapy within a 2-week window before or after SBRT.
After receiving definitive-intent SBRT, patients were generally followed with a physical examination and 3D imaging, including CT, PET/CT, and/or MRI, of at least the treated site every 3 months. Patients treated with palliative-intent SBRT were typically evaluated with physical examination and imaging obtained at the discretion of the treating physician. Acute and late toxicities were graded according to CTCAE v4.0 (available at: http://ctep.info.nih.gov/reporting/ctc.html), assessed during RT and at each follow-up visit, and defined as early (occurring within 3 months after the start of RT) or late (occurring more than 3 months after the start of RT).
Statistical analysis
Comparisons of continuous data were made using the Wilcoxon rank-sum test; a chi-square test or Fisher’s exact test was used to compare categorical data. The duration of best radiographic response was defined as the time from the date of best response after SBRT to LF or death, whichever occurred first. The duration of symptom improvement was defined as the time from the date of symptom improvement after SBRT in patients with symptoms prior to SBRT until symptom progression. Progression-free survival (PFS) was defined as the time from the start of first SBRT to progression, i.e., to LF, distant failure, or death, whichever occurred first. Overall survival (OS) was defined as the time from the start of first SBRT to death from any cause. The probabilities of PFS and OS and the median duration of best radiographic response were estimated by the Kaplan–Meier method. Patients who did not experience an event were censored at the last follow-up date, which was defined as the last imaging date for all outcomes except symptom control, toxicity, and OS, which were based on the last clinical follow-up. The cumulative incidence of LF and the cumulative incidence of acute/late toxicity were estimated by a sub-distribution hazard model accounting for a competing risk event (death)17 and were compared using the Gray test. Cases with less than 3 months of follow-up imaging after SBRT were excluded from the analyses of the cumulative incidence of LF and PFS. Patients who died within 3 months of the end of SBRT were not included in the analysis of late toxicity. A joint frailty model that simultaneously models clustered risk and competing risk (death, in this case) was used to identify significant predictors of LF.18 A variable selection criteria of P < 0.3 was used to eliminate the nonsignificant effects in the variable selection process for the final multivariable model. Risk estimates, estimated by hazard ratios (HRs) and P values, and 95% confidence intervals (CIs) were calculated. The correlation of equivalent total dose in 2-Gy fractions (EQD2Gy),19 assuming an α/β ratio of 10, and the durations of radiographic response, symptom response, and local tumor control were evaluated in patients who experienced an event (radiographic response, symptomatic progression, LF, or death) and were assessed by the correlation coefficient r and plotted. Statistical analyses were performed using R version 3.6.0. A 2-sided significance level of P < 0.05 was considered statistically significant.
RESULTS
Clinical and treatment characteristics
In total, 55 children and young adults with recurrent or metastatic extra-CNS solid tumors were treated with SBRT to a total of 107 lesions at the 2 institutions between 2010 and 2016. Baseline characteristics of the overall study population and the study population of each institution are summarized in Table 1. The median durations of clinical and imaging follow-up were 11.0 months (range, 0.23–64.2 months) and 9.5 months (range, 0.07–79.2 months), respectively, and did not differ by institution. Except for race, demographic variables did not differ significantly between the institutions. The most commonly treated tumor histologies included non-rhabdomyosarcoma soft tissue sarcoma (n = 16), Ewing sarcoma (n = 13), and osteosarcoma (n = 13), while extra-pulmonary lesions comprised the majority of soft tissue lesions (67.4%). The tumor type treated and SBRT intent differed between the institutions, with bone lesions and definitive treatments being more frequent at Institution 1 and soft tissue lesions and palliative treatments being more frequent at Institution 2. Several treatment parameters differed significantly between the institutions, including the cumulative RT dose, BED10, EQD2Gy, number of fractions employed, and PTV expansions (Table 1).
Table 1.
Patient, tumor, and treatment characteristics
| Total cohort | Institution 1 | Institution 2 | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | N | % or median (range) | N | % or median (range) | N | % or median (range) | P |
| Age @ first SBRT (years) | 55 | 16.7 (5.5–25.6) | 30 | 15.0 (5.5–25.6) | 25 | 17.1 (9.9–25.2) | 0.18 |
| <12 | 10 | 10.2 (5.5–11.9) | 6 | 8.7 (5.5–10.9) | 4 | 11.3 (9.9–11.9) | |
| ≥12 | 45 | 17.8 (12.5–25.6) | 24 | 17.1 (12.5–25.6) | 21 | 18.1(13.5–25.2) | |
| Sex | 55 | -- | 30 | -- | 25 | -- | 0.33 |
| Male | 37 | 67% | 18 | 60% | 19 | 76% | |
| Female | 18 | 33% | 12 | 40% | 6 | 24% | |
| Race | 55 | -- | 30 | -- | 25 | -- | 0.002 |
| Black | 8 | 15% | 8 | 27% | 0 | 0% | |
| White | 42 | 76% | 18 | 60% | 24 | 96% | |
| Other | 5 | 9% | 4 | 13% | 1 | 4% | |
| Tumor histology | 55 | -- | 30 | -- | 25 | -- | 0.38 |
| Osteosarcoma | 13 | 24% | 8 | 27% | 5 | 20% | |
| Ewing sarcoma | 15 | 27% | 9 | 30% | 6 | 24% | |
| NRST | 16 | 29% | 9 | 30% | 7 | 28% | |
| Rhabdomyosarcoma | 5 | 9% | 1 | 3% | 4 | 16% | |
| Neuroblastoma | 2 | 4% | 0 | 0% | 2 | 8% | |
| Other | 4 | 7% | 3 | 10% | 1 | 4% | |
| Number of SBRT sites per patient | 55 | 1 (1–8) | 30 | 1 (1–8) | 25 | 1 (1–6) | 0.71 |
| Disease status at SBRT | 107 | -- | 66 | -- | 41 | -- | 0.67 |
| Newly diagnosed | 15 | 14% | 8 | 12% | 7 | 17% | |
| Recurrent | 92 | 86% | 58 | 88% | 34 | 83% | |
| Disease extent at SBRT | 107 | -- | 66 | -- | 41 | -- | 0.16 |
| Locally recurrent, solitary | 8 | 7% | 5 | 8% | 3 | 7% | |
| Metastatic, solitary | 10 | 9% | 9 | 14% | 1 | 2% | |
| Metastatic, multiple | 89 | 83% | 52 | 79% | 37 | 90% | |
| Tumor site receiving SBRT | 107 | -- | 66 | -- | 41 | -- | 0.11 |
| Primary site | 15 | 14% | 6 | 9% | 9 | 22% | |
| Metastatic site | 92 | 86% | 60 | 91% | 32 | 78% | |
| Lesion type receiving SBRT | 107 | -- | 66 | -- | 41 | -- | 0.04 |
| Bone | 64 | 60% | 45 | 68% | 19 | 46% | |
| Soft tissue | 43 | 40% | 21 | 32% | 22 | 54% | |
| SBRT intent | 107 | -- | 66 | -- | 41 | -- | <0.001 |
| Palliative | 49 | 46% | 21 | 32% | 28 | 68% | |
| Definitive | 58 | 54% | 45 | 68% | 13 | 32% | |
| Re-irradiation | 107 | -- | 66 | -- | 41 | -- | 0.58 |
| Yes | 17 | 16% | 12 | 18% | 5 | 12% | |
| No | 90 | 84% | 54 | 82% | 36 | 88% | |
| Concomitant chemotherapy | 107 | -- | 66 | -- | 41 | -- | 0.74 |
| Yes | 24 | 22% | 16 | 24% | 8 | 20% | |
| No | 83 | 78% | 50 | 76% | 33 | 80% | |
| Cumulative RT dose (Gy) | 107 | 35 (12–45) | 66 | 40 (20–45) | 41 | 25 (12 −40) | <0.001 |
| Dose (Gy)/fraction | 107 | 7 (4.5–25) | 66 | 6 (5–8) | 41 | 7 (4.5–25) | 0.12 |
| Fraction number | 107 | 5 (1–9) | 66 | 5 (3–9) | 41 | 5 (1–5) | <0.001 |
| BED10 (Gy) | 107 | 59.5 (26.1–87.5) | 66 | 60.5 (30.0–72.0) | 41 | 48.0 (26.1–87.5) | <0.001 |
| EQD2Gy (Gy) | 107 | 49.6 (21.8–72.9) | 66 | 50.0 (25.0–60.0) | 41 | 40.0 (21.8 – 72.9) | 0.002 |
| PTV expansion (mm) | 107 | 3 (0–10) | 66 | 2 (0–10) | 41 | 3 (3–5) | <0.001 |
| PTV volume (cm3) | 107 | 36.2 (1.1–4,208.0) | 66 | 32.4 (1.1–1,289.6) | 41 | 47.2 (3.8–4,208.0) | 0.14 |
| Clinical follow-up (mo) (patient) | 55 | 8.1 (0.23–64.2) | 30 | 9.6 (1.7–33.1) | 25 | 5.3 (0.23–64.2) | 0.27 |
| Imaging follow-up (mo) (patient) | 55 | 8.2 (0.07–79.2) | 30 | 8.8 (1.3–32.9) | 25 | 4.1 (0.07–79.2) | 0.22 |
| Clinical follow-up (mo) (site) | 107 | 11.0 (0.23–64.2) | 66 | 10.7 (1.7–34.8) | 41 | 13.9 (0.23–64.2) | 0.86 |
| Imaging follow-up (mo) (site) | 107 | 9.5 (0.07–79.2) | 66 | 9.3 (1.3–32.9) | 41 | 13.3 (0.07–79.2) | 0.77 |
Abbreviations: SBRT, stereotactic body radiotherapy; NRST, non-rhabdomyosarcoma soft tissue sarcoma.
Treatment responses
Of the 64 treated bone lesions, 58 (90.6%) had an objective response, with 16 having a CR and 42 exhibiting stable disease (SD) (Table 2). The median duration of objective response for bone lesions was 10.6 months (interquartile range (IQR), 4.2–25.8 months). An objective response was noted in 33 of 43 treated soft tissue lesions (76.7%), with 9 having a CR, 10 having a PR, and 14 exhibiting SD. The median duration of objective response for soft tissue lesions was 4.0 months (IQR, 2.5–9.2 months). Clinical symptoms were associated with 47 treated lesions, 29 (62%) of which showed improvement of symptoms. The median duration of symptom improvement in those patients who experienced symptom progression or death was 4.3 months (IQR, 1.6–6.8 months).
Table 2.
SBRT treatment responses by site
| Characteristic | N (%) or median (IQR or 95% confidence interval) |
|---|---|
| Radiographic response | 107 |
| Bone lesions | 64 (60%) |
| Rate of CR/SD | 58 (90.6%) |
| Duration of best response (mo) | 10.6 (4.2–25.8) |
| Soft tissue | 43 (40%) |
| Rate of CR/PR/SD | 33 (76.7%) |
| Duration of best response (mo) | 4.0 (2.5–9.2) |
| Symptom response | 47 |
| Rate of symptomatic improvement | 29 (62%) |
| Duration of symptom improvement (mo) | 4.3 (1.6–6.8) |
| Dosimetric pattern of local failure | 27 |
| Local failure type | |
| In-field | 14 (52%) |
| Marginal | 9 (33%) |
| Out-of-field | 4 (15%) |
Abbreviations: IQR, interquartile range; CR, complete response; PR, partial response; SD, stable disease; CI, cumulative incidence
Tumor control and survival
The estimated cumulative incidence of LF, with or without synchronous distant failure and with death as a competing risk at 6 months, was 12.5% (95% CI, 7.1%−21.5%), and the 1-year cumulative incidence of LF was 25.2% (95% CI, 17.2%−36.1%) (Fig. 1A). The cumulative incidence of LF was significantly higher for soft tissue lesions than for bone lesions (P = .03) (Fig. 1B). When comparing LF of soft tissue lesions by non-lung or lung vs. bone lesions, a significant increase in the cumulative incidence of LF of lung soft tissue lesions compared to bone lesions was observed (P = 0.03), while the LF of non-lung soft tissue lesions did not significantly differ to that of bone lesions (P = 0.12) (Supplementary Fig. 1). The 6-month and 1-year PFS probabilities were 56.5% (95% CI, 43.5%−73.3%) and 17.5% (95% CI, 9.0%−34.1%), respectively (Fig. 1C). The 6-month and 1-year OS probabilities were 78% (95% CI, 67.3%−89.7%) and 61.0% (95% CI, 48.9%−76.1%), respectively (Fig. 1D).
Figure 1.
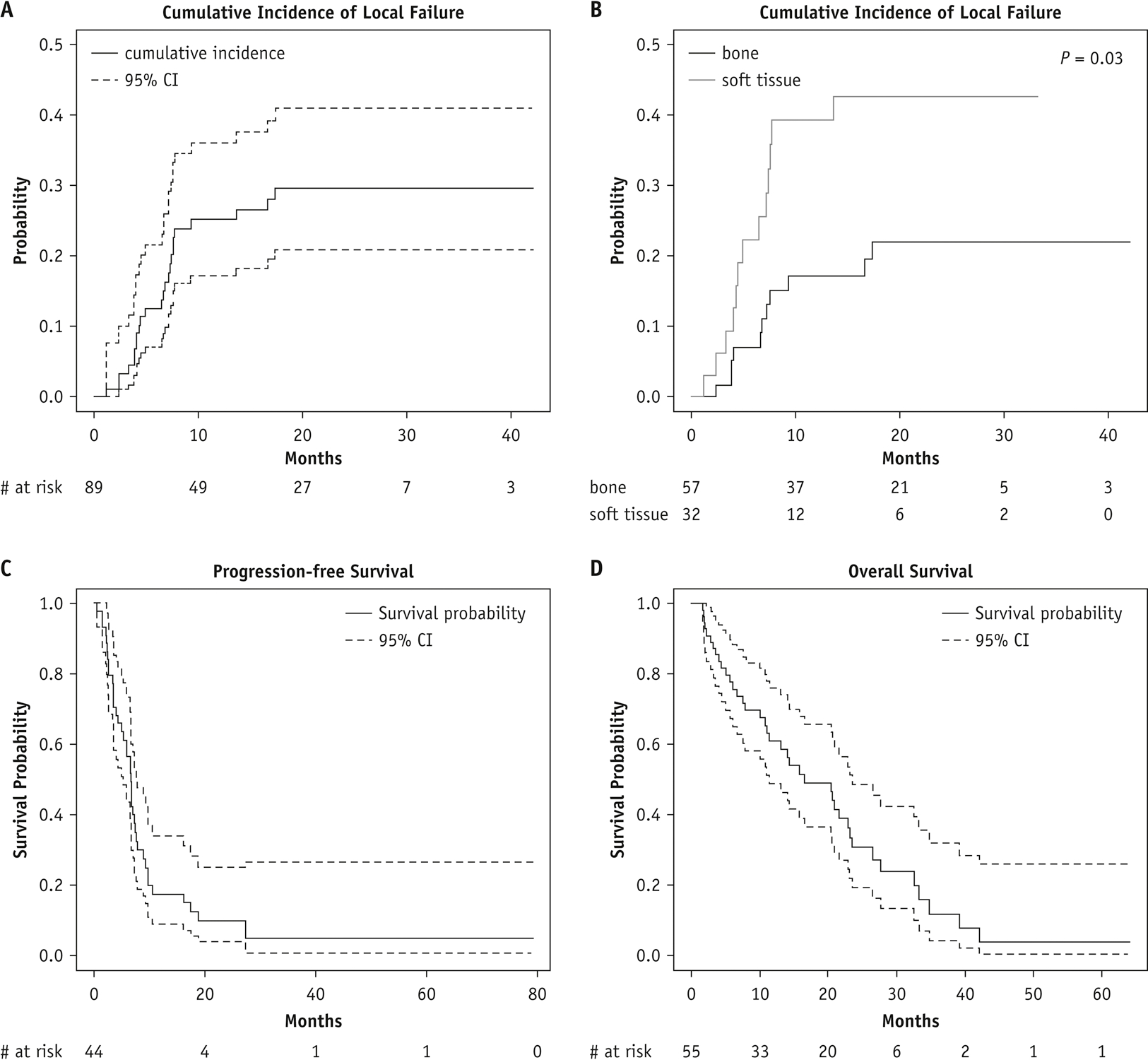
Tumor control and survival outcomes. Cumulative incidence of local failure in the evaluable cohort (A) and by SBRT tumor type (B) and Kaplan–Meier estimates of progression-free survival (C) and overall survival (D). CI, confidence interval. Note that y-axis units differ among panels.
A joint frailty model for clustered competing risks was used to identify significant predictors of LF within the overall patient cohort among the patient, tumor and treatment variables (Table 3). Univariable analysis revealed lesion type to be significantly associated with LF, with soft tissue lesions being at higher risk of LF when compared with bone lesions (HR = 3.12, 95% CI, 1.25–7.77; P = 0.02). On multivariable analysis incorporating variables with a P value of <0.3 on univariable analysis, only lesion type was significantly associated with increased risk of LF (HR = 2.8, 95% CI, 1.04–7.58; P = 0.04). Correlation analysis failed to demonstrate a strong correlation between EQD2Gy and time to LF, duration of best bone or soft tissue response, or duration of symptom improvement (Supplementary Fig. 2).
Table 3.
Univariable and multivariable joint frailty models with competing risk for local failure
| Univariable analysis | |||
|---|---|---|---|
| Covariate | HR | (95% CI) | P |
| Age @ SBRT (years) | 1.00 | (0.92–1.10) | 0.93 |
| Age group (≥12 years vs <12 years) | 1.13 | (0.33–3.88) | 0.84 |
| Sex (female vs male) | 0.62 | (0.21–1.84) | 0.39 |
| Race (black/other vs white) | 1.24 | (0.40–3.85) | 0.71 |
| Disease status (newly diagnosed vs recurrent) | 0.69 | (0.20–2.36) | 0.55 |
| Disease extent (locally recurrent vs metastatic) | 1.34 | (0.34–5.25) | 0.68 |
| Tumor histology (other vs osteosarcoma) | 0.45 | (0.16–1.25) | 0.13 |
| SBRT intent (definitive vs palliative) | 1.10 | (0.48–2.52) | 0.82 |
| SBRT lesion type (soft tissue vs bone) | 3.12 | (1.25–7.77) | 0.02 |
| SBRT tumor site (primary vs metastatic) | 0.55 | (0.15–2.05) | 0.37 |
| PTV volume (≥36.2 cm3 vs <36.2 cm3) | 0.59 | (0.25–1.39) | 0.23 |
| Cumulative RT dose (≥35 Gy vs <35 Gy) | 1.41 | (0.60–3.31) | 0.42 |
| BED10 (≥59.5 Gy vs <59.5 Gy) | 2.24 | (0.90–5.62) | 0.08 |
| EQD2Gy (≥50 Gy vs <50 Gy) | 1.65 | (0.66–4.14) | 0.29 |
| Concomitant chemotherapy (yes vs no) | 0.38 | (0.11–1.28) | 0.12 |
| Re-irradiation (yes vs no) | 1.10 | (0.34–3.56) | 0.87 |
| Treating institution (1 vs 2) | 1.39 | (0.52–3.75) | 0.51 |
| Multivariable analysis | |||
| Covariate | HR | 95% CI | P |
| Tumor histology (other vs osteosarcoma) | 0.41 | (0.12–1.32) | 0.13 |
| SBRT lesion type (soft tissue vs bone) | 2.80 | (1.04–7.58) | 0.04 |
| PTV volume (≥36.2 cm3 vs <36.2 cm3) | 0.43 | (0.16–1.18) | 0.10 |
| BED10 (≥59.5 Gy vs <59.5 Gy) | 2.39 | (0.50–11.51) | 0.28 |
| EQD2Gy (≥50 Gy vs <50 Gy) | 0.78 | (0.17–3.68) | 0.75 |
| PTV volume (≥36.2 cm3 vs <36.2 cm3) | 0.43 | (0.16–1.18) | 0.10 |
| Concomitant chemotherapy (yes vs no) | 0.31 | (0.09–1.08) | 0.07 |
Abbreviations: HR, hazard ratio; CI, confidence interval; SBRT, stereotactic body radiation therapy; EQD2Gy, equivalent total dose in 2-Gy fractions.
Patterns of local disease progression
Of the 107 lesions treated, 27 sites showed local progression over the course of radiographic follow-up. Assessment of the extent of tumor progression relative to the delivered RT dose distribution for these 27 lesions revealed that in-field progression (Vprogression95% > 80%) predominated, with 14 of 27 lesions showing this pattern of recurrence (Table 2 and Fig. 2). Marginal LF (Vprogression95% = 20%−80%) was observed in 9 lesions, and 4 lesions recurred locally with <20% of the progression volume being encompassed by the 95% IDL. Of the 27 sites with local progression, the most common tumor histologies were NRSTS (13 lesions) and osteosarcoma (8 lesions).
Figure 2.
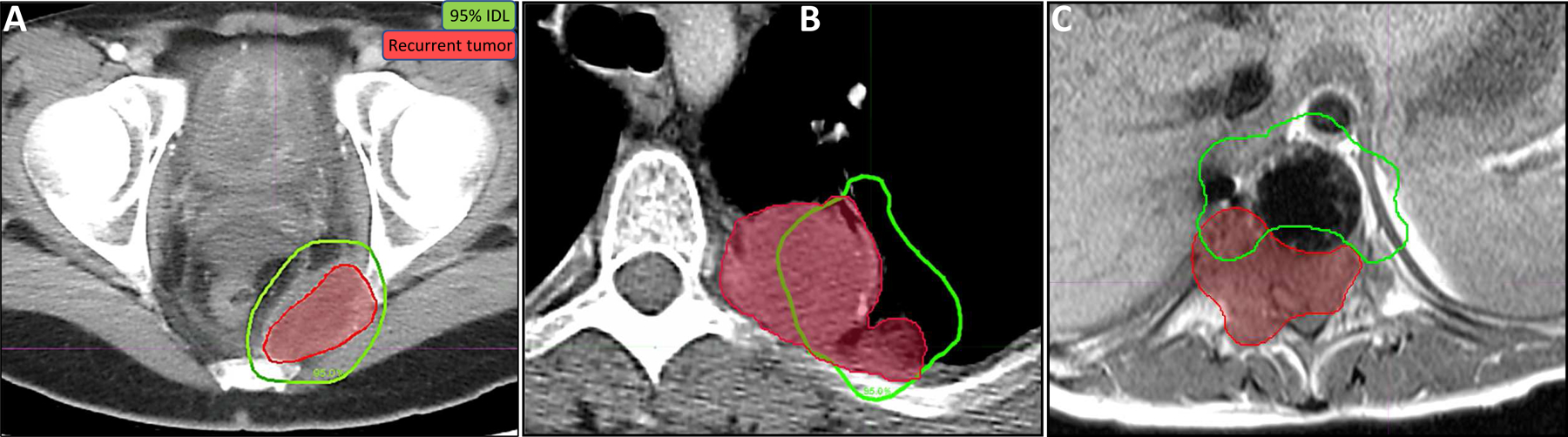
Local failure analysis. Recurrent tumor volumes (red) were delineated on 3D imaging failure scans (MRI, CT, and/or PET/CT) co-registered to CT simulation scans, and SBRT 95% prescription isodose lines (IDL) (green) were overlaid. Clinical examples of the three dosimetric failure types are shown: (A) in-field (>80% of the recurrence volume within the 95% IDL; axial CT); (B) marginal (20%−80% inside the 95% IDL; axial CT); and (C) out-of-field (<20% within the 95% IDL; axial MRI with metal suppression). Among the local failures, in-field failures predominated (A).
Treatment toxicity
The 3-month cumulative incidence of acute toxicity of any grade was 14.0% (95% CI, 8.7%−22.2%) (Fig. 3A). The most common types of grade 1 or 2 acute toxicity were radiation dermatitis (n = 6; grade 1 or 2), odynophagia/esophagitis (n = 3; grade 1 or 2), nausea (n = 3; grade 1 or 2), and chest wall pain (n = 2; grade 1). No grade 3 or higher acute events were observed. The 6-month cumulative incidence of late toxicity was 5.2% (95% CI, 2.2%−12.1%), and the 1-year cumulative incidence of late toxicity was 7.5% (95% CI, 3.6%−15.1%) (Fig. 3B). Grade 1 or 2 late toxicity consisted of myositis (n = 5; grade 1 or 2) and a T9 vertebral body fracture observed in the absence of disease progression at this site (grade 2). Grade 3 events included osteonecrosis of the distal radius (n = 1), which required curettage and cementation for stabilization, and radiation pneumonitis (n = 1), which resolved with prednisone. No grade 4 or 5 late events were observed.
Figure 3.
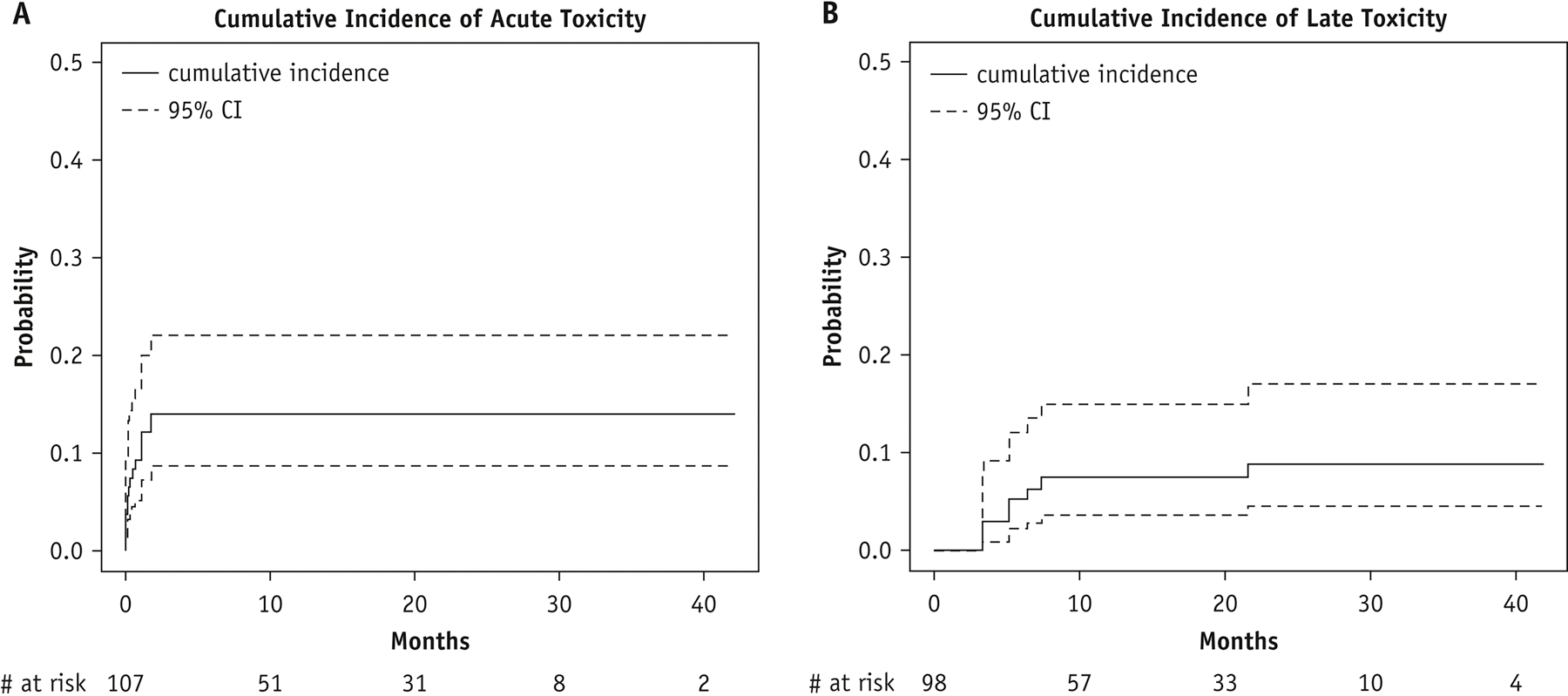
Cumulative incidence of any grade acute (A) and late toxicity (B) by site. CI, confidence interval
DISCUSSION
SBRT is an established treatment approach in the definitive, oligometastatic, and palliative settings for adults with solid tumors requiring RT. The ability to deliver tumor ablative doses accurately and precisely while minimizing exposure of surrounding normal tissues has resulted in primary tumor control rates of >90% in some adult patients with early stage cancer. Longer follow-up has demonstrated that this excellent local control can be accomplished without prohibitive late toxic effects.20,21 Although significant gains in PFS and OS have been observed in patients with limited oligometastatic burden treated with SBRT,3 durable local control rates after SBRT in adults with widely metastatic and/or recurrent disease have been less robust, and ongoing randomized studies (NCT03721341) are exploring the impact of SBRT in patients with more advanced metastatic disease.22–24 Similarly, across the limited reports of SBRT and hypofractionated RT in the pediatric setting, most of which have detailed outcomes in patients with advanced-stage disease, local control rates have ranged from 68% to 85%.8,10,12 Here, in one of the larger cohorts of pediatric patients with tumors managed with SBRT, we found a cumulative incidence of LF of 12.5% (95% CI, 7.1%−21.5%) at 6 months and 25.2% (95% CI, 17.2%−36.1%) at 1 year, comparable to those in adult and pediatric studies with similar study cohorts.
To identify significant predictors of LF and ultimately improve local tumor control outcomes, we developed a joint frailty model for clustered competing risk to better account for unobserved associations in patients treated at multiple tumor sites.25 Soft tissue lesions were associated with a significantly greater hazard of LF when compared with bone lesions in multivariable analysis across a range of patient, tumor, and treatment variables. The lesion type (soft tissue vs bone) also influences local control and survival outcomes in adults treated with SBRT for oligometastatic disease, with patients with bone metastases having improved survival when compared with those with other organ metastases.26–28 Factors postulated to drive this improved survival include enhanced setup immobilization and expanded target volumes that completely encompass a bone (e.g., a vertebral body) or a sub-compartment of a bone, as well as there being fewer and/or less critical organs at risk, with the associated dosimetric constraints.26,29 Although cumulative RT dose, BED10, and total EQD2Gy were not significantly associated with LF in multivariable analysis and did not correlate with radiographic or symptomatic responses, higher SBRT doses have been associated with improved local control in several studies in adults.30,31 This discordance may be related to the distinctiveness of the pediatric and young adult patient population with regard to long-term toxicity risk, which has influenced the adoption of reduced cumulative doses and dose/fraction regimens of SBRT in this setting. For example, the median BED10 for soft tissue lesions in this cohort was 60 Gy, whereas SBRT dose/fraction regimens in adults with primary or metastatic soft tissue lesions have generally resulted in a BED10 of ≥100 Gy.3,30 It is acknowledged that employing an α/β ratio of 10 for this study population may have limitations given that the most represented histology of our cohort was sarcoma and preclinical work suggests a ratio closer to 3–5 for this tumor type.36–38 However, based on the fact that our study population also included patients with non-sarcomatous tumors (carcinoma, NOS; Wilms; lymphoma; etc) and our desire to broadly compare BED values to adult studies employing SBRT where BED values have generally been derived using higher α/β ratios, as well as the understanding that the clinically relevant α/β ratios of the various sarcoma subtypes are still unknown,37,39 we choose to employ the more generalized α/β ratio of 10 for tumors. With continued imaging and technological advances, coupled with encouraging toxicity outcomes reported here and elsewhere, prospective assessment of more aggressive SBRT regimens in pediatric patients may be warranted, particularly for extra-CNS soft tissue lesions.
To further define local tumor progression relative to the delivered dose, as optimal PTV margins are undefined for the pediatric population, we evaluated the dosimetric pattern of failure for all sites with local anatomic progression after SBRT, similar to the conventions used previously for adults with pancreatic adenocarcinoma treated with SBRT and for adults with high-grade glioma.16,32 Importantly, the most common dosimetric failure type was in-field failure, suggesting that the target delineation and treatment targeting were sufficient for most cases. In contrast, of the 27 lesions with local progression, 9 had marginal progression and 4 had local progression with most of the recurrent tumor volume being outside the high-dose irradiated volume. Of these 13 lesions, 7 were paraspinal or vertebral body lesions for which target-volume coverage was limited by the spinal cord or cauda equina (Fig. 2), and we often observed paraspinal disease extension to the epidural space or adjacent vertebral bodies, as recently reported in pediatric patients treated with spine radiosurgery.12 Although the proportion of these marginal and out-of-filed LFs was similar to that in a prior dosimetric analysis in adults,16 consideration should be given to extending the PTV margins or employing conventional fractionation in cases in which paraspinal disease extension is a particular concern in order to improve local control rates. A limitation to this dosimetric analysis includes a lack of detailed assessment of SBRT plan quality, including dose heterogeneity and conformality metrics, which was limited due to the multi-institutional nature of the study. These metrics are relevant for the pediatric population given particular concerns for late toxicities and may have resulted in less heterogenous plans and impacted target coverage. As delivery of SBRT expands and more uniform RT guidelines develop in this population, prospective assessment of these metrics and the correlation with tumor control and acute and late effects will be critical to optimize this treatment approach.
The radiographic and symptomatic response rates observed in this study suggest that the imaging and palliative responses to SBRT are highly favorable, as was observed in several of the limited reports on pediatric SBRT.8,10 The rate of CR or SD for treated bone lesions, as assessed by RECIST criteria, was 90.6%, whereas soft tissue lesion responses of CR, PR, or SD, as assessed by the modified bone response criteria employed in the recently completed AEWS1221 clinical trial, were observed in 76.7% of treated lesions. Similarly, symptomatic improvement at sites presenting with baseline symptoms was observed in 62% of cases. Although the response criteria and the consideration of the imaging and/or clinical response varied, these radiographic and symptom-palliation outcomes compare favorably to those in studies in pediatric patients with metastatic solid tumors treated predominantly with more protracted RT courses.33,34 As has been observed in adults treated with palliative SBRT,2,35 this treatment technique could be an attractive alternative to longer treatment courses to maximize clinical responses and durable local control, as it is delivered in a manner that is convenient for patients and providers alike and that may also minimize interruptions to systemic therapy.
Despite promising local control rates and highly conformal treatment, the use of SBRT in pediatric patients has been limited by concerns over significant early toxicity and potential late effects. Indeed, several of the initial reports of SBRT in children and young adults highlighted particular toxicities with this technique, including severe myositis, sacral plexopathy, myonecrosis, fracture, and avascular necrosis.5,8 Factors possibly contributing to these toxicities included a short interval after prior RT, high-dose treatment, a large treatment volume, re-irradiation, and concomitant chemotherapy. However, subsequent studies found significant toxicity rates similar to those reported for SBRT in adults, including an incidence of any grade 3 or higher toxicity of 6.7% in a cohort of 62 patients with 104 treated lesions, 25% of which were treated with SBRT.10–12 Here, we found a 3-month cumulative incidence of acute toxicity of any grade of 14.0%, with no grade 3 or higher events, and a 1-year cumulative incidence of late toxicity of 7.5%, including 2 grade 3 events, which compare favorably with published outcomes of SBRT in adult and pediatric patients.10,20 In this case, neither of the grade 3 late events involved re-irradiation or concomitant chemotherapy; the SBRT regimens were relatively modest, with 1 site being treated with 8 Gy × 5 fractions and 1 site with 20 Gy in a single fraction; and the tumor volumes were relatively small (14.3 cm3 and 3.8 cm3, respectively). Despite these encouraging findings, as would be expected in a patient population with metastatic and/or recurrent solid tumors, long-term follow-up of our study has been limited, and caution should be exercised with this particularly vulnerable young population for whom, with improvements in treatment and the advancement of SBRT into the primary disease setting, long-term survival may become a more realistic prospect.
Supplementary Material
Acknowledgments:
The authors would like to thank Keith A. Laycock, PhD, ELS, for scientific editing of the manuscript and Melissa Gargone, MHS, RT(T), CMD, for assistance with radiation dosimetry.
Funding:
This work was supported in part by the American Lebanese Syrian Associated Charities (ALSAC), by National Cancer Institute grant P30 CA021765 (St. Jude Cancer Center Support Grant), and by the William M. Wood Foundation (D.H.K). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: S.G.D. has received fees for consulting and advisory board roles from Bayer and Loxo Oncology and has received travel expenses from Loxo Oncology, Roche, and Salarius.
Data sharing: Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
REFERENCES
- 1.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen QN, Chun SG, Chow E, et al. Single-fraction stereotactic vs conventional multifraction radiotherapy for pain relief in patients with predominantly nonspine bone metastases: a randomized phase 2 trial. JAMA Oncol 2019;5:872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. doi: 10.1200/JCO.20.00818 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 4.Marciscano AE, Haimovitz-Friedman A, Lee P, et al. Immunomodulatory effects of stereotactic body radiation therapy: preclinical insights and clinical opportunities. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2019.02.046 [Epub ahead of print] [DOI] [PubMed]
- 5.Taunk NK, Kushner B, Ibanez K, Wolden SL. Short-interval retreatment with stereotactic body radiotherapy (SBRT) for pediatric neuroblastoma resulting in severe myositis. Pediatr Blood Cancer 2016;63:731–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiniker SM, Rangaswami A, Lungren MP, et al. Stereotactic body radiotherapy for pediatric hepatocellular carcinoma with central biliary obstruction. Pediatr Blood Cancer 2017;64:e26330. [DOI] [PubMed] [Google Scholar]
- 7.Deck J, Eastwick G, Sima J, Raymond A, Bogart J, Aridgides P. Efficacy and tolerability of stereotactic body radiotherapy for lung metastases in three patients with pediatric malignancies. Onco Targets Ther 2019;12:3723–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown LC, Lester RA, Grams MP, et al. Stereotactic body radiotherapy for metastatic and recurrent ewing sarcoma and osteosarcoma. Sarcoma 2014;2014:418270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alcorn S, Nilsson K, Rao AD, et al. Practice patterns of stereotactic radiotherapy in pediatrics: results from an international pediatric research consortium. J Pediatr Hematol Oncol 2018;40:522–526. [DOI] [PubMed] [Google Scholar]
- 10.Lazarev S, Kushner BH, Wolden SL. Short hypofractionated radiation therapy in palliation of pediatric malignancies: outcomes and toxicities. Int J Radiat Oncol Biol Phys 2018;102:1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gultekin M, Cengiz M, Sezen D, et al. Reirradiation of pediatric tumors using hypofractionated stereotactic radiotherapy. Technol Cancer Res Treat 2017;16:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsai S, Juloori A, Angelov L, et al. Spine radiosurgery in adolescents and young adults: early outcomes and toxicity in patients with metastatic Ewing sarcoma and osteosarcoma. J Neurosurg Spine. doi: 10.3171/2019.9.SPINE19377 [Epub ahead of print] [DOI] [PubMed]
- 13.Potters L, Kavanagh B, Galvin JM, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2010;76:326–332. [DOI] [PubMed] [Google Scholar]
- 14.Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 2010;37:4078–4101. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 16.Baine MJ, Sleightholm R, Lin C. Incidence and patterns of locoregional failure after stereotactic body radiation therapy for pancreatic adenocarcinoma. Pract Radiat Oncol 2019;9:e29–e37. [DOI] [PubMed] [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 18.Rondeau V, Pignon JP, Michiels S, on behalf of the Mach-NC Collaborative Group. A joint model for the dependence between clustered times to tumour progression and deaths: a meta-analysis of chemotherapy in head and neck cancer. Stat Methods Med Res 2015;24:711–729. [DOI] [PubMed] [Google Scholar]
- 19.Barton M Tables of equivalent dose in 2 Gy fractions: a simple application of the linear quadratic formula. Int J Radiat Oncol Biol Phys 1995;31:371–378. [DOI] [PubMed] [Google Scholar]
- 20.Timmerman RD, Hu C, Michalski JM, et al. Long-term results of stereotactic body radiation therapy in medically inoperable stage I non-small cell lung cancer. JAMA Oncol 2018;4:1287–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King CR, Freeman D, Kaplan I, et al. Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol 2013;109:217–221. [DOI] [PubMed] [Google Scholar]
- 22.Levine AM, Coleman C, Horasek S. Stereotactic radiosurgery for the treatment of primary sarcomas and sarcoma metastases of the spine. Neurosurgery 2009;64:A54–A59. [DOI] [PubMed] [Google Scholar]
- 23.Chang UK, Cho WI, Lee DH, et al. Stereotactic radiosurgery for primary and metastatic sarcomas involving the spine. J Neurooncol 2012;107:551–557. [DOI] [PubMed] [Google Scholar]
- 24.Miller JA, Balagamwala EH, Angelov L, et al. Stereotactic radiosurgery for the treatment of primary and metastatic spinal sarcomas. Technol Cancer Res Treat 2017;16:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hougaard P Frailty models for survival data. Lifetime Data Anal 1995;1:255–273. [DOI] [PubMed] [Google Scholar]
- 26.Milano MT, Katz AW, Zhang H, Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys 2012;83:878–886. [DOI] [PubMed] [Google Scholar]
- 27.Tree AC, Khoo VS, Eeles RA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol 2013;14:e28–e37. [DOI] [PubMed] [Google Scholar]
- 28.Milano MT, Katz AW, Okunieff P. Patterns of recurrence after curative-intent radiation for oligometastases confined to one organ. Am J Clin Oncol 2010;33:157–163. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharya IS, Woolf DK, Hughes RJ, et al. Stereotactic body radiotherapy (SBRT) in the management of extracranial oligometastatic (OM) disease. Br J Radiol 2015;88:20140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:1623–1631. [DOI] [PubMed] [Google Scholar]
- 31.Bibault JE, Dewas S, Vautravers-Dewas C, et al. Stereotactic body radiation therapy for hepatocellular carcinoma: prognostic factors of local control, overall survival, and toxicity. PLoS One 2013;8:e77472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan JL, Lee SW, Fraass BA, et al. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol 2002;20:1635–1642. [DOI] [PubMed] [Google Scholar]
- 33.Paulino AC. Palliative radiotherapy in children with neuroblastoma. Pediatr Hematol Oncol 2003;20:111–117. [DOI] [PubMed] [Google Scholar]
- 34.Rahn DA 3rd, Mundt AJ, Murphy JD, Schiff D, Adams J, Murphy KT. Clinical outcomes of palliative radiation therapy for children. Pract Radiat Oncol 2015;5:183–187. [DOI] [PubMed] [Google Scholar]
- 35.Ryan JF, Rosati LM, Groot VP, et al. Stereotactic body radiation therapy for palliative management of pancreatic adenocarcinoma in elderly and medically inoperable patients. Oncotarget 2018;9:16427–16436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thames HD and Suit HD. Tumor radioresponsiveness versus fractionation sensitivity. Int J Radiat Oncol Biol Phys 1986;12:687–691. [DOI] [PubMed] [Google Scholar]
- 37.Haas RLM, Miah AB, LePechoux C, et al. Preoperative radiotherapy for extremity soft tissue sarcoma; past, present and future perspectives on dose fractionation regimens and combined modality strategies. Radiother Oncol 2016;119:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leeuwan CM, Oei AL, Crezee J, et al. The alfa and beta of tumours: a review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiat Oncol 2018;13:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montero A, Nunez M, Hernando O, Vicente E, et al. Retroperitoneal soft-tissue sarcomas: Radiotherapy experience from a tertiary cancer center and review of current evidence. Rep Pract Oncol Radiother 2020;25:643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


