Abstract
Acute kidney injury (AKI) is common in critically ill patients and renal replacement therapy (RRT) constitutes an important aspect of acute management during critical illness. Continuous renal replacement therapy (CRRT) is frequently utilized in ICU settings, particularly in patients with severe AKI, fluid overload, and hemodynamic instability. The main goal of CRRT is to timely optimize solute control, acid-base, and volume status. Total effluent dose of CRRT is a deliverable that depends on multiple factors and therefore should be systematically monitored (prescribed vs delivered) and iteratively adjusted in a sustainable mode. In this manuscript, we review current evidence of CRRT dosing and provide recommendations for its implementation as a quality indicator of CRRT delivery.
Keywords: acute kidney injury, quality metric, quality indicator, continuous renal replacement therapy, dose, CRRT, AKI
Introduction
Acute kidney injury (AKI) is a frequent complication encountered in critically ill patients admitted to Intensive Care Units (ICU) and is associated with increased morbidity and mortality. About 5 to 15% of critically ill patients with AKI in the ICU require renal replacement therapy (RRT) (1-3), which carries mortality rates up to 70% (4-7). In the multinational prospective AKI-EPI study, 57% of ICU patients had AKI and 13.5% required RRT; most of them (75%) in the form of continuous renal replacement therapy (CRRT) (8).
The dose of CRRT remains a highly variable CRRT deliverable as it encompasses a broad concept of clearance with multiple challenges for its measurement and application (9). Like other extracorporeal support treatment, the provision of CRRT requires a timely prescription and a specific dose to achieve its main goals of solute and volume control. The dose of CRRT relates to clearance measured as the removal rate of urea, which is a small solute with a sieving coefficient of approximately 1 (10,11). There are several patient-and machine-specific factors that influence CRRT dosing in critically ill patients with AKI in the ICU. Therefore, total effluent dose of CRRT should be evaluated frequently to accommodate timely changes according to specific goals of therapy (12).
The optimal delivery of CRRT requires iterative assessment, coordination and communication among multiple stakeholders in the ICU. The systematic evaluation of CRRT quality indicators/metrics (e.g., CRRT dose) is a critical first step in the development of quality assurance systems that support CRRT delivery. The selection of quality indicators and the goals of quality assurance systems should be customized to specific logistics and needs of each institution (13). In this manuscript, we review current evidence of CRRT dosing and provide recommendations for its implementation as a quality indicator of CRRT delivery.
CRRT dose as a novel quality indicator: why is it important?
The optimal delivery of CRRT should be a dynamic, precise and sustainable process that is informed by evidence-based medicine and accommodates precision-medicine in the acutely ill (10). The concept of dynamic CRRT delivery consists of adapting the treatment according to time-varying changes in the clinical status of the critically ill patient, which implies that audits, feedback and quality indicators are incorporated in the decision-making process (14, 15).
There is wide variation in the delivery of CRRT across centers and there are limited data on validation of CRRT quality indicators/metrics and their impact on clinical and patient-centered outcomes (16). Therefore, development of quality assurance programs represents one way to continuously measure, monitor, communicate, and improve specific aspects of CRRT delivery (14). In this context, delivered CRRT dose (Figure 1) represents an ideal quality indicator of CRRT adequacy which can be monitored by the ratio of delivered to prescribed CRRT dose and should be maintained above 0.8 on average (Figure 2) (11, 16).
Figure 1:
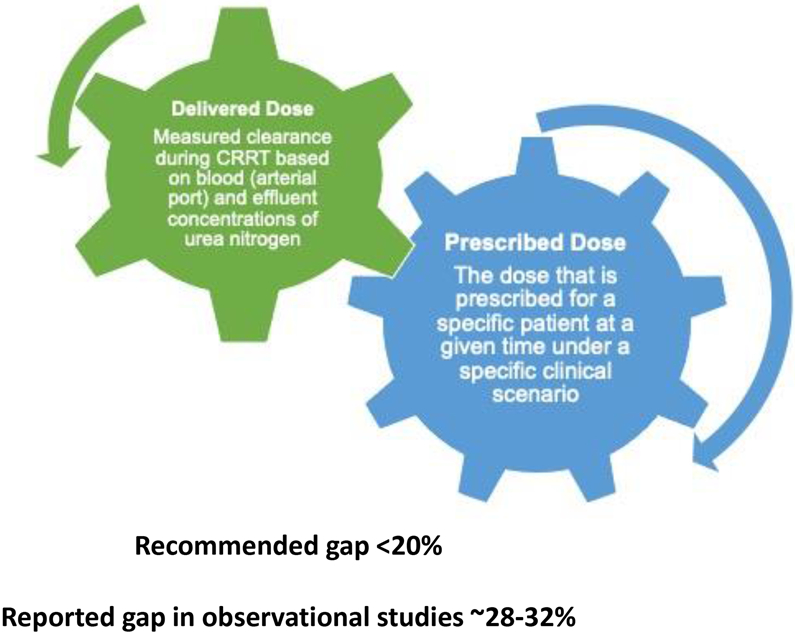
Conceptual difference between prescribed and delivered CRRT dose
Figure 2:
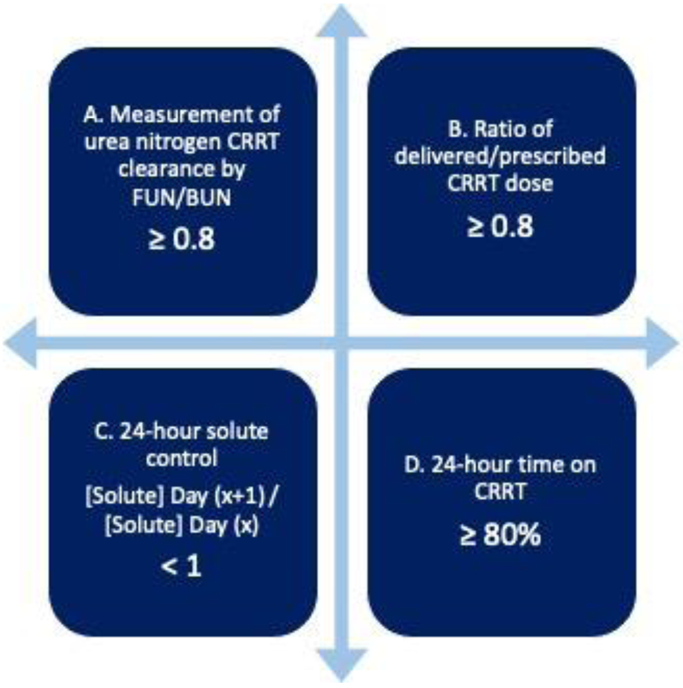
Quality indicators related to delivered CRRT dose. A) Clearance of urea nitrogen delivered with CRRT can be measured by the ratio of effluent urea nitrogen to blood urea nitrogen (arterial port) and should be maintained above 0.8 on average; B) The ratio of delivered to prescribed CRRT dose should be also maintained above 0.8 on average; C) Change in blood concentrations of small solutes (e.g., urea nitrogen) evaluated in 24-hour cycles can also indicate CRRT adequacy for solute removal; D) Time on CRRT is a critical aspect to effectively deliver the prescribed CRRT dose.
A recent study identified 18 potential quality indicators to evaluate CRRT delivery by using the Donabedian domains of structure, processes of care and patient outcomes. The most commonly used quality metrics focused on filter lifespan, small solute clearance, bleeding, delivered dose, and treatment interruptions, although there was significant heterogeneity in the definitions (17). Later, using a Delphi process, a list of 13 quality indicators (including delivered CRRT dose) were proposed for inclusion into routine clinical practice and for development of a quality assurance panel to monitor CRRT delivery (18).
Frequent training of the nursing and clinician staff is a strategy that has been shown to decrease the occurrence of unplanned CRRT interruptions to optimize delivered CRRT dose (19). Mottes et al reported the implementation of a CRRT quality dashboard based on categories such as filter life, prescribed vs. delivered dose and fluid balance for the evaluation of adherence to evidence-based practices and tracking of quality indicators of CRRT. The authors observed that the proportion of patients achieving at least 90% of the prescribed dose increased from 87 to 100% by reinforcement of education strategies and continuous monitoring through the dashboard (20).
Practical considerations to determine CRRT dose
The total effluent fluid rate is a surrogate of solute clearance provided by CRRT and is used to determine the dose of CRRT in diffusive, convective and hybrid modalities. The total effluent rate is typically reported in milliliters per hour and adjusted by the patient’s weight in kilograms (ml/kg/hr). The determination of the total effluent rate varies according to CRRT modality. In continuous venovenous hemofiltration (CVVH, convective clearance), it is equivalent to the total ultrafiltration rate (the sum of pre-filter replacement fluid rate, post-filter replacement fluid rate and the patient’s net fluid removal rate). In continuous venovenous hemodialysis (CVVHD, diffusive clearance), it is the sum of dialysate fluid rate plus the patient’s net fluid removal rate, and in continuous venovenous hemodiafiltration (CVVHDF, convective and diffusive clearance) the sum of dialysate fluid rate and total ultrafiltration rate (1,21) (Table 1).
Table 1.
Calculation of Total Effluent Fluid Rate according to CRRT Modality
| CVVH: Total Ultrafiltration (UF) Rate (ml/hr) + Fluid Removal Rate (ml/hr) |
| ■ Example: CVVH: 1400 ml/hr + 200 ml/hr Effluent flow rate= 22.8 ml/kg/hr |
| CVVHD: Dialysate Rate (ml/hr) + Fluid Removal Rate (ml/hr) |
| ■ Example: CVVHD: 1000 ml/hr + 200 ml/hr. Effluent flow rate= 17.1 ml/kg/hr |
| CVVHDF: Total UF Rate (ml/hr) + Dialysate Rate (ml/hr) + Fluid Removal Rate (ml/hr) |
| ■ Example: CVVHDF: 1400 ml/hr + 800 ml/hr + 200 ml/hr Effluent flow rate= 34.2 ml/kg/hr |
| Dilution factor for Predilution: Plasma flow rate (ml/hr)/ [Plasma Flow Rate (ml/hr) + Pre-Filter Replacement Fluid Rate (ml/hr)] |
| Total Ultrafiltration (UF) Rate (ml/hr) = Pre-Filter Replacement Fluid Rate (ml/hr) + Post-Filter Replacement Fluid Rate (ml/hr) Plasma Flow Rate (ml/hr) = Blood Flow Rate (ml/min) x 60 (min/hr) x (1-HCT); where HCT is the current hematocrit of the patient. |
| Example: Weight 70 kg, HCT= 30%, Blood Flow Rate= 150 ml/min, Pre-Filter Replacement= 1000 ml/hr, Post-Filter Replacement= 400 ml/hr, Dialysate rate 800 ml/hr, Fluid Removal Rate= 200 ml/hr |
When using pre-filter replacement fluid in CVVH or CVVHDF modes, the blood entering the circuit is diluted and therefore clearance is decreased. In this setting, the total effluent fluid rate should be multiplied (adjusted) by a dilution factor that is calculated as follows:
When using diffusive clearance (CVVHD), dialysate flow rate, concentration gradients, and filter surface area -the latter to a lesser extend- are the main determinants of clearance (22). When using convective clearance (CVVH or CVVHDF), one should recognize the concept of filtration fraction (FF), which is the proportion of plasma water entering the filter that is removed by ultrafiltration. FF is inversely proportional to the blood flow rate (BFR). Therefore, a low BFR (<100 ml/min) could increase the risk of clotting due to stasis of blood, while a high BFR (>250 ml/min) could decrease circuit lifespan due to vascular access issues (23). The use of prefilter replacement fluid helps to maintain a lower FF by diluting the blood but to the expense of a decrease in small solute clearance. In a study by Troyanov et al, an efficiency loss for urea clearance of about 35% was reported when using CVVH with prefilter replacement fluid (24). For this reason, it is recommended -in adult patients- to keep a BFR of 150 to 200 ml/min, particularly when using replacement fluid rates of more than 1500 ml/hr (24, 25).
There is no evidence-based consensus in relation to which patient’s weight to use (admission weight, ideal weight, or current weight) when determining CRRT dose. However, it is recommended to use the current weight of the patient (at the time of determining CRRT dose) as it theoretically accommodates acute increases in the volume of distribution due to fluid overload. The KDIGO 2012 clinical practice guidelines suggest delivering an average effluent dose of 20 to 25 ml/kg/hr for patients with AKI requiring CRRT (26). However, the prescribed dose is not always delivered due to CRRT interruptions related to off-room procedures or circuit downtime due to clotting/clogging, replacing filters, bag/tubing changes or dialysis catheter malfunction. Therefore, evaluation of the delivered dose and solute/volume control goals are recommended to adjust the CRRT prescription (27). The CRRT dose could be tailored to accommodate specific patient’s needs at any given time and should account for possible hindrances that potentially preclude the goals of therapy.
One way to measure delivered CRRT dose is using the clearance equation of U/P x V; in which U represents the urea nitrogen concentration in the effluent fluid, P represents the blood urea nitrogen concentration entering the circuit (arterial port), and V represents the total effluent fluid rate (28). The ratio of effluent fluid urea nitrogen [FUN] by blood urea nitrogen [BUN] should be approximately 1 at the beginning of treatment; however, FUN/BUN declines in parallel to a decrease in filter efficiency (29). Multiple factors affect filter efficiency such as solute concentration polarization, solute fouling on the membrane surface (adsorption) and filter degradation/clogging (30, 31). Therefore, FUN/BUN is a valid measure of small solute clearance that can be helpful in certain clinical scenarios in which clearance needs to be properly quantified.
Differentiating prescribed versus delivered CRRT dose
Venkataraman et al found that only 60% of the prescribed dose was effectively delivered in patients on CVVHD (32). In the DO-RE-MI study, a multicenter observational study of RRT practices in critically ill patients, only 22% of patients prescribed a total effluent fluid rate of ≥35 mL/kg/hr received the full dose (33). In another study, Claure Del Granado et al found that the delivered dose was only 73% of the prescribed dose when evaluated by measured urea clearance (FUN/BUN ratio) in patients treated with predilution CVVHDF. The decrease in urea clearance was attributed to circuit downtime and predilution replacement fluid (29). Similarly, Zhang et al demonstrated in 60 patients receiving CVVH that the delivered dose was 9.3% lower than the prescribed dose, and this difference increased progressively over time as filter degraded. Furthermore, the authors showed that the difference between prescribed and delivered CRRT dose positively correlated with transmembrane pressures, indicating that filter clogging may have played a role in the observed reduction in delivered dose (34).
The use of heparin or regional citrate anticoagulation may prevent interruptions due to filter clotting. When using regional citrate anticoagulation (anticoagulant citrate dextrose solution A), a relationship between BFR and citrate rate should be maintained (typically 1:1.5-2) to achieve a blood citrate concentration of 4 to 6 mmol/L in the extracorporeal circuit (circuit ionized calcium of <0.35 mmol/L) (35). When CRRT is delivered without regional citrate anticoagulation, BFR is typically maintained at ≥200 ml/min to theoretically increase filter life by attenuating FF. However, in a randomized controlled trial comparing CRRT without regional citrate anticoagulation with BFR of 150 vs. 250 mL/min there was no difference in circuit clotting or median circuit lifespan of first CRRT circuit in both groups (9.1 vs 10 hours, p=0.37, respectively) (36).
Interruptions during CRRT can unintendedly occur due to machine malfunction alarms, filter clogging/clotting, dialysis catheter dysfunction, bag/tubing changes; or intendedly occur due to interruptions for surgical or radiological procedures that require mobilizing the patient outside the ICU room. However, there are no evidence-based proven strategies to compensate for the decrease in delivered CRRT dose due to circuit downtime. Therefore, one should account for this when prescribing CRRT to accommodate 10-15% of circuit downtime on average, meaning the total prescribed effluent dose should be 25-30 ml/kg/hr (10-15% above the recommended delivered effluent dose) (37). The concepts of average delivered CRRT dose, the dose delivered over the effective treatment time, and quality indicators related to delivered CRRT dose are represented in Figures 1 and 2 (11, 16).
Interventional studies examining CRRT dose in heterogeneous ICU populations
There have been several interventional studies examining the relationship of CRRT dose with overall survival and kidney recovery among critically ill patients on CRRT as shown in Table 2 (38).
Table 2.
Randomized controlled trials assessing dose of CRRT in critically ill patients with AKI
| Study | Population | Dose Comparison | Primary Outcome | Comments |
|---|---|---|---|---|
|
Ronco, et al. (9) SS-RCT |
N= 425 Predominantly postsurgical AKI | Post dilution CVVHF 20 mL/kg/hr vs 35 mL/kg/hr vs 45 mL/kg/hr |
15-day survival 59% vs 43% vs 42% *p<0.002 for comparison of 20 vs 35 and p=NS for comparison of 35 vs 45 |
90-95% recovery of kidney function in survivors |
|
Bouman, et al. (25) 2C-RCT |
N= 106 Postsurgical AKI | CVVH 72-96 L/day early vs 24-36 L/day early vs 24-36 L/day late |
28-day survival 74% vs 69% vs 75% *p=NS for comparisons 24-36 L/day early vs late |
All hospital survivors had kidney recovery at discharge, except for one patient |
|
Saudan, et al. (5) SS-RCT |
N= 206 Multifactorial AKI | CVVH: 1-2.5 L/hr vs CVVHDF: 1-2.5 L/hr HF + 1-1.5 L/h HD |
90-day survival 34% vs 59% (p=0.0005) |
75% of survivors had recovery of kidney function at 90 days |
|
Tolwani, et al. (26) SS-RCT |
N= 200 Multifactorial AKI | Predilution CVVHDF 20 ml/kg/hr vs 35 ml/kg/hr |
30-day survival 49% vs 56%(p=NS) |
No differences in kidney recovery at either ICU or hospital discharge |
|
ATN (29) MC-RCT |
N=1124 Multifactorial AKI | Predilution CVVHDF 35 ml/kg/hr or 6/week SLEDD or 6/week IHD vs Predilution CVVHDF 20 ml/kg/hr or 3/week SLEDD or 3/week IHD |
60-day mortality 46% vs 48% (p=NS) |
At day 28, no differences in complete or partial recovery of kidney function |
|
RENAL (30) MC-RCT |
N=1508Ventilated patients, septic AKI | Postdilution CVVHDF 40 ml/kg/hr vs 25 m/kg/hr |
90-day mortality 55% vs 55% (p=NS) |
No difference in recovery of kidney function or dependence on RRT |
2C-RCT, Two-Center Randomized Controlled Trial. AKI, Acute Kidney Injury. CVVH, Continuous Venovenous Hemofiltration. CVVHD, Continuous Venovenous Hemodialysis. CVVHDF, Continuous Venovenous Hemodiafiltration HD, Hemodialysis; HF, Hemofiltration; ICU, Intensive Care Unit; IHD, Intermittent Hemodialysis; MC-RCT, Multi-Center Randomized Controlled Trial; NS, Not Significant; RRT, Renal Replacement Therapy; SLEDD, Sustained Low Efficiency Daily Dialysis. SOFA, Sequential Organ Failure Assessment; SS-RCT, Single-Center Randomized Controlled Trial.
Ronco et al tested different doses of CVVH and did not find a difference in mortality when effluent doses of 45 vs. 35 ml/kg/hr were compared (10). Similarly, there was no difference in mortality or kidney recovery when higher doses of CVVH or CVVHDF (35-48 ml/kg/hr) were compared to a standard dose of 20 ml/kg/hr (39,40). Only one study reported improved survival at 28 and 90 days with higher doses of CVVH (42 vs. 25 ml/kg/hr) (5). Overall, the aforementioned studies were relatively small and/or single center. In this context, two large multicenter clinical trials, the Acute Renal Failure Trial Network (ATN) and the Randomized Evaluation of Normal versus Augmented Level (RENAL), were conducted and concluded that there were no differences in mortality or kidney recovery outcomes when high intensity (35-40 ml/kg/hr) vs. standard intensity (20-25 ml/kg/hr) CRRT doses were compared (41-44).
The conclusions of these two large interventional studies were further supported by the 2016 Cochrane systematic review stating that higher dose of CRRT does not impact favorably on mortality or recovery of kidney function in critically ill patients with AKI and increases the risk of hypophosphatemia (45). Two subsequent metanalysis concluded that higher intensity RRT does not impact mortality outcome (46, 47). Finally, Combes et al randomized patients with post-cardiac surgery shock and severe AKI to delayed CVVHDF (standard indications) vs. early high-volume hemofiltration (HVHF, 80ml/kg/hr) for 48 h followed by standard-dose CVVHDF (<35 ml/kg/hr) until resolution of shock and kidney recovery and did not observe differences in mortality rates at 30 days (48).
Recent studies have tested the hypothesis that high-volume hemofiltration (HVHF) may improve hemodynamics and mortality in critically ill septic patients with AKI by removal of pro-inflammatory cytokines; however, no difference was found in mortality outcomes (49-52). A recent metanalysis by Clark et al included 4 randomized clinical trials examining potential benefits of HVHF in critically ill septic patients with AKI and concluded that current evidence is insufficient to support the routine use of HVHF in this specific subpopulation. (53) More interventional studies are needed to further assess the non-selective convective clearance of cytokines in specific subpopulations such as critically ill septic patients.
How to operationalize the systematic monitoring of CRRT dose?
The approach to the systematic evaluation of CRRT dose as a quality indicator of CRRT delivery should be supported by evolving implementation science and logistics specific to each institution. Some previously reported interventions for improving the average delivered CRRT dose include the development of electronic flowsheets to continuously report delivered CRRT dose according to pre-determined time intervals, the standardization of CRRT prescription and documentation templates, and the enhancement of education to nurses, clinicians and the overall multidisciplinary CRRT team. (13, 54).
A recent study by Griffin et al demonstrated an improvement in mean delivered CRRT dose in patients receiving CVVH by implementing a quality assurance program. The intervention consisted of 1) incorporating an average 24-hour CRRT delivered dose into the electronic flowsheet; 2) adding CRRT delivered dose to the procedure note; 3) modifying the CRRT order set to display dose calculations; and 4) educational sessions. Before the intervention, only 279 of 837 (33%) treatments achieved an average daily delivered effluent dose of 20 to 25 ml/kg/hr but following the intervention, 631 of 952 (66%) treatments achieved an average daily delivered effluent dose of 20 to 25 ml/kg/hr, as recommended by KDIGO guidelines (13). One should note that local needs and expertise, available logistics, and the specific timeline of goals and implementation strategies should be evaluated before prioritizing quality improvement initiatives at each institution (54).
Summary Statement
There is no evidence to support that average high-intensity CRRT dose (prescribed effluent fluid rate >35-40 ml/kg/hr) favorably impacts mortality or kidney recovery when compared to standard CRRT dose (prescribed effluent fluid rate ~25-30 ml/kg/hr). However, one may consider –albeit transiently– adjustments in CRRT dose to individualize therapy according to specific solute or volume control goals in acutely ill patients with AKI in need of CRRT. Consideration should be given to estimates of circuit downtime, which attenuates the effective delivery of the prescribed dose of CRRT. Finally, delivered CRRT dose constitutes a trackable quality indicator that should be systematically monitored by effective quality assurance systems during the provision of CRRT in the ICU. The impact of adhering to evidence-based practices of delivered CRRT dose on clinical and patient-centered outcomes requires further investigation.
Acknowledgments
Dr. Neyra is currently supported by grants from NIDDK (R56 DK126930 and P30 DK079337) and NHLBI (R01 HL148448-01 and R21 HL145424-01A1). The authors would like to thank Dr. Matthew Sparks for his thoughtful review of this manuscript.
Footnotes
Conflict of Interest
Dr. Neyra has consulted for Baxter Healthcare and Renibus Therapeutics.
References
- 1.Tolwani A. Continuous renal-replacement therapy for acute kidney injury. N Engl J Med. 2012;367(26):2505–2514. [DOI] [PubMed] [Google Scholar]
- 2.Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, McArthur C, McGuinness S, Myburgh J, Norton R, Scheinkestel C, Su S. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 2009; 361: 1627–1638. [DOI] [PubMed] [Google Scholar]
- 3.Ostermann M, Chang R: Correlation between the AKI classification and outcome. Crit Care 2008, 12: R144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu KD, Himmelfarb J, Paganini E, Ikizler TA, Soroko SH, Mehta RL, Chertow GM. Timing of initiation of dialysis in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2006;1(5):915–919. [DOI] [PubMed] [Google Scholar]
- 5.Saudan P, Niederberger M, De Seigneux S, Romand J, Pugin J, Perneger T, Martin PY. Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney Int. 2006;70(7):1312–1317. [DOI] [PubMed] [Google Scholar]
- 6.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. [DOI] [PubMed] [Google Scholar]
- 7.Tandukar S, Palevsky PM. Continuous renal replacement therapy, Who, When, Why, and How. Chest 2019; 155(3):626–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015; 41:1411–23 [DOI] [PubMed] [Google Scholar]
- 9.Claure-Del Granado R, Macedo E, Chertow GM, Soroko S, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL. Toward the optimal dose metric in continuous renal replacement therapy. Int J Artif Organs. 2012; 35(6): 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, La Greca G. Effects of different doses in continuous veno-venous hemofiltration on outcomes of acute renal failure: a prospective randomized trial. Lancet. 2000; 356:26–30. [DOI] [PubMed] [Google Scholar]
- 11.Neri M, Villa G, Garzotto F, Bagshaw S, Bellomo R, Cerda J, Ferrari F, Guggia S, Joannidis M, Kellum J, Kim J, Mehta R, Ricci Z, Trevisani A, Marafon S, Clark W, Vincent J, Ronco C. Nomenclature for renal replacement therapy in acute kidney injury: basic principles. Crit Care. 2016; 20:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prowle JR, Bellomo R. Continuous renal replacement therapy: recent advances and future research. Nat Rev Nephrol 2010; 6: 521–529. [DOI] [PubMed] [Google Scholar]
- 13.Griffin BR, Thomson A, Yoder M, Francis I, Ambruso S, Bregman A, Feller M, Johnson-Bortolotto S, King C, Bonnes D, Dufficy L, Wu C, Bansal A, Tad-y D, Faubel S, Jalal D. Continuous renal replacement therapy dosing in critically ill patients: A quality improvement initiative. Am J Kidney Dis 2019. 74:727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rewa O, Mottes T, Bagshaw SM: Quality measures for acute kidney injury and continuous renal replacement therapy. Curr Opin Crit Care 2015; 21:490–499. [DOI] [PubMed] [Google Scholar]
- 15.Bagshaw SM, Chakravarthi MR, Ricci Z, Tolwani A, Neri M, De Rosa S, Kellum JA, Ronco C: Precision Continuous Renal Replacement Therapy and Solute Control. Blood Purif. 2016;42(3):238–47. [DOI] [PubMed] [Google Scholar]
- 16.Rewa OG, Tolwani A, Mottes T, Juncos LA, Ronco C, Kashani K, Rosner M, Haase M, Kellum J, Bagshaw SM. Quality of care and safety measures of acute renal replacement therapy: Workgroup statements from the 22nd acute disease quality initiative (ADQI) consensus conference. J Crit Care 2019; 54:52–57. [DOI] [PubMed] [Google Scholar]
- 17.Rewa OG, Villeneuve PM, Lachance P, Eurich DT, Stelfox HT, Gibney RTN, Hartling L, Featherstone R, Bagshaw SM. Quality indicators of continuous renal replacement therapy (CRRT) care in critically ill patients: a systematic review. Intensive Care Med 2017;43(6):750–63. [DOI] [PubMed] [Google Scholar]
- 18.Rewa OG, Eurich DT, Gibney RTN, Bagshaw SM. A modified Delphi process to identify, rank and prioritize quality indicators for continuous renal replacement therapy (CRRT) care in critically ill patients. J Crit Care 2018;47:145–52. [DOI] [PubMed] [Google Scholar]
- 19.Lemarie P, Husser-Vidal S, Gergaud S, Verger X, Rineau E, Berton J, Parot-Schinkel E, Hamel JF, Lasocki S. High-Fidelity Simulation Nurse Training Reduces Unplanned Interruption of Continuous Renal Replacement Therapy Sessions in Critically III Patients: The SimHeR Randomized Controlled Trial, Anesthesia & Analgesia 2019; 129(1): 121–128 doi: 10.1213/ANE.0000000000003581 [DOI] [PubMed] [Google Scholar]
- 20.Mottes ΤΑ, Goldstein SL, Basu RK. Process based quality improvement using a continuous renal replacement therapy dashboard. BMC Nephrol 2019. 20: 17. https://doi.ora/10.1186/s12882-018-1195-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerdá J, Ronco C, THE CLINICAL APPLICATION OF CRRT—CURRENT STATUS: Modalities of Continuous Renal Replacement Therapy: Technical and Clinical Considerations. Seminars in Dialysis 2009, 22: 114–122. [DOI] [PubMed] [Google Scholar]
- 22.Brunet S, Leblanc M, Geadah D, Parent D, Courteau S, Cardinal J: Diffusive and convective solute clearances during continuous renal replacement therapy at various dialysate and ultrafiltration flow rates. Am J Kidney Dis. 1999;34(3):486–92. [DOI] [PubMed] [Google Scholar]
- 23.Hatamizadeh P, Tolwani A, Palevsky P. Revisiting Filtration Fraction as an Index of the Risk of Hemofilter Clotting in Continuous Venovenous Hemofiltration. CJASN 15: ccc–ccc, 2020. doi: 10.2215/CJN.02410220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Troyanov S, Cardinal J, Geadah D, Parent D, Courteau S, Caron S, Leblanc M: Solute clearances during continuous venovenous haemofiltration at various ultrafiltration flow rates using Multiflow-100 and HF1000 filters. Nephrol Dial Transplant. 2003;18(5):961–6. [DOI] [PubMed] [Google Scholar]
- 25.Clark WR, Turk JE, Kraus MA, Gao D: Dose determinants in continuous renal replacement therapy. Artif Organs 2003; 27:815–820. [DOI] [PubMed] [Google Scholar]
- 26.KDIGO Clinical Practice Guideline for Acute Kidney Injury: Dose of renal replacement therapy in AKI. Kidney Int 2012; 2:113–115. [Google Scholar]
- 27.Palevsky PM, Liu KD, Brophy PD, Chawla LS, Thakar CV, Tolwani AJ, Walkar SS, Weisbord SD. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013; 61:649–672. [DOI] [PubMed] [Google Scholar]
- 28.Macedo E, Claure del Granado R, Mehta RL. Effluent volume and dialysis dose in CRRT: time for reappraisal. Nat Rev Nephrol 2011. 1;8 (1):57–60. [DOI] [PubMed] [Google Scholar]
- 29.Claure-Del Granado R, Macedo E, Chertow GM, Soroko S, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL. Effluent volume in continuous renal replacement therapy overestimates the delivered dose of dialysis. Clin J Am Soc Nephrol. 2011; 6:467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall MR. Current status of dosing and quantification of acute renal replacement therapy. Part 1: mechanisms and consequences of therapy under-delivery. Nephrology (Carlton) 2006. 11, 171–180. [DOI] [PubMed] [Google Scholar]
- 31.Feldhoff P, Turnham T, Klein E. Effect of plasma proteins on the sieving spectra of hemofilters. Artif. Organs 1984. 8, 186–192. [DOI] [PubMed] [Google Scholar]
- 32.Venkataraman R, Kellum JA, Palevsky P. Dosing patterns for continuous renal replacement therapy at a large academic medical center in the united states. J Crit Care. 2002; 17:246–50. [DOI] [PubMed] [Google Scholar]
- 33.Vesconi S, Cruz DN, Fumagalli R, Kindgen-Milles D, Monti G, Marinho A, Mariano F, Formica M, Marchesi M, Rená R, Livigni S, Ronco C. Delivered dose of renal replacement therapy and mortality in critically ill patients with acute kidney injury. Crit Care. 2009; 13:R57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Ni H, Fan H, Li D, Xu X. Actually, Delivered Dose of Continuous Renal Replacement Therapy Is Underestimated in Hemofiltration. ASAIO Journal 2013; 59:622–626. [DOI] [PubMed] [Google Scholar]
- 35.Davenport A, Tolwani A. Citrate anticoagulation for continuous renal replacement therapy (CRRT) in patients with acute kidney injury admitted to the intensive care unit. NDT Plus. 2009;2(6):439–447. doi: 10.1093/ndtplus/sfp136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fealy N, Aitken L, du Toit E, Lo S, Baldwin I. Faster Blood Flow Rate Does Not Improve Circuit Life in Continuous Renal Replacement Therapy: A Randomized Controlled Trial. Critical Care Medicine. 2017. October;45(10):e1018–e1025. DOI: 10.1097/ccm.0000000000002568 [DOI] [PubMed] [Google Scholar]
- 37.Connor MJ Jr, Karakala N. Continuous renal replacement therapy: reviewing current best practice to provide high-quality extracorporeal therapy to critically ill patients. Adv Chronic Kidney Dis. 2017;24(4):213–218 [DOI] [PubMed] [Google Scholar]
- 38.Prowle JR, Schneider A, Bellomo R: Clinical Review: Optimal dose of continuous renal replacement therapy in acute kidney injury. Critical Care 2011,15: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, Zandstra DF, Kesecioglu J. Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Critical care medicine, 2002. 30(10): 2205–11. [DOI] [PubMed] [Google Scholar]
- 40.Tolwani AJ, Campbell RC, Stofan BS, Robin-Lai K, Oster RA, Wille KM. Standard versus high-dose CVVHDF for ICU-related acute renal failure. JASN, 2008. 19(6): 1233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macedo E, Mehta RL: Continuous Dialysis Therapies: Core Curriculum. Am J Kidney Dis 2016. 68; 645–657. [DOI] [PubMed] [Google Scholar]
- 42.Clark WR, Leblanc M, Ricci Z, Ronco C: Quantification and Dosing of Renal Replacement Therapy in Acute Kidney Injury: A Reappraisal. Blood Purif 2017; 44:140–155. [DOI] [PubMed] [Google Scholar]
- 43.VA/NIH Acute Renal Failure Trial Network, Palevsky PM, Zhang JH, O'Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Vijayan A, Watnick S, Star RA, Peduzzi P: Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 2008; 359:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.RENAL Replacement Therapy Study Investigators., Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, McArthur C, McGuinness S, Myburgh J, Norton R, Scheinkestel C, Su S: Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 2009;361:1627–1638. [DOI] [PubMed] [Google Scholar]
- 45.Fayad Al, Buamscha DG, Ciapponi A. Intensity of continuous renal replacement therapy for acute kidney injury. Cochrane Database Syst Rev 2016; 10:CD010613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Gallagher M, Li Q, Lo S, Cass A, Finfer S, Myburgh J, Bouman C, Faulhaber-Walter R, Kellum JA, Palevsky PM, Ronco C, Saudan P, Tolwani A, Bellomo R. Renal replacement therapy intensity for acute kidney injury and recovery to dialysis independence: a systematic review and individual patient data meta-analysis. Nephrol Dial Transplant 2018. 33(6): p. 1017–1024. [DOI] [PubMed] [Google Scholar]
- 47.Jun M, Heerspink HJ, Ninomiva T, Gallagher m, Bellomo R, Myburgh J, Finfer S, Palevsky PM, Kellum JA, Perkovic V, Cass A. Intensities of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis. Clin J Am Soc Nephrol 2010; 5(6):953–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Combes A, Brechot N, Amour J, Cozic N, Lebreton G, Guidon C, Zogheib E, Thiranos JC, Rigal JC, Bastien O, Benhaoua H, Abry B, Ouattara A, Trouillet JL, Mallet A, Chastre J, Leprince P, Luyt CE. Early High-Volume Hemofiltration versus Standard Care for Post-Cardiac Surgery Shock. The HEROICS Study. Am J Respir Crit Care Med. 2015; 192(10): 1179–90. [DOI] [PubMed] [Google Scholar]
- 49.Joannes-Boyau O, Honorá PM, Perez P, Bagshaw SM, Grand H, Canivet JL, Dewitte A, Flamens C, Pujol W, Grandoulier AS, Fleureau C, Jacobs R, Broux C, Floch H, Branchard O, Franck S, Rozá H, Collin V, Boer W, Calderon J, Gauche B, Spapen HD, Janvier G, Ouattara A. High-volume versus standard volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE study): a multicentre randomized controlled trial. Intensive Care Med. 2013; 39:1535–46. [DOI] [PubMed] [Google Scholar]
- 50.Boussekey N, Chiche A, Faure K, Devos P, Guery B, d’Escrivan T, Georges H, Leroy H. A pilot randomized study comparing high and low volume hemofiltration on vasopressor use in septic shock. Intensive Care Med 2008, 34:1646–1653. 26. [DOI] [PubMed] [Google Scholar]
- 51.Zhang P, Yang Y, Lv R, Zhang Y, Xie W, Chen J: Effect of the intensity of continuous renal replacement therapy in patients with sepsis and acute kidney injury: a single-center randomized clinical trial. Nephrol Dial Transplant 2012, 27:967–973. [DOI] [PubMed] [Google Scholar]
- 52.Park JT, Lee H, Kee YK, Park S, Oh HJ, Han SH, Joo KW, Lim CS, Kim YS, Kang SW, Yoo TH, Kim DK. High-dose versus conventional-dose continuous venovenous hemodiafiltration and patient and kidney survival and cytokine removal in sepsis-associated acute kidney injury: a randomized controlled trial. Am J Kidney Dis. 2016; 68:599–608. [DOI] [PubMed] [Google Scholar]
- 53.Clark E, Molnar AO, Joannes-Boyau O, Honoré PM, Sikora L, Bagshaw SM. High-volume hemofiltration for septic acute kidney injury: a systematic review and meta-analysis. Crit Care, 2014. 18(1): p. R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neyra JA, Tolwani AJ. A quality improvement initiative targeting CRRT delivered dose: the what, the how, and the why. Am J Kidney Dis 2019. 74:721–723. [DOI] [PubMed] [Google Scholar]


