Abstract
Background:
Organophosphates are frequently applied insecticides that inhibit acetylcholinesterase (AChE) activity resulting in cholinergic overstimulation. Limited evidence suggests that organophosphates may alter thyroid hormone levels, although studies have yielded inconsistent findings. We aimed to test the associations between AChE activity, a physiological marker of organophosphate exposure, and thyroid function in adolescents.
Methods:
We included information of 80 adolescent participants (ages 12–17y in 2016, 53% male) growing up in agricultural settings in Ecuador. We measured fingerstick erythrocytic AChE activity and hemoglobin concentration, and concurrent serum thyroid stimulating hormone (TSH) and free-T4 (fT4) concentrations. General linear models were used to test associations adjusting for demographic and anthropometric variables. TSH associations were further adjusted for fT4.
Results:
The mean (SD) AChE, TSH and fT4 levels were 3.77 U/mL (0.55), 2.82 μIU/ml (1.49) and 1.11 ng/dl (0.13), respectively. Lower AChE activity, indicating greater organophosphate exposure, was marginally associated with greater fT4 concentrations (difference per SD decrease in AChE activity (β)=0.03 ng/dL, [90% CI: 0.00, 0.06]) but not with TSH (β=−0.01 μIU/ml, [−0.38, 0.36]). Gender modified the AChE-TSH association (p=0.03). In girls, lower AChE activity was associated with higher fT4 levels (β=0.05 ng/dL [0.01, 0.10]) and lower TSH concentrations (β= −0.51 μIU/ml, [−1.00, −0.023]). No associations were observed in boys.
Discussion:
These cross-sectional findings suggest that alterations in the cholinergic system from organophosphate exposures can increase fT4 levels coupled with a beyond-compensatory downregulation of TSH in female adolescents. This is the first study to characterize these associations in adolescents.
Keywords: Pesticide, organophosphate, adolescent, agriculture, thyroid, endocrine
Introduction
Organophosphates are commonly used insecticides in agricultural production worldwide, and inhibit acetylcholinesterase (AChE) activity resulting in buildup of acetylcholine and altered neurotransmission (Colovic et al., 2013). Evidence suggests that organophosphate pesticides can disrupt the function of endocrine organs (Kitamura et al., 2011). Findings from human studies are limited yet suggest that organophosphate pesticides may disrupt thyroid hormones. However, the type of thyroid hormone alteration and its directionality is not generally consistent among studies. Organophosphate exposure in adolescents and adults has been linked with both higher (Fortenberry et al., 2012; Lacasaña et al., 2010) and lower levels of fT4 (Meeker et al., 2006), and with both lower TSH (Fortenberry et al., 2012; Suhartono et al., 2018) and higher TSH levels (Lacasaña et al., 2010; Meeker et al., 2006). Another study showed no significant associations between organophosphates and thyroid hormone levels (Piccoli et al., 2016). The only two studies to date that have characterized this association in children using urinary pesticide metabolites, have reported lower TSH concentrations with organophosphate exposures (Fortenberry et al., 2012; Suhartono et al., 2018).
There are several pathways through which organophosphate pesticides could disturb thyroid hormone regulation. Thyroid hormone levels are regulated by the hypothalamic-pituitary-thyroid axis. At the central nervous system, hypothalamic neurons stimulate the release of thyroid stimulating hormone (TSH) into systemic circulation. TSH then stimulates the thyroid to produce the thyroid hormones thyroxine (T4) and to a lesser extent, triiodothyronine (T3) (Molina, 2018). T4 and T3 are then converted to their metabolically active forms of free thyroxine (fT4) and free triiodothyronine (fT3) which act on peripheral tissues (Jameson et al., 2018). T3 and T4 provide negative feedback to the hypothalamus which downregulates TSH (Molina, 2018). At the level of the central nervous system, cholinergic overstimulation associated with organophosphate pesticide exposures (via AChE inhibition) can stimulate somatostatin which can inhibit TSH release (Smallridge et al., 1991).
It is well established that thyroid hormones play central roles in growth, organogenesis, and metabolic homeostasis throughout the lifecycle (Jameson et al., 2018). Both thyroid dysfunction and organophosphate pesticide exposures, separately, have been associated with developmental and neurobehavioral delays in children (Aakre et al., 2017; Sapbamrer and Hongsibsong, 2019; Suarez-Lopez et al., 2013).
The objective of the present pilot study was to characterize the association between AChE activity, a physiological marker of cholinesterase inhibitor insecticides, and thyroid hormone levels in adolescents growing up in agricultural communities in Ecuador. The inconsistent associations found in the limited number of existing human observational studies did not allow us to develop a coherent a-priori hypothesis.
Participants and Methods
The Study of Secondary Exposures to Pesticides among Children and Adolescents (ESPINA; Spanish: Estudio de la Exposicion Secundaria a Plaguicidas en Niños y Adolescentes) is a prospective cohort study that seeks to understand the effects of chronic pesticide exposure on children’s health and development. Specimens and survey data were collected from children living in the agricultural county of Pedro Moncayo, Pichincha province, Ecuador. Flower plantations in this county frequently use various pesticides including insecticides (organophosphates, neonicotinoids and pyrethroids), fungicides and, to lesser extent, herbicides (Grandjean et al., 2006; Handal et al., 2016; Harari, 2004; Suarez-Lopez et al., 2017)
In 2008, participants were recruited from the 2004 Survey of Access and Demand of Health Services, a representative sample of the population of Pedro Moncayo County, and via word of mouth. The ESPINA study sought out participants who lived in households with a flower plantation worker as well as those living in households without an agricultural worker. To be eligible to participate, participants must have been between 4–9 years of age and: 1) lived with a flower plantation worker for at least one year, or 2) never lived with an agricultural worker, never inhabited a house where agricultural pesticides were stored and have had no previous direct contact with pesticides. In 2008, 313 boys and girls were examined, of which 48% lived with a floricultural worker. In 2016, participants, previously examined in 2008, were recontacted and new adolescent participants were recruited using the System of Local and Community Information (SILC) developed by Fundación Cimas del Ecuador. A total of 545 participants ages 11–17 years (48% lived with a floricultural worker) were examined in 2016, including 245 participants from 2008. Additional details about the study methodology in 2008 and 2016 have been published elsewhere (Suarez-Lopez et al., 2019, 2012).
The present analyses included 80 adolescent participants examined in 2016 who were selected by their AChE activity levels in 2008: 40 who had the lowest and 40 who had the highest AChE levels. The mean AChE activity in 2008 among participants in the high AChE group was 0.98 U/mL higher (95% CI: 0.87, 1.08) than those in the low AChE category, after adjusting for age, gender, z-score for height-for-age and hemoglobin. This difference across these AChE groups persisted in 2016, with an adjusted difference of 0.72 U/mL (95% CI: 0.53, 0.91). The height of children was measured to the nearest 1 mm following established protocols (World Health Organization, 2008). Z-scores for height-for age were calculated using the World Health Organization (WHO) growth standards (World Health Organization Multicentre Growth Reference Study Group, 2006).
Erythrocytic AChE activity and hemoglobin concentrations (mg/dL) were measured with the EQM Test-mate ChE Cholinesterase Test System 400 (EQM AChE Erythrocyte Cholinesterase Assay Kit 470; EQM Research, Inc, Cincinnati, OH) from a fresh finger-stick blood sample collected in 2016, following standard procedures (EQM Research Inc., 2003). TSH and fT4 levels in serum were measured using enzymatic assays by ALPCO (The Ultrasensitive Thyroid Stimulating Hormone ELISA. Cat No. 25-TSHHUU-E01; and Free Thyroxine ELISA Cat No. 25-FT4HU-E01) at the Altman Clinical and Translational Research Institute laboratory at the University of California San Diego. Thyroid hormones were measured in serum samples collected minutes after the finger-stick sample. Serum samples were stored at −80°C prior to analyses.
Parents provided informed consent to participate in the study and permission for participation for their children. All participants younger than 18 years of age provided assent to participate. This study received approval from the institutional review boards at the University of California, San Diego, and Universidad San Francisco de Quito. Additionally, the Ecuadorian Ministries of Health and Education approved the present study.
Statistical Analysis
Participants’ characteristics were calculated as mean (SD) and stratified by tertiles of AChE activity. Crude linear regression analyses were used to evaluate p-trends of participant characteristics across AChE activity values. Linear regression analyses were used to test associations of AChE activity with TSH and fT4. Models were defined a-priori and adjusted for age, hemoglobin concentration, gender, height-for-age z-score, and parental years of education (the average years of education for both parents). Models that included TSH were further adjusted for fT4 considering that TSH levels fluctuate in response to fT4 concentrations. Associations were tested using a 90% confidence interval due to the limited sample size of the study. The AChE-thyroid hormone associations were calculated per SD decrease in AChE activity since lower AChE reflects greater exposure to cholinesterase inhibitors. We tested for curvilinear associations using a quadratic term (ACHE+AChE*ACHE). We also tested for effect-modification by gender using a multiplicative term (AChE+gender+AChE*gender) and plotted the adjusted least squares means of fT4 and TSH for each quintile of AChE activity.
Results
Participant characteristics
Participants had a mean age of 14.6 years (SD=1.7) and 53% were male. The mean AChE activity was 3.77 U/ml (SD=0.63) and the mean hemoglobin concentration was 12.9 g/L (SD=1.2, Table 1). The mean concentrations for TSH and fT4 were 2.83 μIU/mL (SD=1.62) and 1.10 (SD=0.14) ng/dL, respectively. Participants were on average 1.56 SD shorter (z-score height-for-age) than the WHO normative sample. A greater proportion of boys had higher AChE activity than girls, and age was positively associated with AChE activity.
Table 1.
Participant characteristics by AChE activity tertile.
| Total | AChE Activity Tertiles | ||||
|---|---|---|---|---|---|
| AChE Range, U/mL | 1.97–5.92 | 1.97–3.52 | 3.54–4.04 | 4.06–5.92 | P-trend |
| N | 80 | 26 | 27 | 27 | |
| Age, years | 14.6 (1.7) | 14.4 (1.7) | 13.8 (1.4) | 15.7 (1.4) | 0.01 |
| Gender (male), % | 53 | 31 | 59 | 59 | 0.01 |
| Parental education, years | 9.2 (3.3) | 9.3 (2.70) | 9.1 (3.1) | 9.1 (4.1) | 0.97 |
| Height-for-age z-score, SD | −1.56 (0.88) | −1.42 (0.98) | −1.61 (0.83) | −1.66 (0.84) | 0.28 |
| AChE, U/mL | 3.77 (0.63) | 3.08 (0.38) | 3.79 (0.16) | 4.43 (0.31) | - |
| Hemoglobin, mg/dL | 12.9 (1.2) | 12.3 (1.4) | 12.9 (1.0) | 13.6 (1.0) | <0.01 |
Displayed values are either percent or mean (SD).
AChE and thyroid hormones
Overall, we observed that every SD decrease in AChE activity (β) was marginally associated with higher fT4 (b: 0.03 ng/dL, 90% CI: 0.00 to 0.06) but was not associated with TSH levels (β:−0.01 μIU/mL, 90% CI: −0.38 to 0.36, Figures 1 and 2). There was no evidence for curvilinear associations.
Figure 1:
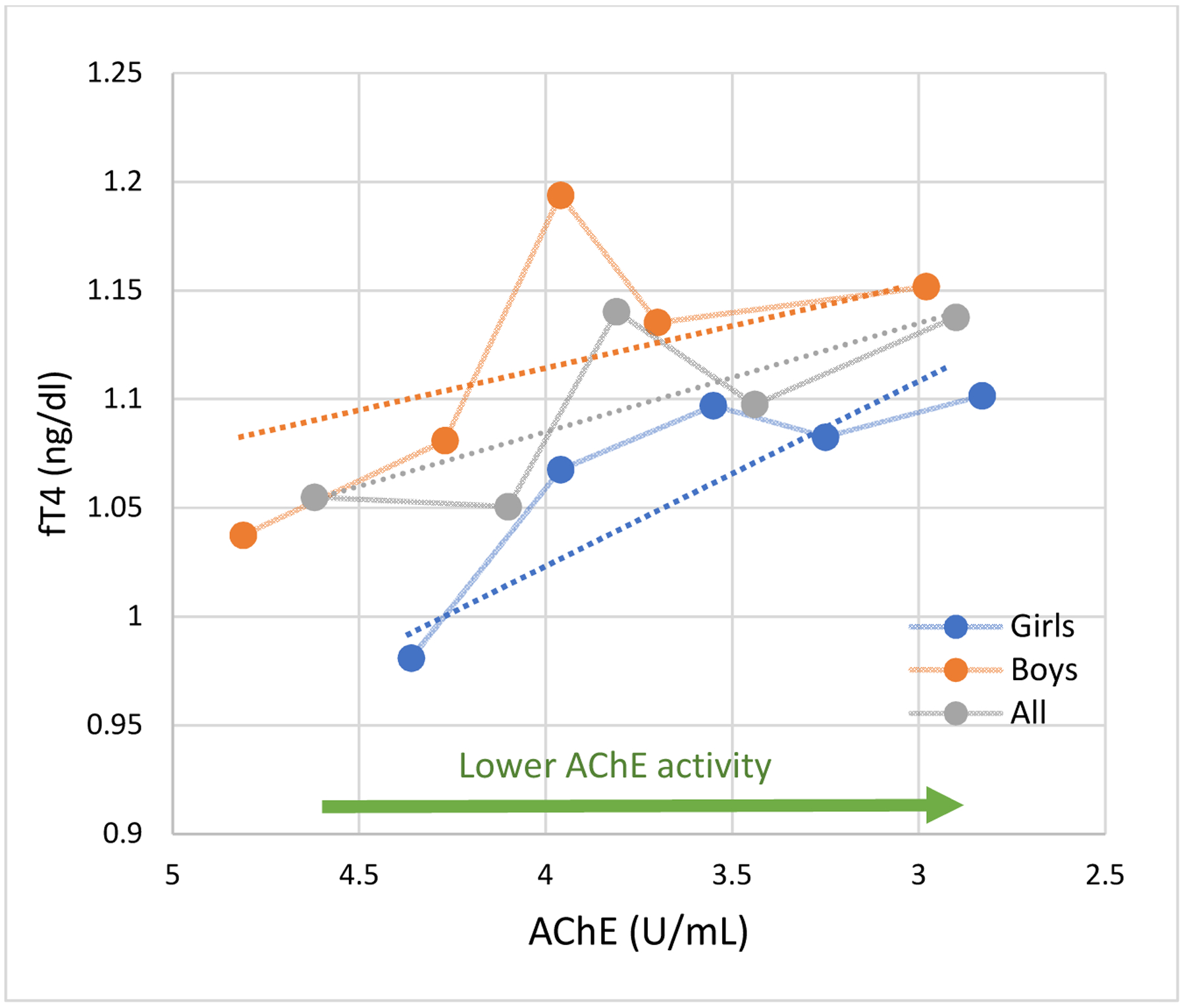
Relationship between fT4 and AChE activity in girls and boys. N=80.
fT4 Difference per SD* decrease in AChE activity (90% CI)
All 0.03 (0.00 to 0.06)
Girls 0.05 (0.01 to 0.10)
Boys 0.02 (−0.02 to 0.07)
* AChE SD=0.63
Circles represent the mean fT4 values for quintiles of AChE activity. Connecting lines are displayed for visualization purposes. Dotted lines are the linear slopes of the adjusted model using continuous dependent and independent variables. Adjusted for age, hemoglobin concentration, gender, height-forage z-score and parental years of education.
Figure 2:
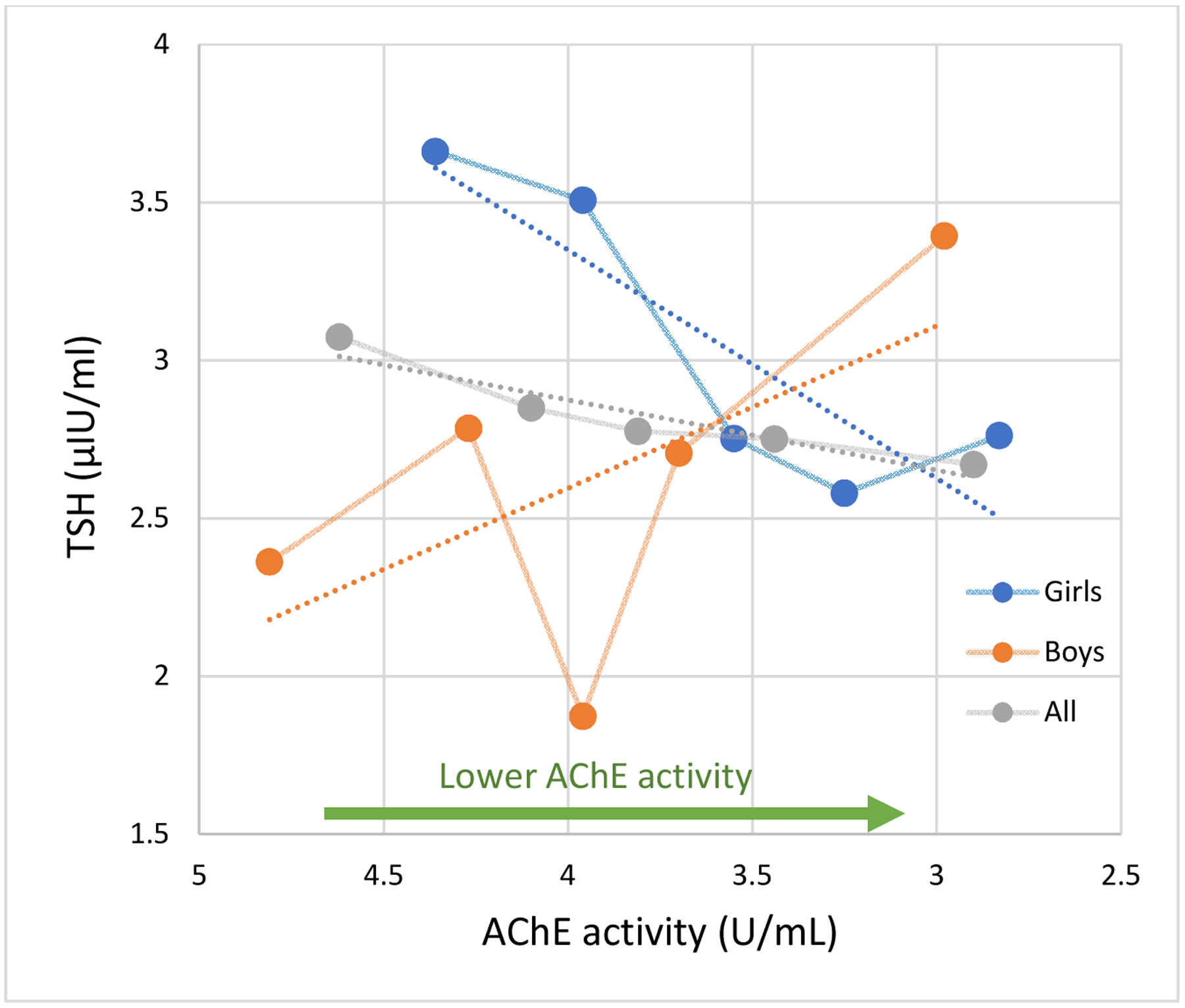
Relationship between TSH and AChE activity in girls and boys. N=80.
TSH Difference per SD* decrease in AChE activity (90% CI)
All** −0.01 (−0.38 to 0.36)
Girls −0.51 (−1.00 to −0.023)
Boys 0.36 (−0.19 to 0.90)
* AChE SD=0.63
** p for gender interaction = 0.03
Circles represent the mean TSH values for quintiles of AChE activity. Connecting lines are displayed for visualization purposes. Dotted lines are the linear slopes of the adjusted model. Adjusted for age, hemoglobin concentration, gender, height-forage z-score, parental years of education and fT4.
There was significant effect modification by gender on the association between AChE activity and TSH (p= 0.03) but not with fT4 (p=0.56). In girls, lower AChE activity was marginally associated with greater fT4 concentrations (β= 0.05 ng/dl, 90% CI: 0.01 to 0.10, Figures 1 and 2) and with lower TSH concentrations (β= −0.51 μIU/ml, 90% CI: −1.00 to −0.023), independently of concurrent fT4 concentrations. Among boys, lower AChE activity was also associated with higher fT4 concentrations, although this association was weaker and non-significant. AChE activity was not associated with TSH in boys.
Discussion
In this pilot study, we examined the cross-sectional relationships between AChE activity, a marker of organophosphate pesticide exposure, and the thyroid hormones fT4 and TSH in adolescents living in an agricultural region of Ecuador. Findings suggest that alterations in the cholinergic system from non-occupational pesticide exposures may alter thyroid hormone levels in female adolescents. We observed that lower AChE activity, indicating greater exposure to cholinesterase inhibitor pesticides, was associated with greater fT4 and lower TSH concentrations among adolescent girls living in agricultural areas of Ecuador. These findings partially indicate that cholinesterase inhibition can increase serum fT4 levels coupled with a downregulation of TSH in girls. The amount of TSH down regulation appears to be at levels beyond what would be expected from the observed higher fT4 levels, considering that the AChE-TSH associations observed were adjusted for concurrent fT4 concentrations. In boys, AChE activity was also positively associated with fT4, but the association was weaker and not statistically significant. To our knowledge, this is the first study in adolescents to characterize the associations between AChE activity and thyroid hormone levels.
Findings from this study are consistent with those from the National Health and Nutrition Examination Survey (NHANES), which included cross-sectional data on adults and children in the United States (Fortenberry et al., 2012). That study reported a positive association between the urinary organophosphate metabolite 3,5,6-Trichloro-2-pyridinol and serum total T4 in adolescent and adult males, and corresponding negative associations with TSH; however, contrary to our findings, no associations were observed among females. The difference may be due to variable levels of exposure among sexes in each study population or differences in the measurement variables. The present study measured free thyroxine (fT4) while the NHANES study measured total thyroxine (T4), which includes both protein-bound T4 and fT4.
The positive association between fT4 and organophosphate exposure as reported in the present study aligns with an observational study of adult floricultural workers in which increased levels of total T4 were observed during high-spraying periods of organophosphate pesticides (Lacasaña et al., 2010). However, TSH levels in that study were found to be increased, which contrasts with our findings
In contrast with our finding of higher fT4 in association with organophosphate pesticide exposure, decreased T4 in association with organophosphate exposure has also been reported in the literature. An observational study of males in the United States seeking infertility treatment showed increased organophosphate urinary metabolites were associated with reduced T4 and increased TSH levels (Meeker et al., 2006). Among adult agricultural laborers in Brazil, there was no significant association between AChE activity, a physiological marker of organophosphate and carbamate pesticide exposure, and thyroid hormone levels (Piccoli et al., 2016).
Besides the findings in NHANES, the only other pediatric study to our knowledge is a cross-sectional study that included 66 children in an agricultural region of Indonesia. This study found that the presence of organophosphate metabolites in urine (vs. not) was significantly associated with hypothyroidism defined as TSH >4.5 μIU/L, but there was no difference in levels of fT4 (Suhartono et al., 2018). That study, however, did not report the linear associations between continuous variables of exposure (e.g. AChE activity or metabolite levels) and thyroid hormones, so we are unable to compare our main findings.
Rodent models demonstrate that organophosphate pesticides can induce changes in thyroid hormone levels, although trends in specific thyroid hormone levels have not been consistent. Rats given a single injection of diisopropyl fluorophosphate, an organophosphate pesticide, had an 80% reduction in TSH levels which aligns with TSH trends seen in the present study (Smallridge et al., 1991). Contrary to our findings, rodents who received an oral dose of the organophosphate malathion and mice pups exposed to organophosphate pesticides in utero and after birth had significantly decreased serum levels of T4 and T3, and increased TSH concentrations (Akhtar et al., 1996; Jeong et al., 2006). Similarly, female mice who received the highest dose of the organophosphate pesticide chlorpyrifos had significantly lower T4 levels than female mice who received lower or no pesticide doses (Angelis et al., 2009). The results reported in these studies differ from those seen in our study population and may be explained by different metabolic pathways of organophosphate pesticides, different thresholds for induction of hormone level changes in humans compared with rodents, or varying effects during different windows of development (early life vs adolescence).
Our findings also suggest that lower cholinesterase activity, marking greater exposure to cholinesterase inhibitor pesticides, is associated with greater concentrations of fT4. It is not well understood how organophosphate pesticides interact with peripheral thyroid hormone levels, but they may alter hepatic metabolism. Conversion of T4 to its more metabolically active form, T3, occurs in the liver and other tissues (Brent and Koenig, 2017). Lacasaña et al (2010) proposed that inhibition of enzymes such as hepatic 5′-deiodinase type II involved in the metabolism of T4 to T3, may drive up levels of T4 while diminishing T3. Higher T4 levels associated with organophosphate exposures was also seen in catfish and thought to be due to decreased T4 to T3 conversion (Sinha 1992). This mechanism may help to explain the association between elevated T4 and increased organophosphate exposure observed in participants in this study. While we did not measure T3 levels, it may be a helpful next step in elucidating a mechanism.
The present study has some limitations. First, the sample size of this study may have limited our ability to detect differences in thyroid hormones in relation to AChE levels. This may be an explanation as to why we only observed borderline significant associations among girls but not boys. Additionally, the present findings are cross-sectional, so we cannot completely discern the directionality of the associations; that is, whether AChE activity influences fT4 production or whether fT4 levels affect AChE activity. However, there is no physiological premise to indicate that circulating thyroid hormones may affect circulating red blood cell AChE activity. Exposure assessment utilizing AChE activity precludes us from identifying specific cholinesterase inhibitor pesticides that may be driving the observed associations. Considering that in agriculture mixtures of multiple classes of pesticides are frequently used, it is plausible that AChE activity may also be indirectly marking exposure to other classes of pesticides besides cholinesterase inhibitors. Erythrocytic AChE activity is less sensitive and specific than most pesticide quantification methods (Barr and Angerer, 2006; He, 1999); however, it has certain benefits over measuring urinary pesticide metabolites. First, AChE is a more stable indicator of exposure to cholinesterase inhibitors than pesticides metabolites in bodily fluids (Lefkowitz et al., 2007; Suarez-Lopez et al., 2017) and is a better indicator of long-term exposure as it is estimated that it may take up to 82 days for AChE levels to return to baseline levels after irreversible inhibition by organophosphates (Mason, 2000). Furthermore, it is a physiological marker of exposure; hence, it informs that pesticide exposures are altering a physiological process in the organism (Taylor, 2011). Additionally, erythrocytic AChE activity is similar to neuronal AChE activity (Aaron CK, 2007) and brain AChE has been reported to be diminished after chlorpyrifos exposure in rats (Dam et al., 2000; Johnson et al., 2009; Qiao et al., 2002; Richardson and E, 2005; Song et al., 1997). This is particularly important as a central component of thyroid hormone regulation is in the central nervous system. Larger prospective studies of children and adolescents that characterize the associations between various pesticide exposure constructs and thyroid hormones are warranted.
Conclusion
In conclusion, the present cross-sectional study is the first to assess the association of AChE activity and thyroid hormones in adolescents to our knowledge. Our findings bring attention to the role of pesticide exposures and the cholinergic system on thyroid hormone homeostasis, as lower AChE activity, marking greater exposure to cholinesterase inhibitor pesticides, was associated with greater fT4 and lower TSH concentrations in girls but not boys. Thyroid hormone disturbances in childhood pose several risks to physiologic development. Findings of this pilot study suggest that larger studies of this relationship are warranted. Further investigation on the effects of chronic pesticide exposure on thyroid hormone function in children is necessary to understand and mitigate potential risks of exposure.
Acknowledgments:
The ESPINA study received funding from the National Institute of Occupational Safety and Health (1R36OH009402) and the National Institute of Environmental Health Sciences (R01ES030378, R01ES025792, R21ES026084). We thank Fundación Cimas del Ecuador, the Parish Governments of Pedro Moncayo County, community members of Pedro Moncayo and the Education District of Pichincha-Cayambe-Pedro Moncayo counties for their support on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- Aakre I, Strand TA, Moubarek K, Barikmo I, Henjum S, 2017. Associations between thyroid dysfunction and developmental status in children with excessive iodine status. PLoS One 12, 1–16. 10.1371/journal.pone.0187241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaron CK, 2007. Organophosphates and carbamates, in: Haddad LM Borron SW, Burns MJ, S. MW (Ed.), Haddad and Winchester’s Clinical Management of Poisoning and Drug Overdose. Saunders/Elsevier, Philadelphia. [Google Scholar]
- Akhtar N, Kayani SA, Ahmadt MM, Shahab M, 1996. Insecticide-induced Changes in Secretory Activity of the Thyroid Gland in Rats 16, 397–400. [DOI] [PubMed] [Google Scholar]
- De Angelis S, Tassinari R, Maranghi F, Eusepi A, Di Virgilio A, Chiarotti F, Ricceri L, Pesciolini AV, Gilardi E, Moracci G, Calamandrei G, Olivieri A, Mantovani A, 2009. Developmental Exposure to Chlorpyrifos Induces Alterations in Thyroid and Thyroid Hormone Levels Without Other Toxicity Signs in Cd1 Mice 108, 311–319. 10.1093/toxsci/kfp017 [DOI] [PubMed] [Google Scholar]
- Barr DB, Angerer J, 2006. Potential Uses of Biomonitoring Data: A Case Study Using the Organophosphorus Pesticides Chlorpyrifos and Malathion. Environ. Health Perspect 114, 1763–1769. 10.1289/ehp.9062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent GA, Koenig RJ, 2017. Thyroid and Antithyroid Drugs, in: Brunton LL, Hilal-Dandan R, Knollmann BC (Eds.), Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13e. McGraw-Hill Education, New York, NY. [Google Scholar]
- Colovic MB, Krstic DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM, Colović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM, 2013. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol 11, 315–335. 10.2174/1570159X11311030006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA, 2000. Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Brain Res. Dev. Brain Res 121, 179–187. [DOI] [PubMed] [Google Scholar]
- EQM Research Inc., 2003. Test-mate ChE Cholinesterase Test System (Model 400). Instruction Manual. [WWW Document]. URL http://www.eqmresearch.com/Manual-E.pdf
- Fortenberry GZ, Hu H, Turyk M, Barr DB, Meeker JD, 2012. Association between urinary 3, 5, 6-trichloro-2-pyridinol, a metabolite of chlorpyrifos and chlorpyrifos-methyl, and serum T4 and TSH in NHANES 1999–2002. Sci. Total Environ 424, 351–355. 10.1016/J.SCITOTENV.2012.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Harari R, Barr DB, Debes F, 2006. Pesticide exposure and stunting as independent predictors of neurobehavioral deficits in Ecuadorian school children. Pediatrics 117, e546–56. 10.1542/peds.2005-1781 [DOI] [PubMed] [Google Scholar]
- Handal AJ, Hund L, Páez M, Bear S, Greenberg C, Fenske RA, Barr DB, 2016. Characterization of Pesticide Exposure in a Sample of Pregnant Women in Ecuador. Arch. Environ. Contam. Toxicol 70, 627–639. 10.1007/s00244-015-0217-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari R, 2004. Seguridad, salud y ambiente en la floricultura. IFA, PROMSA, Quito. [Google Scholar]
- He F, 1999. Biological monitoring of exposure to pesticides: current issues. Toxicol. Lett 108, 277–283. [DOI] [PubMed] [Google Scholar]
- Jameson JL, Mandel SJ, Weetman AP, 2018. Thyroid Gland Physiology and Testing, in: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J (Eds.), Harrison’s Principles of Internal Medicine, 20e. McGraw-Hill Education, New York, NY. [Google Scholar]
- Jeong S, Kim B, Kang H, Ku H, Cho J, 2006. Effect of chlorpyrifos-methyl on steroid and thyroid hormones in rat F0- and F1-generations 220, 189–202. 10.1016/j.tox.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Johnson FO, Chambers JE, Nail CA, Givaruangsawat S, Carr RL, 2009. Developmental chlorpyrifos and methyl parathion exposure alters radial-arm maze performance in juvenile and adult rats. Toxicol. Sci 109, 132–42. 10.1093/toxsci/kfp053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S, Sugihara K, Fujimoto N, Yamazaki T, 2011. Organophosphates as Endocrine Disruptors, in: Anticholinesterase Pesticides: Metabolism, Neurotoxicity, and Epidemiology. John Wiley and Sons, pp. 189–202. 10.1002/9780470640500.ch15 [DOI] [Google Scholar]
- Lacasaña M, López-Flores I, Rodríguez-Barranco M, Aguilar-Garduño C, Blanco-Muñoz J, Pérez-Méndez O, Gamboa R, Bassol S, Cebrian ME, 2010. Association between organophosphate pesticides exposure and thyroid hormones in floriculture workers. Toxicol. Appl. Pharmacol 243, 19–26. 10.1016/j.taap.2009.11.008 [DOI] [PubMed] [Google Scholar]
- Lefkowitz LJ, Kupina JM, Hirth NL, Henry RM, Noland GY, Barbee JY, Zhou JY, Weese CB, 2007. Intraindividual stability of human erythrocyte cholinesterase activity. Clin. Chem 53, 1358–63. 10.1373/clinchem.2006.085258 [DOI] [PubMed] [Google Scholar]
- Mason HJ, 2000. The recovery of plasma cholinesterase and erythrocyte acetylcholinesterase activity in workers after over-exposure to dichlorvos. Occup. Med. (Lond) 50, 343–7. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Barr DB, Hauser R, 2006. Thyroid hormones in relation to urinary metabolites of non-persistent insecticides in men of reproductive age. Reprod. Toxicol 22, 437–442. 10.1016/j.reprotox.2006.02.005 [DOI] [PubMed] [Google Scholar]
- Molina PE, 2018. Thyroid Gland, in: Endocrine Physiology, 5e. McGraw-Hill Education, New York, NY. [Google Scholar]
- Piccoli C, Cremonese C, Koifman RJ, Koifman S, Freire C, 2016. Pesticide exposure and thyroid function in an agricultural population in Brazil. Environ. Res 151, 389–398. 10.1016/j.envres.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Tate C. a., Cousins MM, Slotkin T. a., 2002. Fetal Chlorpyrifos Exposure: Adverse Effects on Brain Cell Development and Cholinergic Biomarkers Emerge Postnatally and Continue into Adolescence and Adulthood. Environ. Health Perspect 111, 536–544. 10.1289/ehp.5828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JR, E C, 2005. Effects of repeated oral postnatal exposure to chlorpyrifos on cholinergic neurochemistry in developing rats. Toxicol. Sci 84, 352–9. 10.1093/toxsci/kfi081 [DOI] [PubMed] [Google Scholar]
- Sapbamrer R, Hongsibsong S, 2019. Effects of prenatal and postnatal exposure to organophosphate pesticides on child neurodevelopment in different age groups: a systematic review. Environ. Sci. Pollut. Res 26, 18267–18290. 10.1007/s11356-019-05126-w [DOI] [PubMed] [Google Scholar]
- Smallridge RC, Carr FE, Fein HG, 1991. Diisopropylfluorophosphate (DFP) reduces serum prolactin, thyrotropin, luteinizing hormone, and growth hormone and increases adrenocorticotropin and corticosterone in rats: Involvement of dopaminergic and somatostatinergic as well as cholinergic pathways. Toxicol. Appl. Pharmacol 108, 284–295. 10.1016/0041-008X(91)90118-X [DOI] [PubMed] [Google Scholar]
- Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin T. a, 1997. Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol. Appl. Pharmacol 145, 158–74. 10.1006/taap.1997.8171 [DOI] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Butcher CR, Gahagan S, Checkoway H, Alexander BH, Al-Delaimy WK, 2017. Acetylcholinesterase activity and time after a peak pesticide-use period among Ecuadorian children. Int. Arch. Occup. Environ. Health 10.1007/s00420-017-1265-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Himes JH, Jacobs DR, Alexander BH, Gunnar MR, 2013. Acetylcholinesterase activity and neurodevelopment in boys and girls. Pediatrics 132, e1649–e1658. 10.1542/peds.2013-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Hood N, Suárez-Torres J, Gahagan S, Gunnar MR, López-Paredes D, 2019. Associations of acetylcholinesterase activity with depression and anxiety symptoms among adolescents growing up near pesticide spray sites. Int. J. Hyg. Environ. Health 222, 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Jacobs DR, Himes JH, Alexander BH, Lazovich D, Gunnar M, 2012. Lower acetylcholinesterase activity among children living with flower plantation workers. Environ. Res 114, 53–9. 10.1016/j.envres.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhartono S, Kartini A, Subagio HW, Budiyono, Utari A, Suratman S, Sakundarno M, 2018. Pesticide exposure and thyroid function in elementary school children living in an agricultural area, Brebes District, Indonesia. Int. J. Occup. Environ. Med 9, 137–144. 10.15171/ijoem.2018.1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P, 2011. Anticholinesterase Agents, in: Brunton L, Chabner B, Knollmann B (Eds.), Goodman & Gilman’s The Pharmacological Basis of Therapeutics. McGraw Hill Medical. [Google Scholar]
- World Health Organization, 2008. Training Course on Child Growth Assessment 7, 25–36. [Google Scholar]
- World Health Organization Multicentre Growth Reference Study Group, 2006. World Health Organization Child Growth Standards based on length/height, weight and age. Acta Paediatr. 450, 76–85. [Google Scholar]


