Abstract
Background:
Primary aldosteronism is a common cause of treatment-resistant hypertension. However, evidence from local health systems suggests low rates of testing for primary aldosteronism.
Objective:
To evaluate testing rates for primary aldosteronism and evidence-based hypertension management in patients with treatment-resistant hypertension.
Design:
Retrospective cohort study.
Setting:
U.S. Veterans Health Administration.
Participants:
Veterans with apparent treatment-resistant hypertension (n = 269 010) from 2000 to 2017, defined as either 2 blood pressures (BPs) of at least 140 mm Hg (systolic) or 90 mm Hg (diastolic) at least 1 month apart during use of 3 antihypertensive agents (including a diuretic), or hypertension requiring at least 4 antihypertensive classes.
Measurements:
Rates of primary aldosteronism testing (plasma aldosterone–renin) and the association of testing with evidence-based treatment using a mineralocorticoid receptor antagonist (MRA) and with longitudinal systolic BP.
Results:
4277 (1.6%) patients who were tested for primary aldosteronism were identified. An index visit with a nephrologist (hazard ratio [HR], 2.05 [95% CI, 1.66 to 2.52]) or an endocrinologist (HR, 2.48 [CI, 1.69 to 3.63]) was associated with a higher likelihood of testing compared with primary care. Testing was associated with a 4-fold higher likelihood of initiating MRA therapy (HR, 4.10 [CI, 3.68 to 4.55]) and with better BP control over time.
Limitations:
Predominantly male cohort, retrospective design, susceptibility of office BPs to misclassification, and lack of confirmatory testing for primary aldosteronism.
Conclusion:
In a nationally distributed cohort of Veterans with apparent treatment-resistant hypertension, testing for primary aldosteronism was rare and was associated with higher rates of evidence-based treatment with MRAs and better longitudinal BP control. The findings reinforce prior observations of low adherence to guideline-recommended practices in smaller health systems and underscore the urgent need for improved management of patients with treatment-resistant hypertension.
Primary Funding Source:
National Institutes of Health.
Hypertension affects 46% of the adult population in the United States and is a leading risk factor for disability, cardiovascular morbidity, and mortality (1, 2). Although treatment reduces morbidity and mortality, approximately 17% to 20% of patients using antihypertensive medications have apparent treatment-resistant hypertension (3), defined as inadequately controlled blood pressure (BP) with 3 antihypertensive medications, including a diuretic, or a requirement for at least 4 antihypertensive medications to achieve adequate control. Compared with patients who require fewer antihypertensive agents, those with apparent treatment-resistant hypertension are at increased risk for cardiovascular and all-cause mortality, independent of BP control (4). High-quality evidence supports the use of mineralocorticoid receptor antagonist (MRA) therapy for management of treatment-resistant hypertension (5, 6).
Primary aldosteronism is a common cause of secondary hypertension and is highly prevalent among patients with treatment-resistant hypertension (7). Primary aldosteronism is associated with a 4- to 12-fold increased risk for adverse cardiovascular events (including atherosclerotic cardiovascular disease and arrhythmias) compared with primary hypertension (8–13) and can be effectively treated with MRAs or surgery (if lateralization is present) (14). Accordingly, several national and international medical societies, including the American College of Cardiology/American Heart Association (2) and the Endocrine Society (14), recommend assessing for primary aldosteronism by measuring plasma aldosterone–renin activity or concentration in patients with treatment-resistant hypertension and those who have hypertension with hypokalemia.
Studies of health systems in California (15), Illinois (16), and New York (17) found that testing rates for primary aldosteronism were less than 3% among patients for whom it is recommended. However, no similar study has been performed on a large scale, and whether testing rates are low in a large, highly integrated health care system is unknown. We aimed to evaluate the frequency of testing for primary aldosteronism in U.S. Veterans with incident apparent treatment-resistant hypertension and factors associated with testing. We also sought to assess whether testing was associated with evidence-based treatment for apparent treatment-resistant hypertension with MRA therapy and with differences in longitudinal BP control.
Methods
Detailed information is provided in the Supplemental Methods section of the Supplement (available at Annals.org).
Data Source
We used national Veterans Health Administration (VHA) data from the VHA Corporate Data Warehouse, which contains detailed diagnostic codes, laboratory results, vital signs, and pharmacy fill records on approximately 9 million Veterans followed by the VHA nationally (18). The Institutional Review Board at the Corporal Michael J. Crescenz VA Medical Center in Philadelphia, Pennsylvania, approved the study.
Patient Population
The study cohort was derived from the Antihypertensives in Obesity Management Cohort, an observational cohort study that includes more than 1 million Veterans with incident hypertension (defined as ≥1 outpatient billing code for essential hypertension and ≥2 consecutive antihypertensive agent pharmacy fills ≤1 year apart) from 1 January 2000 to 31 December 2017 (followed through 31 July 2019) and evidence of receipt of primary care in the VHA (19, 20). Participants were excluded if they had end-stage kidney disease or no body mass index (BMI) documented in the year before incident hypertension.
For the current study, we restricted the cohort to Veterans with incident apparent treatment-resistant hypertension, defined as either 2 successive BPs of at least 140 mm Hg (systolic) or 90 mm Hg (diastolic) at least 1 month apart during use of 3 antihypertensive agents (including a diuretic), or receipt of 4 antihypertensive classes (8, 15). We excluded patients who had testing for primary aldosteronism or initiated MRA treatment before meeting criteria for apparent treatment-resistant hypertension and who had chronic kidney disease stage 4 or 5 or end-stage kidney disease on or before meeting criteria for apparent treatment-resistant hypertension. The index date was defined as the first date that a Veteran met criteria for apparent treatment-resistant hypertension.
Covariates
We determined baseline covariates using previously published algorithms (see the Supplemental Methods section of the Supplement for details) (21–24). Baseline comorbidities were defined as those diagnosed on or anytime before the index date. We used VHA pharmacy fill data to determine antihypertensive and statin use. Baseline systolic BP (SBP) and diastolic BP (DBP) were defined as the mean values during the year before the index date, restricted to BPs measured in primary care and select internal medicine subspecialty practices. For baseline BMI and laboratory values, we recorded values closest to the index date, limited to those collected in the year before the index date. We also reported the minimum serum potassium level between 1 January 2000 and the index date. Adherence was assessed using VHA pharmacy fill data and was calculated as the proportion of days covered by antihypertensive medication fills in the year before cohort entry (25). Provider and VHA medical center were defined by the clinic where the patient was seen on the index date.
End Points
The primary end point was testing for primary aldosteronism, defined as concomitant measurement of blood aldosterone concentration and either plasma renin activity or plasma renin concentration (8, 26). Secondary end points were initiation of MRA treatment and change in SBP over time.
Censoring
We followed patients until they achieved the end point, were lost to follow-up, or died, whichever occurred first. We defined loss to follow-up as no VHA encounters or pharmacy fills for 2 years.
Statistical Analysis
We present demographic, clinical, laboratory, provider, and center characteristics across patients by primary aldosteronism testing status on or after the index date. Continuous variables were compared using the Kruskal–Wallis test and are reported as medians and interquartile ranges (IQRs), and categorical and binary variables were compared using the χ2 test and are reported as numbers and percentages.
We performed multivariable mixed-effects survival modeling to evaluate factors associated with time to primary aldosteronism testing on or after incident apparent treatment-resistant hypertension, as well as the association of time-updated primary aldosteronism testing with initiation of MRA treatment. The analyses applied a random slope and intercept model to account for medical center and provider random effects, with an independent covariance structure (27). We selected covariates a priori based on factors known or suspected to potentially influence management of apparent treatment-resistant hypertension, including patient-level factors (age, sex, race, BMI, SBP, DBP, estimated glomerular filtration rate [eGFR], minimum potassium level, diabetes mellitus, heart failure, arrhythmia, atherosclerotic cardiovascular disease, stroke, smoking history, antihypertensive class, number of antihypertensive agents, cancer, dementia, alcohol misuse, and adherence), provider-level factors (specialty and number of patients with treatment-resistant hypertension), and center-level factors (number of patients seen annually, number of patients with treatment-resistant hypertension, rural location, and academic affiliation) (8, 15). Continuous covariates were assessed for nonlinear relationships with the outcome and incorporated using restricted cubic splines where appropriate.
For the analyses evaluating primary aldosteronism testing and initiation of MRA therapy, incidence rates were calculated per 1000 person-years of follow-up. Restricted mean survival time was used to estimate the difference in average survival time before initiation of therapy across testing status groups at 5 years of follow-up (28, 29). We assessed for effect modification by index year and hypokalemia (minimum prior potassium level ≤3.5 or >3.5 mmol/L) and stratified the analyses by hypokalemia status. In sensitivity analyses, we censored patients at the time of adrenalectomy or at the time of testing for those with biochemical evidence of primary aldosteronism (defined as plasma aldosterone concentration ≥15 ng/dL and aldosterone–renin activity ratio ≥30 or aldosterone–renin concentration ratio ≥4.8, along with suppressed renin) because many of these patients benefit from adrenalectomy rather than MRA treatment and adrenalectomies may have been performed outside the VHA (8, 14).
In secondary analyses, we performed linear mixed-effects modeling to evaluate the association of testing for primary aldosteronism with BP over time, with random intercepts for medical center, provider, and patient. Sensitivity analyses applied marginal structural modeling with stabilized inverse probability weighting to account for time-updated confounding by MRA use, with weight trimming at the first and 99th percentiles (30). We used the same covariates for the secondary analyses as in the primary analyses because of overlapping factors that are likely to influence treatment-resistant hypertension management (8, 15).
We performed all analyses using Stata, version 16.0 (StataCorp), specifically the mestreg (31), strmst2 (32), and mixed (33) packages.
In the year before incident treatment-resistant hypertension, BMI was missing in 304 (0.1%) patients, and eGFR and potassium level were missing in 11 400 (4%) patients. We addressed missing data by multiple imputation with chained equations, using 10 imputed data sets to iterate each analysis (34).
Role of the Funding Source
This research was supported in part by a grant from the National Institutes of Health. The funding source had no role in study design or implementation. The interpretation and reporting of these data are the responsibility of the authors and should in no way be seen as an official policy of or interpretation by the National Institutes of Health.
Results
Baseline Characteristics
A total of 279 376 Veterans met criteria for incident apparent treatment-resistant hypertension during follow-up. After exclusion of patients with advanced chronic kidney disease (n = 8358) and those who had testing for primary aldosteronism (n = 1786) or received an MRA (n = 222) before meeting criteria for apparent treatment-resistant hypertension, 269 010 patients met inclusion criteria (Figure 1). In the overall cohort, the median age was 65 years (IQR, 58 to 72 years), 4% were women, and 19% were Black Non-Hispanic race (Table 1; Supplement Table 1, available at Annals.org). There were 517 patients who had biochemical evidence suggesting primary aldosteronism based on their initial testing.
Figure 1.
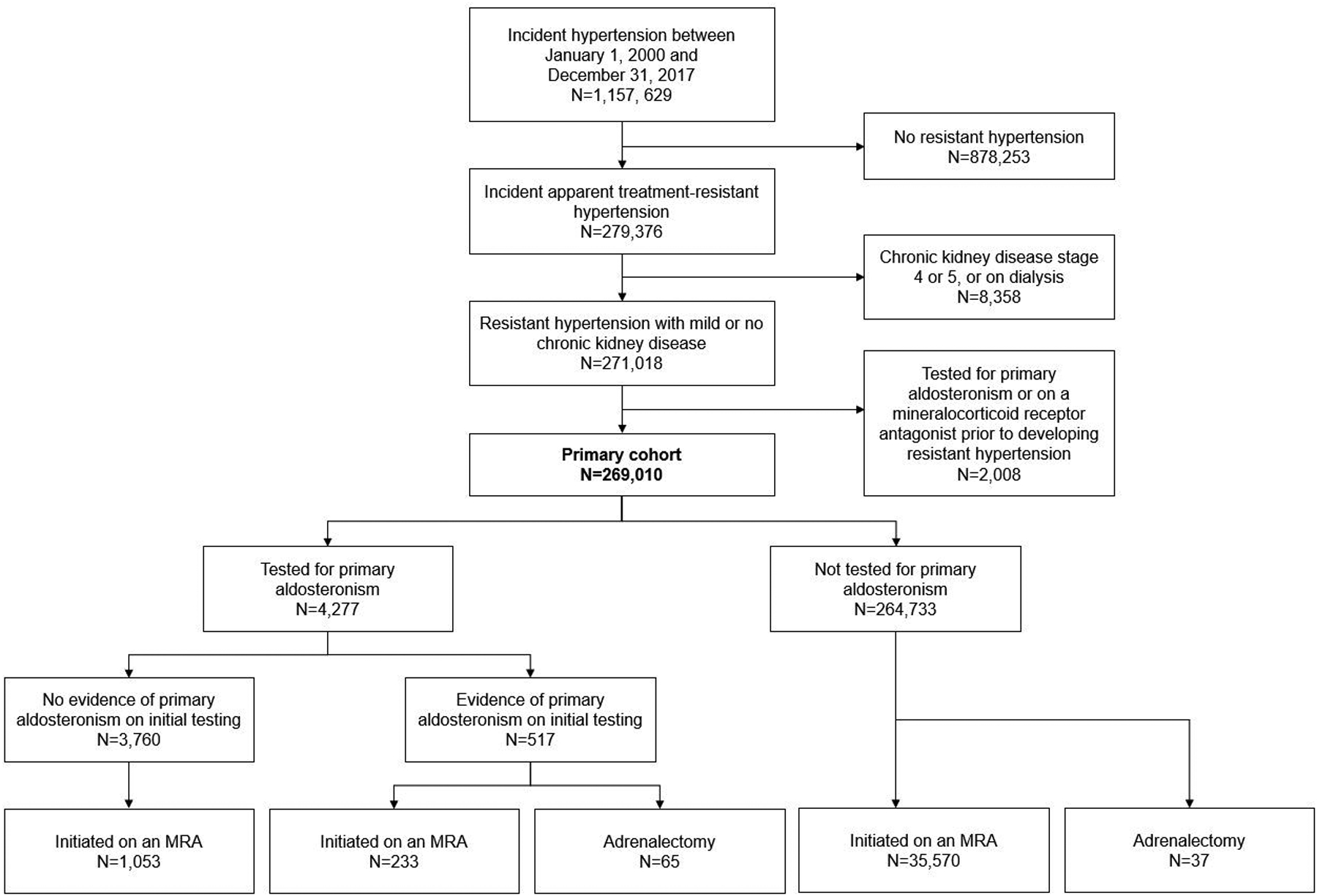
Cohort derivation and primary aldosteronism testing status, initial testing results, MRA use, and adrenalectomy status among participants who met inclusion criteria. MRA = mineralocorticoid receptor antagonist.
Table 1.
Baseline Patient, Provider, and Center Characteristics, by Primary Aldosteronism Testing Status*
| Characteristic | Overall Cohort (n = 269 010) |
No Testing (n = 264 733) |
Testing (n = 4277) |
|---|---|---|---|
| Patient level | |||
| Median age (IQR), y | 65 (58–72) | 65 (58–72) | 59 (52–66) |
| Female, n (%) | 11 009 (4) | 10 675 (4) | 334 (8) |
| Black non-Hispanic, n (%) | 50 883 (19) | 49 517 (19) | 1366 (32) |
| Obese, n (%) | 135 551 (50) | 133 164 (50) | 2387 (56) |
| Median systolic blood pressure (IQR), mm Hg | 140 (132–150) | 140 (132–150) | 145 (136–156) |
| Median diastolic blood pressure (IQR), mm Hg | 79 (72–86) | 79 (72–86) | 84 (76–91) |
| Hypokalemia, n (%) | 8313 (3) | 7878 (3) | 435 (10) |
| Diabetes, n (%) | 106 617 (40) | 104 892 (40) | 1725 (40) |
| Obstructive sleep apnea, n (%) | 28 342 (11) | 27 918 (11) | 424 (10) |
| Heart failure, n (%) | 37 678 (14) | 37 297 (14) | 381 (9) |
| Arrhythmia, n (%) | 66 023 (25) | 65 268 (25) | 755 (18) |
| Atherosclerotic cardiovascular disease, n (%) | 64 479 (24) | 63 677 (24) | 802 (19) |
| Antihypertensive class, n (%) | |||
| ACEI or ARB | 218 059 (81) | 214 571 (81) | 3488 (82) |
| dCCB | 105 656 (39) | 103 562 (39) | 2094 (49) |
| Thiazide or thiazide-like diuretic | 159 219 (59) | 156 306 (59) | 2913 (68) |
| β-Blocker | 173 369 (64) | 170 779 (65) | 2590 (61) |
| Other | 176 815 (66) | 174 380 (66) | 2435 (57) |
| Number of antihypertensive classes, n (%) | |||
| 3 | 218 523 (81) | 215 215 (81) | 3308 (77) |
| 4 | 48 337 (18) | 47 450 (18) | 887 (21) |
| ≥5 | 2372 (1) | 2290 (1) | 82 (2) |
| Median adherence (IQR), % | 89 (68–98) | 89 (68–98) | 88 (63–99) |
| Provider level | |||
| Median number of patients with resistant hypertension (IQR) | 16 (7–31) | 16 (7–31) | 16 (6–32) |
| Index visit provider specialty, n (%) | |||
| Primary care | 237 369 (88) | 233 580 (88) | 3789 (89) |
| Nephrology | 2920 (1) | 2800 (1) | 120 (3) |
| Endocrinology | 912 (<1) | 876 (<1) | 36 (<1) |
| Cardiology | 28 031 (10) | 27 699 (10) | 332 (8) |
| Center level | |||
| Median number of patients seen annually (IQR) | 55 253 (37 221–87 936) | 55 253 (37 221–87 936) | 60 283 (39 914–96 306) |
| Median number of patients with resistant hypertension (IQR) | 2374 (1700–3716) | 2374 (1700–3716) | 2412 (1735–4405) |
| Rural location, n (%) | 17 796 (7) | 17 683 (7) | 113 (3) |
| Academic affiliation, n (%) | 173 969 (65) | 170 793 (64) | 3176 (74) |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin-receptor blocker; dCCB = dihydropyridine calcium-channel blocker; IQR = interquartile range.
All characteristics were measured on or before the date of incident resistant hypertension. Primary aldosteronism testing occurred on or after the date of incident resistant hypertension. Systolic and diastolic blood pressures were calculated as the mean value in the year before baseline. Laboratory values were the closest value obtained within 1 y before baseline. Hypokalemia was defined as a minimum potassium level ≤3.5 mmol/L before the index date. Medications are listed if the patient had an active fill of the medication on or before baseline based on the number of pills prescribed. A more extensive list of baseline characteristics is provided in Supplement Table 1 (available at Annals.org).
Factors Associated With Testing for Primary Aldosteronism
After a median follow-up of 3.3 years (IQR, 1.0 to 6.7 years) after meeting criteria for apparent treatment-resistant hypertension, 4277 (1.6%) patients had testing for primary aldosteronism. Figure 2 (left) shows the proportion of patients with apparent treatment-resistant hypertension who were tested for primary aldosteronism across each VHA medical center (n = 130). Testing rates ranged from 0% to 6%, and the number of patients with apparent treatment-resistant hypertension was not correlated with testing rates across medical centers (r = 0.17). Figure 2 (right) shows testing rates by index year. Testing rates ranged from 1% to 2% per year, with slightly lower rates among patients who met criteria for apparent treatment-resistant hypertension in more recent years and thus had shorter follow-up.
Figure 2.
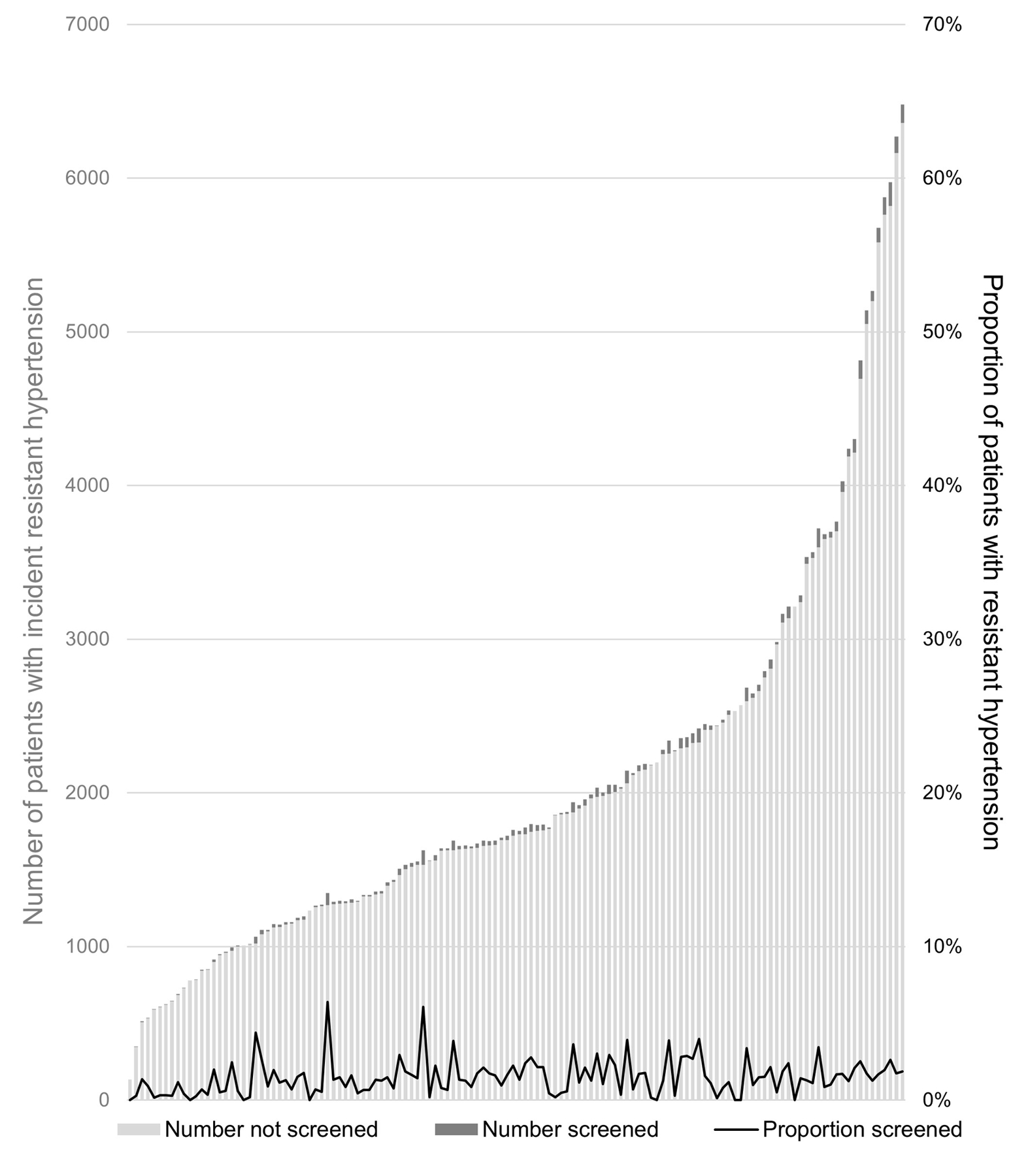
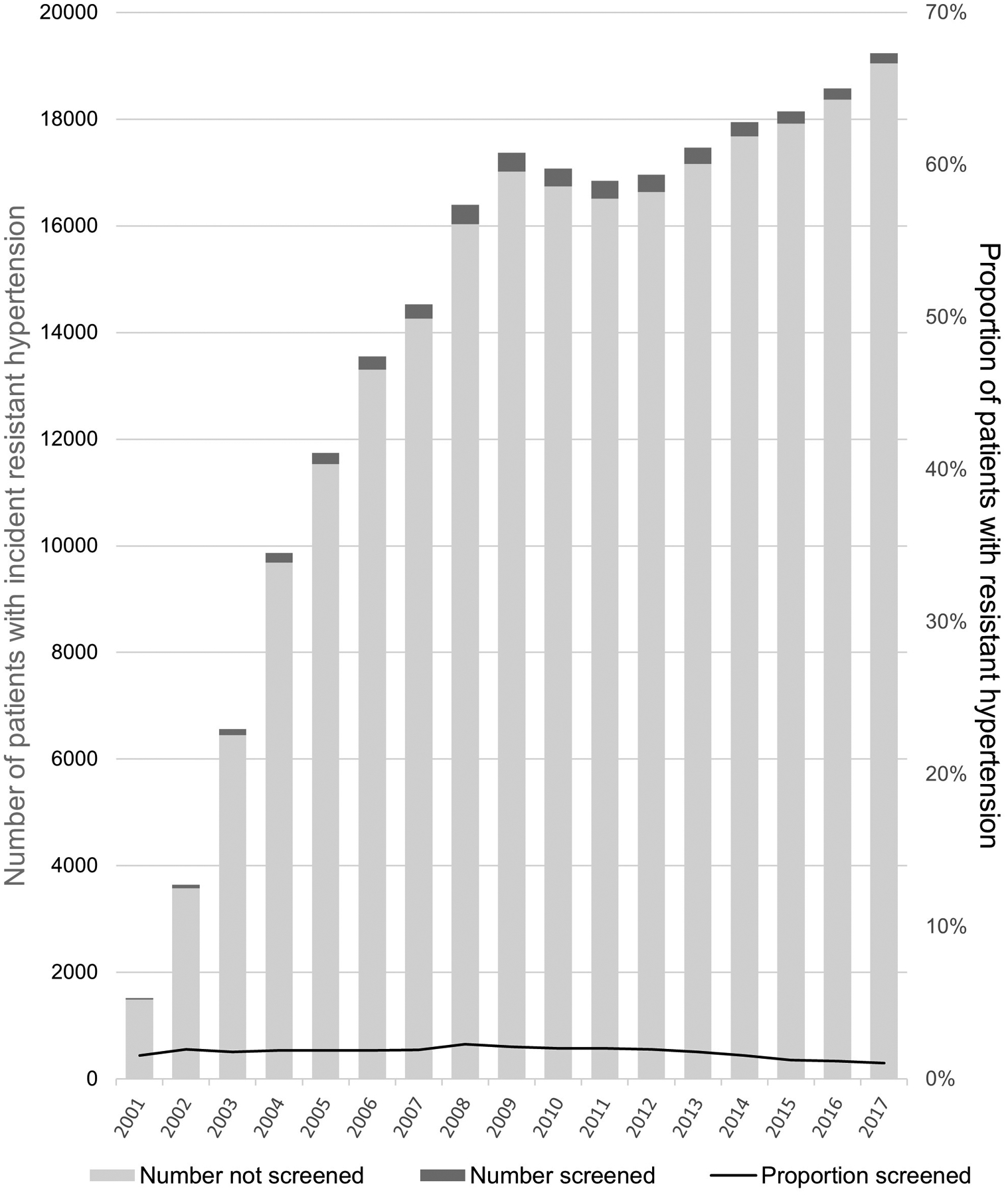
Proportion of patients with apparent treatment-resistant hypertension tested for primary aldosteronism, by medical center (left) and index year (right). The columns depict the total number of patients with treatment-resistant hypertension (light green) and the total number with treatment-resistant hypertension who were tested for primary aldosteronism (dark green), quantified on the left y-axis. The solid black line depicts the proportion of patients with treatment-resistant hypertension who were tested for primary aldosteronism, quantified on the right y-axis.
We performed mixed-effects modeling to evaluate factors associated with primary aldosteronism testing, accounting for differences in testing patterns across providers and centers (Supplement Figure, available at Annals.org). At the patient level, several factors, including hypokalemia (standardized hazard ratio [HR], 1.93 [95% CI, 1.80 to 2.07]) and higher SBP (standardized HR, 1.43 [CI, 1.37 to 1.49]), were associated with a higher likelihood of undergoing testing (Supplement Table 2, available at Annals.org). At the provider level, index visits with a nephrologist (HR, 2.05 [CI, 1.66 to 2.52]) or an endocrinologist (HR, 2.48 [CI, 1.69 to 3.63]), but not a cardiologist, were associated with a higher likelihood of testing compared with primary care. At the center level, rural location was associated with a lower likelihood of testing than nonrural location (HR, 0.53 [CI, 0.31 to 0.91]). The number of patients with treatment-resistant hypertension seen by a provider or center, overall center volume, and center academic affiliation were not meaningfully associated with testing.
Testing for Primary Aldosteronism and Likelihood of Initiating MRA Therapy
We performed mixed-effects survival modeling to evaluate the association of testing for primary aldosteronism with initiation of MRA therapy on or after meeting criteria for apparent treatment-resistant hypertension (Table 2). Similar to the prior analyses, these analyses accounted for differences in testing patterns across providers and centers. At 5 years of follow-up, patients who underwent testing had an average 1.1-year shorter survival time (CI, 0.9 to 1.2 years) before MRA therapy initiation compared with those who were not tested. In models adjusted for patient-, provider-, and center-level factors, testing for primary aldosteronism was associated with a 4-fold higher likelihood of subsequently starting MRA therapy compared with no testing (HR, 4.10 [CI, 3.68 to 4.55]). There was effect modification by hypokalemia status but not index year. Patients with a history of hypokalemia who underwent testing were more likely to be treated with an MRA (HR, 7.11 [CI, 6.25 to 8.10]) than those without hypokalemia (HR, 4.21 [CI, 3.59 to 4.94]). Sensitivity analyses that censored patients at the time of adrenalectomy or at the time of demonstrating biochemical evidence of primary aldosteronism showed similar results.
Table 2.
Likelihood of Initiating MRA Therapy on or After Testing for Primary Aldosteronism Versus No Testing*
| Group | Incidence per 1000 Person-Years | Average Survival Time Before Starting MRA Therapy (95% CI), y† | Adjusted Hazard Ratio (95% CI)‡ | |||
|---|---|---|---|---|---|---|
| No Testing | Testing | No Testing | Testing | Difference | ||
| Overall cohort | 35 | 57 | 4.5 (4.4–4.5) | 3.4 (3.2–3.6) | 1.1 (0.9–1.2) | 4.10 (3.68–4.55) |
| Patients with hypokalemia§ | 43 | 82 | 4.3 (4.2–4.3) | 3.0 (2.8–3.3) | 1.3 (1.0–1.5) | 7.11 (6.25–8.10) |
| Patients without hypokalemia§ | 29 | 44 | 4.5 (4.5–4.5) | 3.8 (3.5–4.0) | 0.7 (0.5–1.0) | 4.21 (3.59–4.94) |
| Censored at positive test result or adrenalectomy | 35 | 52 | 4.5 (4.4–4.5) | 3.6 (3.4–3.8) | 0.9 (0.7–1.1) | 3.75 (3.34–4.20) |
MRA = mineralocorticoid receptor antagonist.
All adjusted analyses included baseline age, sex, race/ethnicity, body mass index, systolic and diastolic blood pressures (mean in previous year), estimated glomerular filtration rate, minimum potassium level, diabetes mellitus, heart failure, arrhythmia, atherosclerotic cardiovascular disease, stroke, smoking history, angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker use, dihydropyridine calcium-channel blocker use, thiazide/thiazide-like diuretic use, β-blocker use, other antihypertensive use, cancer, dementia, alcohol misuse, adherence, provider- and center-level number of patients with resistant hypertension, provider specialty, annual center volume, rural center location, and center academic affiliation. The survival models used mixed-effects modeling with random intercepts for provider and center.
Estimated using restricted mean survival time at 5 y.
Estimated using time-varying survival models evaluating the association of primary aldosteronism testing with initiation of MRA therapy.
Hypokalemia was defined as a minimum potassium level ≤3.5 mmol/L before the index date.
Testing for Primary Aldosteronism and BP Over Time
Using linear mixed-effects modeling, we evaluated the association of testing for primary aldosteronism and longitudinal BPs (Table 3). These analyses accounted for differences in patterns of BP control across patients, providers, and centers. Patients had a median of 17 BPs (IQR, 8 to 31) measured over 4.2 years (IQR, 1.7 to 7.8 years) of follow-up and had a mean SBP of 136.7 mm Hg (CI, 136.4 to 136.9 mm Hg) during follow-up. Although patients who underwent testing had higher baseline SBP (mean, 146.7 vs. 140.7 mm Hg), testing was associated with an average 1.32–mm Hg (CI, −1.50 to −1.14 mm Hg) lower SBP over time in unadjusted analyses (mean decrease in SBP, 10.7 vs. 3.3 mm Hg). In analyses adjusted for patient-, provider-, and center-level covariates (including baseline BP), compared with no testing, testing for primary aldosteronism was associated with an average 1.47–mm Hg (CI, −1.64 to −1.29 mm Hg) lower SBP over time. The results were similar after adjustment for MRA use.
Table 3.
Difference in Systolic Blood Pressure Over Time With Primary Aldosteronism Testing Versus No Testing*
| Variable | Average Systolic Blood Pressure During Follow-up (95% CI), mm Hg | Difference in Systolic Blood Pressure (95% CI), mm Hg |
|---|---|---|
| Unadjusted | 136.7 (136.4 to 136.9) | −1.32 (−1.50 to −1.14) |
| Adjusted for baseline covariates | 136.6 (136.4 to 136.7) | −1.47 (−1.64 to −1.29) |
| Adjusted for mineralocorticoid receptor antagonist use† | 136.7 (136.4 to 137.0) | −1.30 (−1.93 to −0.66) |
All analyses used linear mixed-effects modeling with random intercepts for patient, provider, and center. Adjusted analyses included baseline age, sex, race/ethnicity, body mass index, systolic and diastolic blood pressures (mean in previous year), estimated glomerular filtration rate, minimum potassium level, diabetes mellitus, heart failure, arrhythmia, atherosclerotic cardiovascular disease, stroke, smoking history, angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker use, dihydropyridine calcium-channel blocker use, thiazide/thiazide-like diuretic use, β-blocker use, other antihypertensive use, cancer, dementia, alcohol misuse, adherence, provider- and center-level number of patients with resistant hypertension, provider specialty, annual center volume, rural center location, and center academic affiliation.
These analyses used inverse probability weights to account for time-updated confounding by mineralocorticoid receptor antagonist use.
Discussion
This is, to our knowledge, the first large-scale multicenter study of testing practices for primary aldosteronism. We observed that fewer than 2% of patients with incident apparent treatment-resistant hypertension underwent guideline-recommended testing for primary aldosteronism. Testing rates ranged from 0% to 6% across medical centers and did not correlate to population size of patients with apparent treatment-resistant hypertension. Testing rates also did not change meaningfully over nearly 2 decades of follow-up despite an increasing number of guidelines recommending testing for primary aldosteronism in this population. Our finding of infrequent testing among patients with apparent treatment-resistant hypertension accords with prior studies in smaller health systems (15–17). We also found that consultation with a nephrologist or an endocrinologist and nonrural center location were independently associated with a higher likelihood of testing. In addition, we observed that testing for primary aldosteronism was associated with a substantially higher likelihood of initiation of evidence-based MRA therapy for management of apparent treatment-resistant hypertension (even in the absence of biochemical evidence of primary aldosteronism) and with greater improvement in BP over time.
Although this may be the first large-scale study of testing practices for primary aldosteronism in patients with apparent treatment-resistant hypertension, many studies have established that the disease is not rare. Recent data showed that the incidence of primary aldosteronism in persons evaluated with confirmatory testing (24-hour urinary aldosterone levels after sodium loading) is 11% in patients with normotension and 20% to 22% in those with apparent treatment-resistant hypertension (7, 35). We observed that hypokalemia was associated with a higher likelihood of testing for primary aldosteronism. However, testing was infrequent even among patients with hypokalemia (5% testing rate [Table 1]), for whom testing has been recommended in guidelines for decades (36). Moreover, most patients with primary aldosteronism have a normal serum potassium level, such that a large proportion fall outside the classic description of the disease. Although a reliable testing approach to identify normotensive patients with primary aldosteronism is not available, testing of patients with apparent treatment-resistant hypertension is recommended by guidelines and is achievable in their routine care (26).
We considered several reasons for the low rates of testing. Various barriers to testing for primary aldosteronism have been identified via focus group from the perspective of German primary care clinicians (37). For example, some clinicians preferred an empirical trial of spironolactone without making a diagnosis, and others noted that stopping β-blockers before testing was impractical. However, we observed relatively low rates of MRA therapy initiation after patients met criteria for apparent treatment-resistant hypertension, particularly among those who were not tested for primary aldosteronism. Only 13% of patients with incident apparent treatment-resistant hypertension ultimately started MRA therapy, even though MRAs are recommended in approximately 70% of patients with treatment-resistant hypertension (8). Our results show that empirical MRA therapy in patients with apparent treatment-resistant hypertension is widely underused, particularly among those who are not tested for primary aldosteronism. In addition, testing for primary aldosteronism can proceed without stopping medication use under most circumstances (26).
Generally, failure to test patients with apparent treatment-resistant hypertension for primary aldosteronism may reflect a lack of familiarity with this common and treatable condition or a broader propensity for treatment inertia in this patient population (38). We found no relationship between center or provider volume of patients with apparent treatment-resistant hypertension and likelihood of testing for primary aldosteronism. Nonetheless, we did see substantial variation in testing practices across centers and providers. We observed higher rates of testing among patients seen by endocrinologists and nephrologists (who typically perform confirmatory testing for and oversee management of primary aldosteronism) compared with those seen by primary care providers or cardiologists. We also saw higher rates of evidence-based MRA treatment and better BP control over time among those who had testing, despite higher baseline BPs. These findings support the hypothesis that testing coincides with familiarity with primary aldosteronism and complex hypertension management. Further investigation is needed into barriers to testing for primary aldosteronism and ways to better implement best practices and guidelines among providers and medical centers that care for patients with treatment-resistant hypertension.
Testing for primary aldosteronism in patients with apparent treatment-resistant hypertension is cost-effective and may substantially improve long-term outcomes (39). In patients with positive results who undergo adrenalectomy (for an aldosterone-secreting adenoma), compared with usual care, adrenalectomy is associated with lower risk for all-cause mortality (HR, 0.23 [CI, 0.13 to 0.26]) (40), atrial fibrillation (HR, 0.55 [CI, 0.32 to 0.93]) (41), and chronic kidney disease (difference in cumulative incidence, 6.5 [CI, 2.7 to 10.4]) (42) and an improvement in quality-adjusted life-years (39). In addition to detecting primary aldosteronism, testing can identify patients with suppressed renin and normal aldosterone levels who may particularly benefit from treatment with an MRA or another potassium-sparing diuretic (5, 43). Testing can also help to identify apparent mineralocorticoid excess syndrome and Liddle syndrome (in which patients tend to have low renin and aldosterone levels) (8).
Our study has several strengths. Because of the detailed information in the VHA electronic health record, we were able to carefully identify patients with new-onset apparent treatment-resistant hypertension who were followed at the VHA for their primary care since the time of incident hypertension diagnosis, thus minimizing misclassification of testing rates (for example, due to long-standing apparent treatment-resistant hypertension with prior work-up or outmigration). The highly integrated nature of the care in the VHA makes it unlikely that the testing rates are lower than in health systems with more fragmented care delivery systems. We also had access to pharmacy fill data, facilitating classification of treatment-resistant hypertension based on medications that were more likely administered than if they had been obtained from a standard electronic health record as well as determination of adherence based on the proportion of days with medications filled.
There are also important limitations. Because of the large proportion of men in the VHA, the findings may not be fully generalizable to female patients, although the cohort included more than 11 000 women with apparent treatment-resistant hypertension. Because this is a retrospective cohort study, it is prone to unmeasured confounding. We were unable to measure serum antihypertensive medication levels or directly observe therapy to confirm treatment resistance. In addition, BPs in the electronic health record are susceptible to misclassification of hypertension, in contrast to, for example, out-of-office BP monitoring or research-quality office BPs (44, 45). To mitigate this, we restricted BP measurements to those obtained in practices focused on hypertension management, including primary care and select internal medicine subspecialty practices. Finally, in the absence of individual-level chart reviews, we were not able to determine whether confirmatory testing (such as salt loading, fludrocortisone suppression test, or captopril challenge test) was performed for primary aldosteronism or the results of confirmatory testing, and adrenalectomies are suspected to have been undercaptured (8, 14, 26).
In conclusion, our data provide evidence of considerable underperformance of guideline-recommended testing for primary aldosteronism in a national cohort of Veterans with apparent treatment-resistant hypertension, bolstering similar observations in smaller health systems (15–17). We also showed that testing practices are strongly associated with evidence-based treatment of apparent treatment-resistant hypertension with MRAs and better BP control over time. The reasons for these widespread missed opportunities for testing and treatment are unclear. The consequences of undertesting for primary aldosteronism and underuse of MRAs in patients with apparent treatment-resistant hypertension may be substantial, potentially increasing morbidity and mortality. We identified several factors associated with undertesting. Given the unique infrastructure of the VHA, our findings suggest an opportunity to introduce innovative practices to meaningfully improve education of providers and increase testing to enhance management in this high-risk patient population. Future studies should evaluate implementation of tools to identify patients via the electronic health record and alert providers to their appropriateness for testing.
Supplementary Material
Supplement Figure. Association of patient-, provider-, and center-level factors with testing for primary aldosteronism. The circles represent multivariable-adjusted hazard ratios, and the bars represent 95% CIs. The corresponding values and additional patient-level factors are tabulated in Supplement Table 2 (available at Annals.org). For continuous variables, the hazard ratios were calculated on the basis of each SD change in the variable. All variables were measured at baseline, the date of incident treatment-resistant hypertension. The analyses were adjusted for age, sex, race/ethnicity, body mass index, systolic and diastolic blood pressures (mean in previous year), estimated glomerular filtration rate, hypokalemia (defined as a minimum potassium level ≤3.5 mmol/L before the index date), diabetes mellitus, heart failure, arrhythmia, atherosclerotic cardiovascular disease, stroke, smoking history, angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker use, dihydropyridine calcium-channel blocker use, thiazide/thiazide-like diuretic use, β-blocker use, number of antihypertensive agents, cancer, dementia, alcohol misuse, adherence, provider- and center-level number of patients with treatment-resistant hypertension, provider specialty, annual center volume, rural center location, and center academic affiliation. Hazard ratios were estimated using mixed-effects survival modeling with random intercepts for provider and center.
Grant Support:
Dr. Jordana Cohen was supported by a grant from the National Institutes of Health, National Heart, Lung, and Blood Institute (K23-HL133843).
Footnotes
Disclosures: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M20-4873.
Reproducible Research Statement: Study protocol and statistical code: Available from Dr. Jordana Cohen (jco@pennmedicine.upenn.edu). Data set: Available with appropriate approvals from Dr. Jordana Cohen.
Publisher's Disclaimer: Disclaimer: Department of Veterans Affairs Health Services Research and Development Service work was supported using resources and facilities at the VA Informatics and Computing Infrastructure, VA HSR RES 13–457. The contents of this article do not represent the views of the U.S. Department of Veterans Affairs or the U.S. government.
Contributor Information
Jordana B. Cohen, Perelman School of Medicine, University of Pennsylvania, and Corporal Michael J. Crescenz VA Medical Center, Philadelphia, Pennsylvania.
John T. Leppert, Stanford University School of Medicine, Stanford, California, and Veterans Affairs Palo Alto Health Care System, Palo Alto, California.
James Brian Byrd, University of Michigan Medical School, Ann Arbor, Michigan.
Vivek Bhalla, Stanford University School of Medicine, Stanford, California.
References
- 1.GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–94. doi: 10.1016/S0140-6736(18)32225-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 3.Carey RM, Sakhuja S, Calhoun DA, et al. Prevalence of apparent treatment-resistant hypertension in the United States. Hypertension. 2019;73:424–31. doi: 10.1161/HYPERTENSIONAHA.118.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Sande NGC, de Beus E, Bots ML, et al. ; SMART study group. Apparent resistant hypertension and the risk of vascular events and mortality in patients with manifest vascular disease. J Hypertens. 2018;36:143–50. doi: 10.1097/HJH.0000000000001494 [DOI] [PubMed] [Google Scholar]
- 5.Williams B, MacDonald TM, Morant S, et al. ; British Hypertension Society’s PATHWAY Studies Group. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–68. doi: 10.1016/S0140-6736(15)00257-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao D, Liu H, Dong P, et al. A meta-analysis of add-on use of spironolactone in patients with resistant hypertension. Int J Cardiol. 2017;233:113–7. doi: 10.1016/j.ijcard.2016.12.158 [DOI] [PubMed] [Google Scholar]
- 7.Brown JM, Siddiqui M, Calhoun DA, et al. The unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann Intern Med. 2020;173:10–20. doi: 10.7326/M20-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey RM, Calhoun DA, Bakris GL, et al. ; American Heart Association Professional/Public Education and Publications Committee of the Council on Hypertension; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Genomic and Precision Medicine; Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research; and Stroke Council. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53–e90. doi: 10.1161/HYP.0000000000000084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SM, Huo T, Delia Johnson B, et al. Cardiovascular and mortality risk of apparent resistant hypertension in women with suspected myocardial ischemia: a report from the NHLBI-sponsored WISE Study. J Am Heart Assoc. 2014;3:e000660. doi: 10.1161/JAHA.113.000660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898–902. doi: 10.1161/HYPERTENSIONAHA.110.168948 [DOI] [PubMed] [Google Scholar]
- 11.Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635–42. doi: 10.1161/CIRCULATIONAHA.111.068064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egan BM, Zhao Y, Axon RN, et al. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124:1046–58. doi: 10.1161/CIRCULATIONAHA.111.030189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57:1076–80. doi: 10.1161/HYPERTENSIONAHA.111.170308 [DOI] [PubMed] [Google Scholar]
- 14.Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–916. doi: 10.1210/jc.2015-4061 [DOI] [PubMed] [Google Scholar]
- 15.Jaffe G, Gray Z, Krishnan G, et al. Screening rates for primary aldosteronism in resistant hypertension: a cohort study. Hypertension. 2020;75:650–9. doi: 10.1161/HYPERTENSIONAHA.119.14359 [DOI] [PubMed] [Google Scholar]
- 16.Ruhle BC, White MG, Alsafran S, et al. Keeping primary aldosteronism in mind: deficiencies in screening at-risk hypertensives. Surgery. 2019;165:221–7. doi: 10.1016/j.surg.2018.05.085 [DOI] [PubMed] [Google Scholar]
- 17.Sivarajah M, Beninato T, Fahey TJ 3rd. Adherence to consensus guidelines for screening of primary aldosteronism in an urban healthcare system. Surgery. 2020;167:211–5. doi: 10.1016/j.surg.2019.05.087 [DOI] [PubMed] [Google Scholar]
- 18.Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33:1203–11. doi: 10.1377/hlthaff.2014.0054 [DOI] [PubMed] [Google Scholar]
- 19.Suarez J, Cohen JB, Potluri V, et al. Racial disparities in nephrology consultation and disease progression among Veterans with CKD: an observational cohort study. J Am Soc Nephrol. 2018;29:2563–73. doi: 10.1681/ASN.2018040344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serper M, Weinberg EM, Cohen JB, et al. Mortality and hepatic decompensation in patients with cirrhosis and atrial fibrillation treated with anticoagulation. Hepatology. 2020. doi: 10.1002/hep.31264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. 2004;57:131–41. [DOI] [PubMed] [Google Scholar]
- 22.Bullano MF, Kamat S, Willey VJ, et al. Agreement between administrative claims and the medical record in identifying patients with a diagnosis of hypertension. Med Care. 2006;44:486–90. [DOI] [PubMed] [Google Scholar]
- 23.Quan H, Li B, Saunders LD, et al. ; IMECCHI Investigators. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43:1424–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suchard MA, Schuemie MJ, Krumholz HM, et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis. Lancet. 2019;394:1816–26. doi: 10.1016/S0140-6736(19)32317-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sattler EL, Lee JS, Perri M 3rd. Medication (re)fill adherence measures derived from pharmacy claims data in older Americans: a review of the literature. Drugs Aging. 2013;30:383–99. doi: 10.1007/s40266-013-0074-z [DOI] [PubMed] [Google Scholar]
- 26.Byrd JB, Turcu AF, Auchus RJ. Primary aldosteronism: practical approach to diagnosis and management. Circulation. 2018;138:823–35. doi: 10.1161/CIRCULATIONAHA.118.033597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Littell RC, Pendergast J, Natarajan R. Modelling covariance structure in the analysis of repeated measures data. Stat Med. 2000;19:1793–819. [DOI] [PubMed] [Google Scholar]
- 28.Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013;13:152. doi: 10.1186/1471-2288-13-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uno H, Wittes J, Fu H, et al. Alternatives to hazard ratios for comparing the efficacy or safety of therapies in noninferiority studies. Ann Intern Med. 2015;163:127–34. doi: 10.7326/M14-1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–64. doi: 10.1093/aje/kwn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crowther MJ. Multilevel mixed-effects parametric survival analysis: estimation, simulation, and application. Stata J. 2019;19:931–49. doi: 10.1177/1536867X19893639 [DOI] [Google Scholar]
- 32.Cronin A, Tian L, Uno H. strmst2 and strmst2pw: New commands to compare survival curves using the restricted mean survival time. Stata J. 2016;16:702–16. doi: 10.1177/1536867X1601600310 [DOI] [Google Scholar]
- 33.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata, Third Edition. Stata Pr; 2012. [Google Scholar]
- 34.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–99. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 35.Calhoun DA, Nishizaka MK, Zaman MA, et al. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40:892–6. [DOI] [PubMed] [Google Scholar]
- 36.Chobanian AV, Bakris GL, Black HR, et al. ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- 37.Hannemann A, Bidlingmaier M, Friedrich N, et al. Screening for primary aldosteronism in hypertensive subjects: results from two German epidemiological studies. Eur J Endocrinol. 2012;167:7–15. doi: 10.1530/EJE-11-1013 [DOI] [PubMed] [Google Scholar]
- 38.Harle CA, Harman JS, Yang S. Physician and patient characteristics associated with clinical inertia in blood pressure control. J Clin Hypertens (Greenwich). 2013;15:820–4. doi: 10.1111/jch.12179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lubitz CC, Economopoulos KP, Sy S, et al. Cost-effectiveness of screening for primary aldosteronism and subtype diagnosis in the resistant hypertensive patients. Circ Cardiovasc Qual Outcomes. 2015;8:621–30. doi: 10.1161/CIRCOUTCOMES.115.002002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu VC, Wang SM, Chang CH, et al. Long term outcome of aldosteronism after target treatments. Sci Rep. 2016;6:32103. doi: 10.1038/srep32103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossi GP, Maiolino G, Flego A, et al. ; PAPY Study Investigators. Adrenalectomy lowers incident atrial fibrillation in primary aldosteronism patients at long term. Hypertension. 2018;71:585–91. doi: 10.1161/HYPERTENSIONAHA.117.10596 [DOI] [PubMed] [Google Scholar]
- 42.Hundemer GL, Curhan GC, Yozamp N, et al. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension. 2018;72:658–66. doi: 10.1161/HYPERTENSIONAHA.118.11568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams B, MacDonald TM, Morant SV, et al. ; British Hypertension Society programme of Prevention And Treatment of Hypertension With Algorithm based Therapy (PATHWAY) Study Group. Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies. Lancet Diabetes Endocrinol. 2018;6:464–75. doi: 10.1016/S2213-8587(18)30071-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen JB, Lotito MJ, Trivedi UK, et al. Cardiovascular events and mortality in white coat hypertension: a systematic review and meta-analysis. Ann Intern Med. 2019;170:853–62. doi: 10.7326/M19-0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myers MG. The great myth of office blood pressure measurement. J Hypertens. 2012;30:1894–8. doi: 10.1097/HJH.0b013e3283577b05 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure. Association of patient-, provider-, and center-level factors with testing for primary aldosteronism. The circles represent multivariable-adjusted hazard ratios, and the bars represent 95% CIs. The corresponding values and additional patient-level factors are tabulated in Supplement Table 2 (available at Annals.org). For continuous variables, the hazard ratios were calculated on the basis of each SD change in the variable. All variables were measured at baseline, the date of incident treatment-resistant hypertension. The analyses were adjusted for age, sex, race/ethnicity, body mass index, systolic and diastolic blood pressures (mean in previous year), estimated glomerular filtration rate, hypokalemia (defined as a minimum potassium level ≤3.5 mmol/L before the index date), diabetes mellitus, heart failure, arrhythmia, atherosclerotic cardiovascular disease, stroke, smoking history, angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker use, dihydropyridine calcium-channel blocker use, thiazide/thiazide-like diuretic use, β-blocker use, number of antihypertensive agents, cancer, dementia, alcohol misuse, adherence, provider- and center-level number of patients with treatment-resistant hypertension, provider specialty, annual center volume, rural center location, and center academic affiliation. Hazard ratios were estimated using mixed-effects survival modeling with random intercepts for provider and center.


