Abstract
While the neurotoxic effects of heavy metals at even low levels have been well-studied, few studies have examined the cardiovascular effects of heavy metals on resting heart rate and these have focused on adult populations. The present study aimed to examine the association between low-level environmental lead and mercury exposure and resting heart rate in community adolescents. As part of the China Jintan Cohort Study, 532 adolescents aged 12 years (SD = 0.6) were tested for blood levels of lead (BLL) and mercury (BML) and resting heart rate (RHR). Generalized linear models were conducted to test the relationship between BLL and BML and RHR, controlling for children’s sex, age, and socioeconomic status. Analyses were clustered at the preschool level when the children were recruited to adjust for standard error. The mean (SD) BLL and BML were 3.14 (SD = 1.19) μg/dL and and 1.26 (SD = 0.68) μg/L at age 12 years, respectively. After adjusting for confounders, we found a significant interaction between BML and BLL in predicting RHR in boys (B = −1.27, SE = 0.49, p < .01, n = 289). We created BLL and BML groups in boys based on median cut-offs. Boys in the High BLL/High BML group had significantly lower RHR (mean = 84.22 beats per minute [bpm], SD = 8.77, n = 61) than boys in the Low BLL/Low BML group (mean = 89.03 bpm, SD = 10.75, n = 69; p < 0.05). BML and BLL did not interact to predict RHR in girls (B = −0.18, SE = 0.88, p > 0.05, n = 242). Combined high BLL and BML were associated with low RHR in community adolescent boys. Low RHR is an indication of chronic under-arousal and has been implicated to psychopathology, particularly for externalizing behavior. Our findings may stimulate further communication and research in this area.
Keywords: lead, mercury, heavy metal resting heart rate, cardiovascular, autonomic function
1. Introduction
Despite concerted efforts in reducing heavy metal pollution in the past few decades, lead and mercury exposure continues to be a significant threat to public health, particularly in countries with fast economic growth yet inadequate environmental regulation (Grandjean et al., 2016). This is particularly true in China (Ji et al., 2011; Kim et al., 2020; Wang et al., 2019). While high levels of either lead or mercury exposure have long been well known as detrimental to cognitive functioning and behavioral outcomes (Bellinger, 2017; Chen et al., 2007; Grandjean & Landrigan, 2014; Liu et al., 2014; Pan et al., 2018; Sripada, 2017), in the past few decades, increasing attention has been given to how low exposure of such heavy metals adversely affects physical health (Arnold & Liu, 2020; Kim et al., 2013; Liu, Liu, et al., 2015; Shi et al., 2017). A recent epidemiological study in a sample of 14,289 American adults reported a positive association between low-level lead exposure (mean blood lead level: 2.71 μg/dL) and cardiovascular disease mortality and ischameic heart disease mortality (Lanphear et al., 2018). Similarly, a recent study done in a high-fish consuming population reported that mean male blood mercury levels of 8.06 μg/L diminished cardio-protective effects of omega-3 (Hu et al., 2017). These studies strengthen the need of research on the effects of low lead and mercury levels on physical health, which have been largely underreported in the past, particularly in children.
Emerging research supports the association between lead and mercury exposure and adverse cardiovascular outcomes (Gribble et al., 2015; Poręba et al., 2011). Studies have reported an association between lead exposure and sympathetic overactivation (Simões et al., 2016), reduced heart rate recovery (Evranos et al., 2016), and diminished autonomic regulation (Gump et al., 2011). Similarly, mercury exposure has been associated with reduced heart rate variability (Lim et al., 2010; Beatriz Valera et al., 2011), reduced heart rate recovery (Yilmaz et al., 2016), and diminished parasympathetic activity (Valera et al., 2008). However, an association between mercury exposure and long-term cardiovascular outcomes is unclear as previous epidemiological studies did not identify a significant relationship (Hu et al., 2018; Karagas et al., 2012; Mozaffarian et al., 2011).
However, while increasing attention has been directed towards the cardiovascular effects of heavy metal exposure, several gaps remain in the literature. Firstly, few studies have examined the effects of lead and mercury exposure on resting heart rate (Böckelmann et al., 2011; Gump et al., 2011; Tajik et al., 2018). Resting heart rate has been increasingly associated as a biological predictor for increased risk for adverse cardiovascular and behavioral outcomes (Fox et al., 2007; Ortiz & Raine, 2004; Williams et al., 2015). Secondly, previous research examining the heavy metal-resting heart rate relationship have reported mixed findings and have been largely limited to adult samples (Böckelmann et al., 2011; Virtanen et al., 2007). The findings of these studies cannot be generalized to children, as childhood represents a unique time of somatic and neurodevelopment (Falck et al., 2015; Faustman et al., 2000). Thirdly, few studies have assessed the effect of low-level lead exposure (<10 μg/dL) or low-level mercury exposure (<5 μg/L) on resting heart rate (Gregory et al., 2016) and no previous study has assessed the combined effects of lead and mercury exposure on resting heart rate (Gambelunghe et al., 2016; Gump et al., 2011; Gump et al., 2005; Piikivi, 1989; Valera et al., 2012). Utilizing a large community sample of Chinese adolescents at age 12 years, the present study seeks to address these gaps by examining the relationship between low blood lead and mercury levels and resting heart rate.
2. Materials and Methods
2.1. Design, Participants and Procedure
The present study is part of the China Jintan Child Cohort Study, which is an ongoing longitudinal project that aimed to study the effect of environmental exposures, such as lead, on children’s neurobehavioral outcomes. The cohort study originally invited 1656 children aged 3 to 5 years from 4 preschools in Jintan, Jiangsu Province, China during 2004–2005 who were representative of the preschoolers in the city in terms of sex, age, and residential regions. A total of 1100 children were retained during the Wave I (2005–2007) and were followed up during the Wave II data collection (2011–2013). All children in Wave II were invited to receive a physical examination which included a blood draw that assessed heavy metal concentrations. They were also invited to participate in psychophysiology testing which would occur in a controlled laboratory in Jintan Hospital. Attrition data is available in the Supplemental Figure. Detailed information on this cohort, including subjects, recruitment and procedures is reported elsewhere (Liu, Cao, et al., 2015; Liu et al., 2011; Liu et al., 2010). The present analysis utilizes information on blood metal levels and resting heart rate collected during Wave II. Institutional Review Board approval was obtained from the University of Pennsylvania and the ethical committee for research at Jintan Hospital.
The research team obtained written informed consent from parents, and verbal informed assent from adolescents prior to second wave of data collection. Children provided general information about their sex and age in classrooms during school hours. Parents provided information about their education levels, family region, and income. Blood samples were collected by trained pediatric nurses at Jintan People’s Hospital. Resting heart rate was recorded in a psychophysiological lab in Jintan People’s Hospital that was sound-proofed and was air-conditioned to 72° F (22°C).
2.2. Measures
2.2.1. Blood lead level (BLL) and blood mercury level (BML)
Blood samples were obtained at school health clinics located at each elementary school attended by cohort children. Trained pediatric nurses collected approximately 0.5 mL of venous blood utilizing a standardized protocol to avoid contamination. Samples were frozen and shipped to the Research Center for Environmental Medicine of Children at Shanghai Jiaotong University where they were analyzed using inductively coupled plasma mass spectrometry. Each specimen was analyzed twice and Kaulson Laboratories provided blood lead reference materials for quality control. The limit of detection was 1.0 μg/dL. More details on this process are provided in Liu et al. (Liu et al., 2013; Liu, Liu, et al., 2015; Liu et al., 2014). Data were available for 1048 subjects.
2.2.2. Resting heart rate (RHR)
Children in Wave II who participated in psychophysiology testing underwent a resting heart rate assessment. RHR was collected in a controlled psychophysiology lab, and full protocol was implemented for every child. Specifically, cohort children were invited to a controlled lab for psychophysiological testing. The laboratory is located on the diagnostic floor of Jintan Hospital. Upon arrival to the laboratory with limited noise level, all children were asked to sit and relax for 3–5 minutes. Then we collected other physical health information and asked a few general health questions for approximately 15 minutes to further relax the children. Afterwards, earphones were given to children to watch popular cartoon clips (Xi Yang Yang). Electrocardiogram leads were then attached. Children watched the video clips for 3 minutes prior and during the RHR collection. A comparison of the 30-second epochs during the 1st and 2nd minute of heartrate showed no considerable differences (F = 1.10, p = 0.35), indicating that children’s activity prior and during the data collection did not elevate “resting” heart rate and thereby did not produce a measure of reactivity rather than rest.
Trained research assistants collected resting heart rate measurements using electrocardiographs (ECG) that were recorded axially on the left and right ribs at the level of the heart (to avoid movement artifact) using silver/silver chloride (Ag/AgCl) adhesive disposable electrodes when children were sitting still. Prior to attaching electrodes, skin was prepared using NuPrep abrasive skin prepping paste. Biopac isotonic recording gel was used as the electrolyte medium. Impedance for ECG was kept below 10kΩ. Data were recorded using a bandpass of 0.5–35 Hz and a 50 Hz notch filter, and the recording was digitized at 1000 Hz. Data were acquired using a BIOPAC MP150 with AcqKnowledge version 4.1 software (BIOPAC Systems, Inc.). The recording period was 2 minutes.
After data collection, ECG data were cleaned for artifacts manually after using AcqKnowledge analytic tools to identify unusually large changes in heart rate. Heart rate was then quantified using AcqKnowledge analytic tools, and custom scripts in MATLAB were used to calculate heart rate in beats per minute (bpm) during the 2-minute resting period. RHR was averaged over 30 seconds epochs and the average of RHR of the four 30-second epochs was used in analysis.
2.2.3. Sociodemographic variables and covariates
Covariates included child sex, age, family region in preschool (i.e. urban, suburban, or rural), and socioeconomic status which was calculated as the standardized score (z score) of the sum of the standardized scores for fathers’ and mothers’ number of years of education, and fathers’ and mothers’ monthly wage (i.e. <500 RMB, 500–1000 RMB, 1000–2000 RMB, 2000–3000 RMB, 3000–5000 RMB, 5000–10000 RMB, and >=10000 RMB, 1RMB ≈ 0.15 USD). Missing values of parental education years and wage were replaced with means of the corresponding variable. Missing values of parental education or wage were imputed for 33.6% of children in the current sample.
2.3. Statistical analysis
The differences between these children and the children excluded/dropped out were examined using t tests or chi-square tests and results are available in Table 1. Sample characteristics were summarized by descriptive statistics. Independent t tests, chi square tests and Pearson’s correlations were used to test bivariate associations among sociodemographic variables, BLL, BML, and RHR.
Table 1.
Comparisons between the present sample and excluded children
| Present sample (n = 532) Mean (SD)/n (%) | Excluded Children Mean (SD)/n (%) | t/χ2 | p | |
|---|---|---|---|---|
| Age (ne = 565), mean (SD) | 12.08 (0.42) | 12.07 (0.42) | 0.49 | 0.62 |
| Sex (ne = 568) | 0.03 | 0.86 | ||
| Boys, n (%) | 290 (54.5) | 469 (55.0) | ||
| Girls, n (%) | 242 (45.5) | 384 (45.0) | ||
| Family Region | 2.62 | 0.27 | ||
| Urban, n (%) | 217 (40.8) | 337 (39.5) | ||
| Suburban, n (%) | 218 (40.1) | 330 (38.7) | ||
| Rural, n (%) | 97 (18.2) | 186 (21.8) | ||
| SES, mean (SD) | −0.01 (1.12) | −0.01 (1.03) | −0.01 | 0.99 |
| RHR (bpm), mean (SD) | 89.28 (11.41) | |||
| BLL (μg/dL; ne = 577), mean (SD) | 3.14 (1.19) | 3.12 (1.17) | −0.27 | 0.79 |
| BML (μg/L; ne = 576), mean (SD) | 1.26 (0.68) | 1.24 (0.61) | −0.39 | 0.70 |
Notes. The present sample utilizes data from Wave II of the China Jintan Child Cohort (Wave 1 n = 1100). ne represents the sample size of excluded children with relevant data. SD, standard deviation; SES, z score of socioeconomic status; BLL, blood lead level; BML, blood mercury level; RHR, resting heart rate; bpm, beats per minute.
Generalized linear models (GLM) were used to test the adjusted relationship between BLL and BML and RHR, as well their interactive effects with child sex. We constructed six GLMs in the full sample. Models 1 and 2 included BLL and BML as the independent variable in the model, respectively. Models 3 and 4 were built on Models 1 and 2 by adding the interaction term of sex with BLL and BML, respectively. Model 5 included the interaction term of BLL with BML. Model 6 added the 3-way interaction term of sex with BLL and BML. We next conducted GLM models separately for males and females. In these analyses, Models a and b included BLL and BML as the independent variable in the model, respectively. Model c included the interaction term of BLL with BML. When interactions involving sex were non-significant, we interpreted main effects from Models 1 and 2 in the full sample. Significant interactions involving sex were probed by interpreting coefficients from the male- and female-only GLM models. We used several methods to probe these significant interactions. First, significant interactions in the male-and female-only GLM models were probed by comparing RHR across groups based on median-splits of BLM and BLL (i.e. Low BLL/Low BML; High BLL/Low BML; Low BLL/High BML; High BLL/High BML). These cut-offs were determined separately by sex. We used one-way ANOVA to determine whether there was a significant between-group difference in mean RHR. Post-hoc group comparisons were performed using the Tukey test in order to determine which group mean differences were significant. We then conducted GLM analyses with the groups entered as dummy variables to determine whether the groups significantly predicted RHR, adjusted for age, region, and SES (with the High BLL/High BML group as the reference category). Finally, we probed significant interactions using the Johnson-Neyman technique (Johnson & Neyman, 1936; Hayes & Matthes, 2009). The Johnson-Neyman technique determines the exact range of values of the moderator for which the relationship between the independent and dependent variable is statistically significant. We determined regions of significance for values of the moderator within three standard deviations of the moderator mean to avoid interpreting extreme values with few cases (Carden et al., 2017).
As a supplemental analysis, we examined whether children in these groups differed by family region, age, or SES. As an additional supplemental analysis, we performed GLM models with BLL, BML, and covariates predicting RHR and calculated the standardized coefficients (betas) in order to determine the relative effects of the predictors.
All GLM analyses were clustered at family region where the children were originally recruited to adjust standard error. We report unstandardized coefficients, as standardized coeffecients are not recommended when reporting moderated regression analyses (Fairchild & McQuillin, 2010; Hayes, 2017). The α level was set at 0.05. A p value higher than 0.05 but less than 0.10 is regarded as marginally significant. All analyses were performed in STATA 16.0 package (College Station, TX).
3. Results
3.1. Representativeness of Groups
During Wave II, 1048 out of 1100 children’s BLL and BML were measured and 552 adolescents (mean age = 12.08, SD = 0.42) participated in psychophysiological tests that included RHR recording. Among the 552 adolescents, 19 were excluded due to the presence of physical health problems (i.e. cold, pyrosis on hand and arrhythmia, n = 7), excessive movement during examination (n = 7), technical problems (n = 1), a RHR higher than 130 bpm (n = 3) or a RHR lower than 60 bpm (n = 1). Out of the 534 adolescents with valid RHR, two were excluded because of missing values on BLL and BML. Therefore, data from the 532 children were used for analysis. The 532 children included in the analysis did not differ from those who were excluded in terms of age, sex, family region, SES, BLL, or BML. The results of these analyses are shown in Table 1. Furthermore, due to the imputation of SES data, as a sensitivity check, we conducted the GLM analyses without SES. Results were substantively unchanged.
3.2. Descriptive analysis and bivariate analyses
Characteristics of the children and their families are described in Table 1. One subject with a BML that was a high outlier (greater than 8 standard deviations from the mean) was excluded. Removing this outlier, the mean BLL and BML in the sample were 3.14 (SD = 1.19) μg/dL and 1.26 (SD = 0.68) μg/L, respectively. As shown in Table 2, we did not find any significant sex differences with respect to the following variables: mean BLL, family region, or socioeconomic status. However, we did find an association between sex and BML, whereby boys had significantly higher BML (t = −2.82, p = 0.005). In addition, boys showed significant lower RHR than girls (t = 4.96, p < 0.0001). Next, we examined bivariate correlations between continuous variables. Results can be found in Supplemental Table 1. RHR was not significantly correlated with BLL or BML in girls or boys (p > 0.10). In bivariate analyses, SES was positively associated with BLL in girls (r = 0.14, p = 0.04).
Table 2:
Blood lead, blood mercury and sociodemographic variables stratified by sex
| Boys (n = 289) Mean (SD)/% | Girls (n = 242) Mean (SD)/% | t/χ2 | p | |
|---|---|---|---|---|
| BLL (μg/dL), mean (SD) | 3.17 (1.17) | 3.09 (1.21) | t = −0.76 | 0.45 |
| BML (μg/L), mean (SD) | 1.33 (0.72) | 1.17 (0.61) | t = −2.82 | 0.005 |
| RHR (bpm), mean (SD) | 87.09 (10.23) | 91.90 (12.19) | t = 4.96 | < 0.0001 |
| Family Region | χ2 = 0.95 | 0.62 | ||
| Urban (%) | 40.5% | 40.9% | ||
| Suburban (%) | 42.6% | 39.3% | ||
| Rural (%) | 17.0% | 19.8% | ||
| SES, mean (SD) | −0.01 (1.20) | 0.01 (1.19) | t = 0.22 | 0.82 |
Notes. The median (interquantile range) of blood lead level was 3.0 (2.4–3.7) μg/dL for boys and 2.9 (2.3–3.7) μg/dL for girls. The median (interquantile range) of blood mercury level was 1.19 (0.9–1.7) μg/L for boys and 1.08 (0.7–1.5) μg/L for girls. SD, standard deviation;SES, z score of social economic status; BLL, blood lead level; BML, blood mercury level; RHR, resting heart rate; bpm, beats per minute.
3.3. Adjusted associations of RHR with BLL and BML
Results of the generalized linear models in the full sample are shown in Table 3. In Models 1 and 3, we found that BLL (B = 0.28, SE = 0.43, p = 0.51) and the interaction between BLL and sex (B = 0.30, SE = 0.86, p = 0.73) did not significantly predict RHR. In Model 2, we found that BML did not significantly predict RHR (B = −0.34, SE = 0.40, p = 0.39). In Model 4, we found that the interaction between BML and sex was marginally significant in predicting RHR (B = −2.19, SE = 1.21, p = 0.071). In Model 5, the interaction between BLL and BML was non-significant in predicting RHR (B = −0.41, SE = 0.73, p = 0.57). In Model 6, the 3-way interaction between sex, BLL, and BML was marginally significant in predicting RHR (B = −0.97, SE = 0.54, p = 0.076).
Table 3.
The adjusted associations of RHR (bpm) with BLL (μg/dL) and BML (μg/L) in the full sample (N = 531)
| Predictors | Model 1 B (SE) |
Model 2 B (SE) |
Model 3 B (SE) |
Model 4 B (SE) |
Model 5 B (SE) |
Model 6 B (SE) |
|---|---|---|---|---|---|---|
| BLL | 0.28 (0.43) | 0.13 (0.89) | 0.84 (1.33) | 0.39 (1.98) | ||
| BML | −0.34 (0.40) | 1.04 (1.21) | 0.94 (2.06) | 1.75 (2.12) | ||
| Age | 0.82 (2.09) | 0.93 (2.25) | 0.82 (2.08) | 0.90 (2.18) | 0.87 (2.13) | 0.86 (2.05) |
| Sex | −4.87 (1.21)*** | −4.80 (1.23)*** | −5.80 (2.52)* | −2.12 (2.50) | −4.86 (1.29)*** | −7.10 (2.63)** |
| Suburban | −2.32 (0.32)*** | −2.31 (0.30)*** | −2.34 (0.29)*** | −2.22 (0.29)*** | −2.31 (0.31)*** | −2.18 (0.28)*** |
| Rural | −2.49 (0.43)*** | −2.53 (0.48)*** | −2.51 (0.43)*** | −2.47 (0.46)*** | −2.47 (0.48)*** | −2.41 (0.45)*** |
| SES | 0.10 (0.58) | 0.11 (0.59) | 0.12 (0.53) | 0.09 (0.55) | 0.11 (0.59) | 0.10 (0.51) |
| BLL × Sex | 0.30 (0.86) | 1.66 (1.50) | ||||
| BML × Sex | −2.19 (1.21) † | 0.58 (0.85) | ||||
| BLL × BML | −0.41 (0.73) | −0.22 (0.96) | ||||
| BLL × BML × Sex | −0.97 (0.54) † |
Notes. Dependent variable is resting heart rate (measured in beats per minute). The reference level for sex and location are girls and urban, respectively. BLL, blood lead level (μg/dL); BML, blood mercury level (μg/L); SES, z score of social economic status; bpm, beats per minute; RHR, resting heart rate. Model adjusted for age, family region, and socioeconomic status. The cells display the unstandardized coefficient and robust standard errors (SE).
p < 0.10
p < 0.05
p < 0.001.
We probed the marginally significant Model 4 interaction between BML and sex by interpreting male- and female-only GLM models (see Table 4, Model b). In girls, BML did not significantly predict RHR net of covariates (B = 0.94, SE = 1.21, p = 0.78, n = 242). In boys, higher BML was significantly associated with lower RHR net of covariates (B = −1.14, SE = 0.26, p < .001, n = 289). Model b showed that BLL was positively associated with RHR in boys (B = 0.41, SE = .20, p = 0.041), but not in girls (B = 0.09, SE = 0.89, p = 0.92), although the BLL x Sex interaction was non-signficicant in the full sample.
Table 4.
The adjusted associations of RHR (bpm) with BLL (μg/dL) and BML (μg/L) in boys and girls
| Boys Only (n = 289) | Girls Only (n = 242) | |||||
|---|---|---|---|---|---|---|
| Predictors | Model a B (SE) |
Model b B (SE) |
Model c B (SE) |
Model a B (SE) |
Model b B (SE) |
Model c B (SE) |
| BLL | 0.41 (0.20)* | 2.16 (0.53)*** | 0.09 (0.89) | 0.32 (1.95) | ||
| BML | −1.14 (0.26)*** | 2.57 (1.59) | 0.94 (1.21) | 1.54 (1.92) | ||
| Age | 0.24 (0.73) | 0.40 (0.71) | 0.34 (0.77) | 1.10 (4.34) | 1.06 (4.32) | 1.06 (4.26) |
| Suburban | −1.18 (0.28)*** | −1.01 (0.24)*** | −0.91 (0.20)*** | −3.73 (0.44)*** | −3.67 (0.48)*** | −3.68 (0.44)*** |
| Rural | 0.35 (0.16)* | 0.32 (0.18) † | 0.42 (0.16)** | −5.38 (0.49)*** | −5.31 (0.43)*** | −5.28 (0.51)*** |
| SES | 0.23 (0.61) | 0.18 (0.57) | 0.19 (0.53)*** | −0.01 (0.68) | −0.02 (0.74) | −0.02 (0.68) |
| BLL × BML | −1.27 (0.49)** | −0.18 (0.88) | ||||
Notes. Dependent variable is resting heart rate (measured in beats per minute). The reference level for location is urban. BLL, blood lead level (μg/dL); BML, blood mercury level (μg/L); SES, z score of social economic status; bpm, beats per minute; RHR, resting heart rate.. Model adjusted age, family region, and socioeconomic status. The cells display the unstandardized coefficient and the robust standard error (SE).
p < 0.10
p < 0.05
p < 0.01
p < 0.001.
We probed the marginally significant BLL x BML x Sex interaction using several steps. First, we analyzed the BLL x BML interaction separately by sex. As shown in Table 4 (Model c), the interaction between BML and BLL was significant in boys (B = −1.27, SE = 0.49, p = 0.009, n = 289), but not in girls (B = −0.18, SE = 0.88, p = 0.84, n = 242).
Next, we probed the significant interaction between BML and BLL in boys. The medians of BLL (3.0 μg/dL) and BML (1.19 μg/dL) for boys were used as cut-off scores to dichotimize BLL and BML into groups in boys: low (BLL ≤ 3.0 μg/dL) vs. high BLL group (BLL > 3.0 μg/dL), and low (BML ≤ 1.19 μg/L) vs. high BML group (BML > 1.19 μg/L). These splits were then used to create four groups in boys based on BLL and BML (i.e. Low BLL/Low BML; High BLL/Low BML; Low BLL/High BML; High BLL/High BML). Mean RHR for the groups are shown in Figure 1. A one-way ANOVA showed there was a significant between-group difference in RHR, F(3, 285) = 2.66, p = 0.049. Post-hoc comparisons using the Tukey test indicated that the boys in the High BML/High BLL group had significantly lower RHR (mean = 84.22 bpm, SD = 8.77, n = 61) than boys in the Low BLL/Low BML group (mean = 89.03 bpm, SD = 10.75, n = 69; p < 0.05). All other group comparisons were non-significant (p > 0.10). We also conducted GLM analyses with the groups entered as dummy variables predicting RHR, adjusted for age, region, and SES (with the High BLL/High BML group as the reference category). Boys in the Low BLL/Low BML group (B = 4.86, SE = 1.63, p = 0.003) and High BLL/Low BML group (B = 3.55, SE = 0.65, p < 0.001) had significantly higher RHR than boys in the High BLL/High BML group. Boys in the Low BLL/High BML group did not significantly differ from boys in the High BLL/High BML group (B = 2.63, SE = 2.53, p = 0.30).
Figure 1.

Mean RHR (bpm) by BLL and BML groups based on median cut-offs in boys
Notes. Error bars represent 95% confidence intervals. Low BLL (BLL ≤ 3.0 μg/dL); High BLL (BLL > 3.0 μg/dL); Low BML (BML ≤ 1.19 μg/L); High BML (BML > 1.19 μg/L). Low BLL/Low BML mean RHR (SD) = 89.03 (10.75), n = 69; High BLL/Low BML mean RHR (SD) = 87.94 (10.50), n = 75; Low BLL/High BML mean RHR (SD) = 86.83 (10.24), n = 84; High BLL/High BML mean RHR (SD) = 84.22 (8.77), n = 61. Boys with High BML/High BLL had significantly lower RHR in than boys with Low BLL/Low BML (p < 0.05). SD, standard deviation; BLL, blood lead level; BML, blood mercury level; RHR, resting heart rate; bpm, beats per minute.
Finally, we probed this significant interaction in boys using the Johnson-Neyman technique. We determined the exact range of values of BLL for which the relationship between BML and RHR was statistically significant in boys. We found that among boys with BLL of 2.6 μg/dL or greater (n = 191), the relationship between BML and RHR was negative and statistically significant (p < 0.05). Among boys with BLL less than 2.6 μg/dL, the relationship between BML and RHR was non-significant (p > 0.05). This is illustrated in Figure 2.
Figure 2.
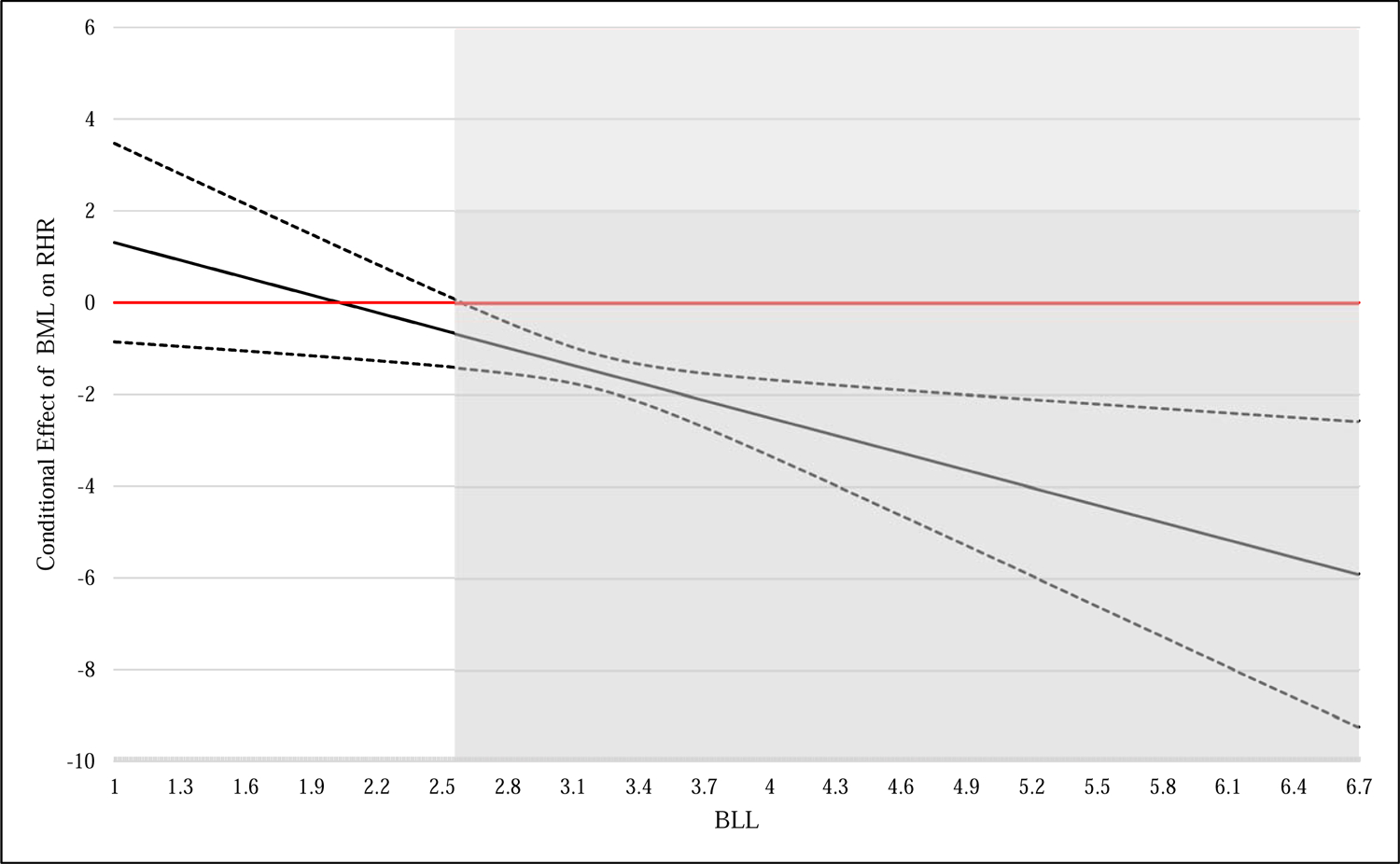
Conditional effects of BML (μg/L) on RHR (bpm) at levels of BLL (μg/dL) in boys
Notes. Y-axis displays the unstandardized coefficient of BML (μg/L) on RHR (measured in beats per minute). Region of BLL (μg/dL) for which the relationship between BML and RHR is signficiant (p < .05, 2-tailed) is shown in gray. The region of significance begins at BLL = 2.6 μg/dL and includes n = 191 boys. Dotted lines represent the upper and lower 95% confidence intervals. Values of BLL within 3 standard deviations of the mean are displayed, though results were unchanged when the full range of values was probed. BLL, blood lead level; BML, blood mercury level; RHR, resting heart rate.
3.4. Supplemental analyses
As a supplemental analysis, we compared whether boys in the four groups differed on family region, SES, or age. Results are shown in Supplemental Table 2. Although the between-group difference in SES was marginally significant in boys (p = 0.098), none of the post-hoc group comparisons were statistically significant (p > 0.10). The groups did not significantly differ by age or family region in boys (p > 0.10).
As an additional supplemental analysis, we compared the relative effects of BLL, BML, and covariates on RHR in boys. As shown in Supplemental Table 3, BML (β = −0.07, SE = 0.01, p < 0.001) was the strongest predictor of RHR in boys, followed by suburban region (β = −0.05, SE = 0.01, p < 0.001).
4. Discussion
Previous studies examining the impact of heavy metal exposure on cardiovascular functioning have largely focused on heart rate variability (Lim et al., 2010; Beatriz Valera et al., 2011) and few studies have investigated its impact on children’s resting heart rate (Grandjean et al., 2004; Gump et al., 2011). In this laboratory-controlled, community sample of 532 adolescents (mean age = 12.08 years), we found that the combination of higher blood mercury and lead levels was associated with lower resting heart rate in boys but not in girls. Results remained significant after controlling for age, family region, and SES. Furthermore, boys with both high BLL and BML had significantly lower RHR than boys with low BLL and BML group. To our knowledge, this is the first study to document an effect of heavy metal exposure on resting heart rate in children. While our results are not clinically significant, as they fall within the normal heart rate for the appropriate age group (Wallis et al., 2005), our findings revealed that exposure to environmental heavy metal pollutants such lead and mercury, even at very low levels, may affect resting heart rate which, in turn, could have adverse effects on children’s physical health.
4.1. Heavy metal exposure and resting heart rate
Our study adds novel evidence to the current literature examining the heavy metal-resting heart rate relationship. Until now, few studies have examined heavy metal exposure and resting heart rate and the results of these have been largely inconclusive. Several studies have reported similar findings of decreased resting heart rate in chronic occupationally lead-exposed workers (Böckelmann et al., 2011; Kosmider & Petelenz, 1961). However, cross-sectional studies examining mercury-exposed populations have largely reported either increases in average heart rate (OKA et al., 2002) or the absence of an effect on heart rate (Choi et al., 2009; Grandjean et al., 2004; B Valera et al., 2011; Yaginuma-Sakurai et al., 2010). Disagreements between our results and those of previous studies may be a result of differences in age at blood lead/mercury analysis, variation in study population, length of exposure, differences in exposure assessment, source of exposure (occupational or general population), failure of prior studies to examine lead/mercury interactions, and residual confounding.
Previous studies in children did not find a significant effect of heavy metal exposure on resting heart rate. In a 2004 study, Grandjean et al. found no effect of prenatal methylmercury or hair mercury levels on heart rate in a prospective study of a Faroese birth cohort (N = 1022) at ages 7 and 14 years. In contrast, our study examined the effect of postnatal mercury exposure as measured by blood mercury levels, which may have a more direct effect on heart rate. On the other hand, due to the cross-sectional nature of this study, we were unable to tease out whether the association of mercury with heart rate is a short-term effect concurrently or if it is due to long-term exposure throughout childhood. Sources of exposure may have contributed to differences as well, as the Faroese birth cohort was predominantly exposed to methylmercury from fish consumption in the diet whereas the sources of mercury exposure in China often include both methylmercury from seafood consumption as well as inorganic mercury emissions from metal smelting and resulting biocontamination (Zhang & Wong, 2007). Furthermore, hair mercury level is prone to misinterpretation and contamination and is often not correlated with blood mercury concentrations or symptoms of mercury (Nuttall, 2006). Moreover, age has been shown to be inversely related to heart rate until age 10 years (Silvetti et al., 2001), which could have made it difficult for researchers to delineate mercury-induced reductions in heart rate from those resulting from increased age. A study done in a sample of 140 American children aged 9–11 years with very low levels of blood lead (mean = 1.01 μg/dL) reported that no significant association was found between blood lead levels and heart rate (Gump et al., 2011). However, this study was done in a relatively small sample that was predominantly White. Variances in behaviors leading to exposure or cardiovascular responses to exposure could be influenced by cultural or racial differences. Sources of exposure may have also contributed to differences in the sample. In our current sample, sources of lead exposure were likely due to air pollution resulting from factory emissions (Chen et al., 2012) which may differ from exposure sources in samples outside of China. Moreover, the mean level of blood lead in our sample was 3.14 μg/dL, which is over five times the mean BLL of recent samples of American children age 6 to 19 years (Tsoi et al., 2016).
Furthermore, animal studies have also reported conflicting results in both lead and mercury-exposed rats. A recent study reported increased heart rate in rats exposed to mercury for 30 days (Simões et al., 2016). In a 2015 study, Wildemann et al. reported that neither lead nor mercury exposure had a significant impact on heart rate in rats after 4 weeks of exposure (Wildemann et al., 2015). However, the short duration of exposure may have not been long enough for the manifestation of the adverse cardiovascular effects of lead. Moreover, the results of these animal studies may not be generalizable to humans.
4.2. Interaction effects of combined exposure and sex
Boys with both high blood lead level and high blood mercury level had lower resting heart rates. To our knowledge, no study has examined the effect of combined blood lead and blood mercury exposure on heart rate. Both lead and mercury exposure have been found to disrupt myocardial contractibility (Hechtenberg & Beyersmann, 1991; Moreira et al., 2003; Vassallo et al., 2008) and autonomic function (Andrzejak et al., 2004; Jhun et al., 2005; Lim et al., 2010; Muzi et al., 2005; B Valera et al., 2011). Furthermore, our findings showed gender as a modifier in the relationship between mercury exposure and heart rate, as well as the interaction beween lead and mercury exposure and heart rate. While mean blood lead levels did not differ in boys and girls, there were significant sex differences in both mean blood mercury levels and resting heart rate. This may be a possible explanation as to why gender played a modifying role only in the relationship between blood mercury and resting heart rate, as well as the relationship between combined exposure and resting heart rate. The underlying reason is likely largely due to pre-existing gender differences rather than differential responses to heavy metal exposure, as several studies in child and adolescent samples have reported lower resting heart rate in males compared to females (Choy et al., 2017; Rautaharju, 2015). In addition, a 2015 study done in an international sample of 6539 children aged 9–11 years reported that boys are more likely to engage in more minutes of moderate-to-vigorous physical activity per day than girls (Katzmarzyk et al., 2015). As physical activity has been shown to decrease resting heart rate (Carnevali & Sgoifo, 2014; Fox et al., 2007), this difference in physical activity levels may be responsible for the gendered differences in heart rate found in our sample.
4.3. Potential mechanisms of heavy metal-induced decreased resting heart rate
The mechanism of action underlying the effect of heavy metal exposure on heart rate is unclear but research has shown modulation of both intrinsic (Simões et al., 2016; Simões et al., 2017) and extrinsic regulators (Karimi et al., 2016; Lopes et al., 2016) of heart rate by lead and mercury. With respect to intrinsic regulation, decreased resting heart rate could result from a reduced intrinsic heart rate wherein the sinoatrial node-generated heart rate is depressed. While animal studies have shown an inconsistent relationship between intrinsic heart rate and resting heart rate (Simões et al., 2016; Simões et al., 2017), Jose and Collison (1970) reported that resting heart rate was lower than intrinsic heart rate in a sample of 432 healthy adults. These findings have also been extended to child samples (Marcus et al., 1990), thus establishing a linear relationship between intrinsic heart rate and resting heart rate in human populations. Changes to intrinsic heart rate occur through decreased myocardial contractability,as seen in both lead and mercury-exposed animals (Hechtenberg & Beyersmann, 1991; Moreira et al., 2003; Vassallo et al., 2008). Both myosin ATPase activity (Moreira et al., 2003; Vassallo et al., 2008) and calcium ATPase activity (Cheng et al., 2012) have been shown to reduce tetanic force development and atrioventricular heart rate, respectively, thus offering potential mechanisms through which mercury and lead exposure may depress heart rate.
Regarding the effects of lead and mercury on extrinsic regulation of heart rate, decreased heart rate may be partly mediated by heavy-metal induced autonomic dysregulation. While the mechanism through which heavy metal exposure elicits autonomic dysfunction is unclear, it may be linked to cellular dysfunction resulting from oxidative stress. As lead and mercury have been shown to induce oxidative stress (Ercal et al., 2001; Karimi et al., 2016; Lopes et al., 2016; Martinez et al., 2014), it is possible that heavy metal-induced resting heart rate depression is partially mediated by autonomic dysfunction and inflammation caused by reactive oxygen species. Future animal and observational studies must be done to elucidate the mechanism underlying heavy-metal-induced alterations in heart rate. Furthermore, longitudinal studies are necessary to understand the implications of chronic exposure to lead or mercury.
4.4. Strengths and Limitations
While this study has several strengths, our findings must be interpreted in light of its limitations. The strengths of this paper include the relatively large community sample size given the laboratory-controlled psychophysiological testing of resting heart rate and the evaluation of low blood lead and mercury levels. However, several limitations remain: Firstly, the cross-sectional design of the study incurs the inability to differentiate the directionality of the association. It is possible that cardiovascular functioning affects the uptake or clearance of lead or mercury. An alternative explanation is that pre-existing low resting heart rate may predispose individuals to behaviors that would increase exposure to lead or mercury. Secondly, although we controlled for possible confounders such as socioeconomic status and residential details, there are still unassessed potential confounders which could have potentially impacted resting heart rate, such as physical activity levels, body mass index, pre-existing conditions such as asthma or obesity, and consumption of medication that may affect heart rate (e.g. methylphenidate, amphetamine). However, methylphenidate (i.e. Ritalin) and amphetamine (i.e. Adderall) are rarely prescribed in China due to strict government regulation. Thirdly, the likelihood of exposure misclassification was increased by the single measure of lead exposure and mercury exposure. Blood lead measurements reflect relatively recent exposure and are poor indicators of chronic lead exposure, which are better assessed through bone lead measurements. (Josephson, 2004). Similarly, blood mercury is mainly a measure of methyl mercury levels, whereas inorganic mercury is found in the urine (Nhanes, 2009). Studies have found that inorganic mercury measurement is a more accurate indicator of mercury accumulation and deposition in the human body than methyl mercury measurement (Laks, 2009). Utilization of these additional measures would have strengthened the quantification of lead exposure to include chronic heavy metal exposure.
5. Conclusion
To our knowledge, the present study is the first examination of the effect of lead and mercury exposure as well as the combined effect of lead and mercury exposure on resting heart rate in community children. In a community sample of 532 Chinese adolescents, we found that boys with higher blood mercury levels and higher blood lead levels had lower resting heart rates. Resting heart rate has been increasingly identified as a biological predictor for increased risk of cardiovascular disease (Fox et al., 2007), poor emotional regulation (Williams et al., 2015), childhood antisocial behavior (Ortiz & Raine, 2004) and later aggressive behavior (Armstrong et al., 2009; Raine et al., 1997). Understanding the role of heavy metal exposure is significant in the prevention of long-term adverse cognitive, behavioral and physical outcomes (Bellinger et al., 2017). The long term cardivocascular effect of decreased resting heart rate during childhood is unclear. While most studies have identified increase risk for adverse cardiovascular outcomes with high resting heart rate (Böhm et al., 2015), these studies have focused on adult populations which may differ from child populations, wherein the range of normal resting heart rate is much larger (Wallis et al., 2005). Further studies are needed to examine the biological mechanisms through which heavy metal exposure exerts its effects on cardiovascular health as well as the long-term clinical significance of these findings.
Supplementary Material
Highlights.
This is the first study to demonstrate the effect of heavy metal exposure on resting heart rate in adolescents.
Combined higher blood lead and mercury levels were associated with lower resting heart rate in boys but not in girls.
Lower resting heart rate may indicate chronic under-arousal and has been implicated in psychopathology.
Acknowledgements
Thanks are extended to the participating children and their families from Jintan City, and to the Jintan Cohort Study Group. We are very grateful to the Jintan city government and the Jintan Hospital for their support and assistance.
Funding
This work was supported by the National Institutes of Environmental Health Sciences and the National Institutes of Health (R01-ES-018858, K02-ES-019878, K01-ES015877 and P30-ES013508;).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
None
References
- Andrzejak R, Poreba R, & Derkacz A (2004). Effect of chronic lead poisoning on the parameters of heart rate variability. Medycyna pracy, 55(2), 139–144. [PubMed] [Google Scholar]
- Armstrong TA, Keller S, Franklin TW, & Macmillan SN (2009). Low resting heart rate and antisocial behavior: a brief review of evidence and preliminary results from a new test. Criminal Justice and Behavior, 36(11), 1125–1140. [Google Scholar]
- Arnold OM, & Liu J (2020). Blood lead levels≤ 10 micrograms/deciliter and executive functioning across childhood development: A systematic review. Neurotoxicology and teratology, 106888. [DOI] [PMC free article] [PubMed]
- Bellinger DC (2017). Childhood lead exposure and adult outcomes. Jama, 317(12), 1219–1220. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Chen A, & Lanphear BP (2017). Establishing and achieving national goals for preventing lead toxicity and exposure in children. JAMA pediatrics, 171(7), 616–618. [DOI] [PubMed] [Google Scholar]
- Böckelmann I, Pfister E, & Darius S (2011). Early effects of long-term neurotoxic lead exposure in copper works employees. Journal of toxicology, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm M, Reil J-C, Deedwania P, Kim JB, & Borer JS (2015). Resting heart rate: risk indicator and emerging risk factor in cardiovascular disease. The American journal of medicine, 128(3), 219–228. [DOI] [PubMed] [Google Scholar]
- Carden SW, Holtzman NS, & Strube MJ (2017). CAHOST: An excel workbook for facilitating the Johnson-Neyman technique for two-way interactions in multiple regression. Frontiers in Psychology, 8, 1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevali L, & Sgoifo A (2014). Vagal modulation of resting heart rate in rats: the role of stress, psychosocial factors, and physical exercise. Frontiers in physiology, 5, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Cai B, Dietrich KN, Radcliffe J, & Rogan WJ (2007). Lead exposure, IQ, and behavior in urban 5-to 7-year-olds: does lead affect behavior only by lowering IQ? Pediatrics, 119(3), e650–e658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Xu Z, Liu M, Huang Y, Fan R, Su Y, Hu G, Peng X, & Peng X (2012). Lead exposure assessment from study near a lead-acid battery factory in China. Science of the total environment, 429, 191–198. [DOI] [PubMed] [Google Scholar]
- Cheng H, Smith GL, Orchard CH, Hancox JC, & Burton FL (2012). Inhibition of sarcoplasmic reticulum Ca2+-ATPase decreases atrioventricular node-paced heart rate in rabbits. Experimental physiology, 97(10), 1131–1139. [DOI] [PubMed] [Google Scholar]
- Choi AL, Weihe P, Budtz-Jørgensen E, Jørgensen PJ, Salonen JT, Tuomainen T-P, Murata K, Nielsen HP, Petersen MS, & Askham J (2009). Methylmercury exposure and adverse cardiovascular effects in Faroese whaling men. Environmental health perspectives, 117(3), 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy O, Raine A, Venables PH, & Farrington DP (2017). Explaining the gender gap in crime: The role of heart rate. Criminology, 55(2), 465–487. [Google Scholar]
- Ercal N, Gurer-Orhan H, & Aykin-Burns N (2001). Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Current topics in medicinal chemistry, 1(6), 529–539. [DOI] [PubMed] [Google Scholar]
- Evranos B, Onay EE, Aytürk M, & Öztürk MT (2016). Exercise heart rate recovery assessment of the cardiac autonomic nervous system in workers occupationally exposed to lead. Turk Kardiyol Dern Ars, 44(5), 371–379. [DOI] [PubMed] [Google Scholar]
- Fairchild AJ, & McQuillin SD (2010). Evaluating mediation and moderation effects in school psychology: A presentation of methods and review of current practice. Journal of school psychology, 48(1), 53–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck AJ, Mooney S, Kapoor SS, White KM, Bearer C, & El Metwally D (2015). Developmental exposure to environmental toxicants. Pediatric Clinics, 62(5), 1173–1197. [DOI] [PubMed] [Google Scholar]
- Faustman EM, Silbernagel SM, Fenske RA, Burbacher TM, & Ponce RA (2000). Mechanisms underlying Children’s susceptibility to environmental toxicants. Environmental health perspectives, 108(Suppl 1), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Sendon JLL, Steg PG, Tardif J-C, Tavazzi L, & Tendera M (2007). Resting heart rate in cardiovascular disease. Journal of the American College of Cardiology, 50(9), 823–830. [DOI] [PubMed] [Google Scholar]
- Gambelunghe A, Sallsten G, Borné Y, Forsgard N, Hedblad B, Nilsson P, Fagerberg B, Engström G, & Barregard L (2016). Low-level exposure to lead, blood pressure, and hypertension in a population-based cohort. Environmental research, 149, 157–163. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Barouki R, Bellinger DC, Casteleyn L, Chadwick LH, Cordier S, Etzel RA, Gray KA, Ha E-H, & Junien C (2016). Life-long implications of developmental exposure to environmental stressors: new perspectives. Endocrinology, 2016(1), 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, & Landrigan PJ (2014). Neurobehavioural effects of developmental toxicity. The Lancet Neurology, 13(3), 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Murata K, Budtz-Jørgensen E, & Weihe P (2004). Cardiac autonomic activity in methylmercury neurotoxicity: 14-year follow-up of a Faroese birth cohort. The Journal of Pediatrics, 144(2), 169–176. [DOI] [PubMed] [Google Scholar]
- Gregory S, Iles-Caven Y, Hibbeln JR, Taylor CM, & Golding J (2016). Are prenatal mercury levels associated with subsequent blood pressure in childhood and adolescence? The Avon prebirth cohort study. BMJ open, 6(10), e012425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble MO, Cheng A, Berger RD, Rosman L, & Guallar E (2015). Mercury exposure and heart rate variability: a systematic review. Current environmental health reports, 2(3), 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump BB, MacKenzie JA, Bendinskas K, Morgan R, Dumas AK, Palmer CD, & Parsons PJ (2011). Low-level Pb and cardiovascular responses to acute stress in children: The role of cardiac autonomic regulation. Neurotoxicology and teratology, 33(2), 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump BB, Stewart P, Reihman J, Lonky E, Darvill T, Matthews KA, & Parsons PJ (2005). Prenatal and early childhood blood lead levels and cardiovascular functioning in 9½ year old children. Neurotoxicology and teratology, 27(4), 655–665. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2017). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford publications. [Google Scholar]
- Hayes AF, & Matthes J (2009). Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behavioral Resesearch Methods, 41, 924–936. [DOI] [PubMed] [Google Scholar]
- Hechtenberg S, & Beyersmann D (1991). Inhibition of sarcoplasmic reticulum Ca2+-ATPase activity by cadmium, lead and mercury. Enzyme, 45, 109–115. [DOI] [PubMed] [Google Scholar]
- Hu XF, Laird BD, & Chan HM (2017). Mercury diminishes the cardiovascular protective effect of omega-3 polyunsaturated fatty acids in the modern diet of Inuit in Canada. Environmental research, 152, 470–477. [DOI] [PubMed] [Google Scholar]
- Hu XF, Singh K, & Chan HM (2018). Mercury exposure, blood pressure, and hypertension: a systematic review and dose–response meta-analysis. Environmental health perspectives, 126(07), 076002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhun H-J, Kim H, & Paek D-M (2005). The association between blood metal concentrations and heart rate variability: a cross-sectional study. International archives of occupational and environmental health, 78(3), 243–247. [DOI] [PubMed] [Google Scholar]
- Ji A, Wang F, Luo W, Yang R, Chen J, & Cai T (2011). Lead poisoning in China: a nightmare from industrialisation. The Lancet, 377(9776), 1474–1476. [DOI] [PubMed] [Google Scholar]
- Johnson PO, & Neyman J (1936). Tests of certain linear hypotheses and their application to some educational problems. Statistical Research Memoirs, 1, 57–93. [Google Scholar]
- Jose AD, & Collison D (1970). The normal range and determinants of the intrinsic heart rate in man. Cardiovascular research, 4(2), 160–167. [DOI] [PubMed] [Google Scholar]
- Josephson J (2004). Measuring Lead Effects: Blood and Bone Together Are Better. Environmental health perspectives, 112(11), A636. [Google Scholar]
- Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E, Cowell W, Grandjean P, & Korrick S (2012). Evidence on the human health effects of low-level methylmercury exposure. Environmental health perspectives, 120(6), 799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi R, Vacchi-Suzzi C, & Meliker JR (2016). Mercury exposure and a shift toward oxidative stress in avid seafood consumers. Environmental research, 146, 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmarzyk PT, Barreira TV, Broyles ST, Champagne CM, Chaput J-P, Fogelholm M, Hu G, Johnson WD, Kuriyan R, & Kurpad A (2015). Physical activity, sedentary time, and obesity in an international sample of children. Medicine & Science in Sports & Exercise, 47(10), 2062–2069. [DOI] [PubMed] [Google Scholar]
- Kim S, Arora M, Fernandez C, Landero J, Caruso J, & Chen A (2013). Lead, mercury, and cadmium exposure and attention deficit hyperactivity disorder in children. Environmental research, 126, 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Xu X, Zhang Y, Zheng X, Liu R, Dietrich KN, Reponen T, Xie C, Sucharew H, Huo X, & Chen A (2020, 2020/04/01/). Birth outcomes associated with maternal exposure to metals from informal electronic waste recycling in Guiyu, China. Environment international, 137, 105580. 10.1016/j.envint.2020.105580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmider S, & Petelenz T (1961). Electrocardiographic studies in cases of chronic occupational lead poisoning. Polskie Archiwum Medycyny Wewnetrznej, 31, 1349. [PubMed] [Google Scholar]
- Laks DR (2009). Assessment of chronic mercury exposure within the US population, National Health and Nutrition Examination Survey, 1999–2006. Biometals, 22 (6), 1103–1114. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Rauch S, Auinger P, Allen RW, & Hornung RW (2018). Low-level lead exposure and mortality in US adults: a population-based cohort study. The Lancet Public Health, 3(4), e177–e184. [DOI] [PubMed] [Google Scholar]
- Lim S, Chung H-U, & Paek D (2010). Low dose mercury and heart rate variability among community residents nearby to an industrial complex in Korea. Neurotoxicology, 31(1), 10–16. [DOI] [PubMed] [Google Scholar]
- Liu J, Cao S, Chen Z, Raine A, Hanlon A, Ai Y, Zhou G, Yan C, Leung PW, McCauley L, Pinto-Martin J, & Group, t. J. C. S. (2015, October 1, 2015). Cohort Profile Update: The China Jintan Child Cohort Study. International Journal of Epidemiology, 44(5), 1548–1548l. 10.1093/ije/dyv119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Li L, Wang Y, Yan C, & Liu X (2013). Impact of low blood lead concentrations on IQ and school performance in Chinese children. PloS one, 8(5), e65230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu X, Pak V, Wang Y, Yan C, Pinto-Martin J, & Dinges D (2015). Early blood lead levels and sleep disturbance in preadolescence. Sleep, 38(12), 1869–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu X, Wang W, McCauley L, Pinto-Martin J, Wang Y, Li L, Yan C, & Rogan WJ (2014). Blood lead concentrations and children’s behavioral and emotional problems: a cohort study. JAMA pediatrics, 168(8), 737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, McCauley L, Leung P, Wang B, Needleman H, & Pinto-Martin J (2011, July). Community-based participatory research (CBPR) approach to study children’s health in China: experiences and reflections. Int J Nurs Stud, 48(7), 904–913. 10.1016/j.ijnurstu.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, McCauley LA, Zhao Y, Zhang H, & Pinto-Martin J (2010, June). Cohort Profile: The China Jintan Child Cohort Study. Int J Epidemiol, 39(3), 668–674. 10.1093/ije/dyp205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes ACBA, Peixe TS, Mesas AE, & Paoliello MM (2016). Lead exposure and oxidative stress: a systematic review. In Reviews of Environmental Contamination and Toxicology Volume 236 (pp. 193–238). Springer. [DOI] [PubMed] [Google Scholar]
- Marcus B, Gillette PC, & Garson A Jr (1990). Intrinsic heart rate inchildren and young adults: an index of sinus node function isolated from autonomic control. American heart journal, 119(4), 911–916. [DOI] [PubMed] [Google Scholar]
- Martinez CS, Escobar AG, Torres JGD, Brum DS, Santos FW, Alonso MJ, Salaices M, Vassallo DV, Peçanha FM, & Leivas FG (2014). Chronic exposure to low doses of mercury impairs sperm quality and induces oxidative stress in rats. Journal of Toxicology and Environmental Health, Part A, 77(1–3), 143–154. [DOI] [PubMed] [Google Scholar]
- Moreira C, Oliveira E, Bonan C, Sarkis J, & Vassallo D (2003). Effects of mercury on myosin ATPase in the ventricular myocardium of the rat. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 135(3), 269–275. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Shi P, Morris JS, Spiegelman D, Grandjean P, Siscovick DS, Willett WC, & Rimm EB (2011). Mercury exposure and risk of cardiovascular disease in two US cohorts. New England Journal of Medicine, 364(12), 1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzi G, Murgia N, Dell’Omo M, Fiordi T, Sposini F, Argentino A, Apostoli P, & Abbritti G (2005). Effects of inorganic lead exposure on the autonomic nervous system and on the variability of heart rate among workers at a battery plant. Giornale italiano di medicina del lavoro ed ergonomia, 27, 46–50. [PubMed] [Google Scholar]
- Nhanes I (2009). Fourth national report on human exposure to environmental chemicals. Department of Health and Human Services Centers for Disease Control and Prevention. Atlanta, Georgia. [Google Scholar]
- Nuttall KL (2006). Interpreting hair mercury levels in individual patients. Annals of Clinical & Laboratory Science, 36(3), 248–261. [PubMed] [Google Scholar]
- OKA T, MATSUKURA M, Okamoto M, HARADA N, Kitano T, Miike T, & Futatsuka M (2002). Autonomic nervous functions in fetal type Minamata disease patients: assessment of heart rate variability. The Tohoku journal of experimental medicine, 198(4), 215–221. [DOI] [PubMed] [Google Scholar]
- Ortiz J, & Raine A (2004). Heart rate level and antisocial behavior in children and adolescents: A meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry, 43(2), 154–162. [DOI] [PubMed] [Google Scholar]
- Pan S, Lin L, Zeng F, Zhang J, Dong G, Yang B, Jing Y, Chen S, Zhang G, & Yu Z (2018). Effects of lead, cadmium, arsenic, and mercury co-exposure on children’s intelligence quotient in an industrialized area of southern China. Environmental Pollution, 235, 47–54. [DOI] [PubMed] [Google Scholar]
- Piikivi L (1989). Cardiovascular reflexes and low long-term exposure to mercury vapour. International archives of occupational and environmental health, 61(6), 391–395. [DOI] [PubMed] [Google Scholar]
- Poręba R, Gać P, Poręba M, & Andrzejak R (2011). Environmental and occupational exposure to lead as a potential risk factor for cardiovascular disease. Environmental toxicology and pharmacology, 31(2), 267–277. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, & Mednick SA (1997). Low resting heart rate at age 3 years predisposes to aggression at age 11 years: Evidence from the Mauritius Child Health Project. Journal of the American Academy of Child & Adolescent Psychiatry, 36(10), 1457–1464. [DOI] [PubMed] [Google Scholar]
- Rautaharju PM (2015). Gender Differences Emerging During Adolescence in Heart Rate, QRS Duration, QT Interval and ST Elevation. In The Female Electrocardiogram (pp. 11–21). Springer. [Google Scholar]
- Shi Z, Zhen S, Orsini N, Zhou Y, Zhou Y, Liu J, & Taylor AW (2017). Association between dietary lead intake and 10-year mortality among Chinese adults. Environmental Science and Pollution Research, 24(13), 12273–12280. [DOI] [PubMed] [Google Scholar]
- Silvetti MS, Drago F, & Ragonese P (2001). Heart rate variability in healthy children and adolescents is partially related to age and gender. International journal of cardiology, 81(2), 169–174. [DOI] [PubMed] [Google Scholar]
- Simões M, Azevedo B, Fiorim J, Freire D, Covre E, Vassallo D, & Santos L (2016). Chronic mercury exposure impairs the sympathovagal control of the rat heart. Clinical and Experimental Pharmacology and Physiology, 43(11), 1038–1045. [DOI] [PubMed] [Google Scholar]
- Simões MR, Preti SC, Azevedo BF, Fiorim J, Freire DD, Covre EP, Vassallo DV, & dos Santos L (2017). Low-level chronic lead exposure impairs neural control of blood pressure and heart rate in rats. Cardiovascular toxicology, 17(2), 190–199. [DOI] [PubMed] [Google Scholar]
- Sripada K (2017). “Beginning with the Smallest Intake”: Children’s Brain Development and the Role of Neuroscience in Global Environmental Health. Neuron, 95(6), 1242–1245. [DOI] [PubMed] [Google Scholar]
- Tajik B, Kurl S, Tuomainen T-P, Savonen K, & Virtanen JK (2018). Associations of the serum long-chain n-3 PUFA and hair mercury with resting heart rate, peak heart rate during exercise and heart rate recovery after exercise in middle-aged men. British Journal of Nutrition, 119(1), 66–73. [DOI] [PubMed] [Google Scholar]
- Tsoi M-F, Cheung C-L, Cheung TT, & Cheung BMY (2016). Continual decrease in blood lead level in Americans: United States National Health Nutrition and examination survey 1999–2014. The American journal of medicine, 129(11), 1213–1218. [DOI] [PubMed] [Google Scholar]
- Valera B, Dewailly E, & Poirier P (2008). Cardiac autonomic activity and blood pressure among Nunavik Inuit adults exposed to environmental mercury: a cross-sectional study. Environmental Health, 7(1), 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera B, Dewailly E, & Poirier P (2011). Impact of mercury exposure on blood pressure and cardiac autonomic activity among Cree adults (James Bay, Quebec, Canada). Environmental research, 111(8), 1265–1270. [DOI] [PubMed] [Google Scholar]
- Valera B, Dewailly É, Poirier P, Counil E, & Suhas E (2011). Influence of mercury exposure on blood pressure, resting heart rate and heart rate variability in French Polynesians: a cross-sectional study. Environmental Health, 10(1), 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera B, Muckle G, Poirier P, Jacobson SW, Jacobson JL, & Dewailly E (2012). Cardiac autonomic activity and blood pressure among Inuit children exposed to mercury. Neurotoxicology, 33(5), 1067–1074. [DOI] [PubMed] [Google Scholar]
- Vassallo D, Lebarch E, Moreira C, Wiggers G, & Stefanon I (2008). Lead reduces tension development and the myosin ATPase activity of the rat right ventricular myocardium. Brazilian Journal of Medical and Biological Research, 41(9), 789–795. [DOI] [PubMed] [Google Scholar]
- Virtanen JK, Rissanen TH, Voutilainen S, & Tuomainen T-P (2007). Mercury as a risk factor for cardiovascular diseases. The Journal of nutritional biochemistry, 18(2), 75–85. [DOI] [PubMed] [Google Scholar]
- Wallis L, Healy M, Undy MB, & Maconochie I (2005). Age related reference ranges for respiration rate and heart rate from 4 to 16 years. Arch Dis Child, 90(11), 1117–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, El-Fahmawi A, Yan C, & Liu J (2019). Childhood lead poisoning from domestic products in China: A case study with implications for practice, education, and policy. Public Health Nursing, 36(6), 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildemann TM, Mirhosseini N, Siciliano SD, & Weber LP (2015). Cardiovascular responses to lead are biphasic, while methylmercury, but not inorganic mercury, monotonically increases blood pressure in rats. Toxicology, 328, 1–11. [DOI] [PubMed] [Google Scholar]
- Williams DP, Cash C, Rankin C, Bernardi A, Koenig J, & Thayer JF (2015). Resting heart rate variability predicts self-reported difficulties in emotion regulation: a focus on different facets of emotion regulation. Frontiers in psychology, 6, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaginuma-Sakurai K, Murata K, Shimada M, Nakai K, Kurokawa N, Kameo S, & Satoh H (2010). Intervention study on cardiac autonomic nervous effects of methylmercury from seafood. Neurotoxicology and teratology, 32(2), 240–245. [DOI] [PubMed] [Google Scholar]
- Yilmaz OH, Karakulak UN, Tutkun E, Bal C, Gunduzoz M, Onay EE, Ayturk M, Ozturk MT, & Alaguney ME (2016). Assessment of the cardiac autonomic nervous system in mercury-exposed individuals via post-exercise heart rate recovery. Medical Principles and Practice, 25(4), 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, & Wong M (2007). Environmental mercury contamination in China: sources and impacts. Environment international, 33(1), 108–121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


