Abstract
Stimulant-induced neurochemical changes may occur at different times for different brain regions or neurotransmitter systems. This study sought to examine the behavioral and neurochemical effects of extended access to α-pyrrolidinopentiophenone (α-PVP) and 4-methylmethcathinone (4MMC). Male and female Sprague-Dawley rats were trained to self-administer α-PVP (0.1 mg/kg/infusion) or 4MMC (0.5 mg/kg/infusion) through autoshaping, and then self-administered for 21 days during 1 h (short access; ShA) or 6 h (long access; LgA) sessions. Separate rats were assigned to a naïve control group. Amygdala, hippocampus, hypothalamus, prefrontal cortex (PFC), striatum, and thalamus were extracted, and tissue was analyzed with electrochemical detection and liquid chromatography mass spectrometry. Rats acquired self-administration of α-PVP and 4MMC, and LgA rats showed more escalation of self-administration than ShA rats. Synthetic cathinone administration produced several effects on neurotransmitters. LgA self-administration of α-PVP increased 5-HIAA levels in all brain regions, compared to control. In contrast, both LgA and ShA 4MMC self-administration decreased 5-HT and 5-HIAA levels in most brain regions. LgA exposure to both synthetic cathinones increased DOPAC levels in hypothalamus and striatum, and increased HVA levels in striatum compared to control. LgA self-administration of either synthetic cathinone produced region-specific increases in NE levels, whereas ShA self-administration lowered NE levels in select locations compared to control. These alterations in neurotransmitter levels indicate that synthetic cathinone use may produce differential neurochemical changes during the transition from use to abuse, and that 21 days of self-administration only models the beginning stages of dysregulated drug intake.
Keywords: alpha-PVP, mephedrone, neurotransmitter, self-administration
1.0. Introduction
Synthetic cathinones are widely available throughout the U.S. (Baumann, 2014; DEA, 2017; Madras, 2017), and unintentional ingestion of synthetic cathinones is rising (Oliver et al., 2019). Identifications of synthetic cathinones in seized drug products (DEA, 2017, 2019; NDEWS, 2018b) and identification of new synthetic cathinones are rising in some parts of the U.S. (NDEWS, 2019). High school students continue to use α-pyrrolidinopentiophenone (α-PVP) (Palamar et al., 2019), and α-PVP has caused multiple medical emergencies and deaths (NDEWS, 2015, 2018a).
Synthetic cathinones produce stimulant-like effects. 4-Methylmethcathinone (4MMC; mephedrone) releases dopamine (DA), norepinephrine (NE), and serotonin (5-HT) (Baumann et al., 2012; Cameron et al., 2013; Simmler et al., 2013). In contrast, α-PVP inhibits uptake of DA and NE transport (Glennon and Young, 2016; Koob and Volkow, 2010; Marusich et al., 2014). Both α-PVP and 4MMC cause hyperactivity and stereotyped behavior (Gatch et al., 2015;Gregg and Rawls, 2014; Marusich et al., 2014; Marusich et al., 2012; Marusich et al., 2016), and α-PVP also produces toxic effects not observed with cocaine or methamphetamine (Marusich et al., 2014). α-PVP and 4MMC are readily self-administered by rats, attesting to their reinforcing effects (Aarde et al., 2015; Creehan et al., 2015; Gannon et al., 2018; Marusich et al., 2019a; Marusich et al., 2019b; Marusich et al., 2013; Nguyen et al., 2017a; Nguyen et al., 2016; Vandewater et al., 2015).
The Koob and Volkow hypothesis of drug abuse posits that treatment needs may differ based on the amount of drug consumed over time, and that stimulant-induced neurochemical changes may occur at different times for different brain regions or neurotransmitter systems (Koob and Volkow, 2010). To test this model, we showed that 21 days of α-PVP and 4MMC self-administration during 1-h sessions (short access; ShA) had little effect on DA or DA metabolite levels in striatum, changes thought to accompany the binge and intoxication stage of drug abuse (Koob and Volkow, 2010; Marusich et al., 2019a; Marusich et al., 2019b). Similarly, ShA self-administration of α-PVP and 4MMC had little effect on GLU levels in hippocampus, PFC, or striatum, or on NE levels in amygdala or hypothalamus, which are hypothesized to change during the anticipation stage or negative reinforcement stage of drug abuse, respectively (Koob and Volkow, 2010; Marusich et al., 2019a; Marusich et al., 2019b). Thus, ShA self-administration does not model the expected changes in neurochemistry for stages of drug abuse based on Koob and Volkow’s hypothesized model (Koob, 2010; Koob and Volkow, 2010; Marusich et al., 2019a).
This study sought to examine effects of long access (LgA) to α-PVP and 4MMC, using procedures similar to those employed for ShA exposure (Marusich et al., 2019a; Marusich et al., 2019b). Neurotransmitter levels were measured to investigate if neuronal signaling changed as a function of duration of synthetic cathinone exposure and modeled different stages of drug abuse (Koob and Volkow, 2010). 4MMC shows escalation of self-administration during LgA conditions (Nguyen et al., 2017b; Watterson et al., 2014). To our knowledge, α-PVP has not been examined for escalation during LgA conditions.
2.0. Materials and Methods
2.1. Subjects
Adult male and female Sprague-Dawley rats (Envigo, Frederick, MD, USA) (total n=48), aged approximately 65-70 days at the start of the experiment, were housed individually in polycarbonate cages with hardwood bedding. Rats were housed in temperature-controlled conditions (20-24°C) with a 12 h standard light-dark cycle (lights on at 0700). Rats had free access to water in the home cage and were lightly food restricted (e.g. 20 g for males and 17 g for females daily). Experiments were approved by Mispro Biotech’s Institutional Animal Care and Use Committee (Protocol 2017-07-24-RTI-40) and complied with the ARRIVE guidelines.
All research was conducted as humanely as possible, and followed the principles of laboratory animal care (National Research Council, 2011).
2.2. Drugs
α-PVP and 4MMC were synthesized in house using standard synthetic procedures. They were formulated as recrystalized salt and were > 97% pure. The purity was assessed by carbon, hydrogen, nitrogen (CHN) combustion analysis, and proton nuclear magnetic resonance spectroscopy. Compounds were dissolved in saline (Patterson Veterinary Supply, Columbus, OH, USA). Gentamicin and heparin, used for maintaining catheter patency, were purchased from Patterson Veterinary Supply.
2.3. Apparatus
Experimental sessions were conducted in operant conditioning chambers for rats (MED Associates, St. Albans, VT, USA) housed inside sound-attenuating chambers (MED Associates). Each chamber contained two retractable levers, with a stimulus light above each lever, and a house light. One lever was designated as the active lever and the other lever was designated as inactive. The side of the chamber associated with the active lever was counterbalanced across subjects. Fans ventilated each chamber and speakers provided white noise. Infusion pumps (Med Associates) were located outside the chamber. Experimental events were arranged and recorded by MED-PC software (Med-Associates).
A Thermo Scientific CoulArray Multi-Channel ECD Array system (model 5600A; Thermo Scientific, Waltham, MA, USA) was used to analyze neurotransmitter concentrations. The array detector contained 16 coulometric electrochemical cells that provided quantitation of multiple neurotransmitters and metabolites simultaneously. An Agilent 1100 HPLC System (Santa Clara, CA, USA) and an Applied Biosystems API 4000 Triple Quadrupole liquid chromatography mass spectrometer (LC-MS) with Turbo Ion Spray source (Foster City, CA, USA) were used for quantitation of GLU.
2.4. Surgical Procedures
Rats were surgically implanted with chronic indwelling jugular catheters under general anesthesia as previously described (Marusich et al., 2019a; Marusich et al., 2019b). The external end of the catheter was secured by a quick connect harness. Rats were given a minimum of 7 days to recover from surgery before beginning the experiment. Catheters were flushed daily with saline prior to the session, and with 0.2 ml of a solution containing 0.96% gentamicin, 2.88% heparin, and 96.2% saline after the session to maintain patency. All catheters were checked for patency prior to the start of the experiment.
2.5. Drug Self-Administration
Rats were randomly assigned to one of three drug groups: α-PVP (0.1 mg/kg/infusion), 4MMC (0.5 mg/kg/infusion), or drug- and experimentally-naïve control (n=8/sex/group). These doses led to acquisition of self-administration in rats using the same schedule of reinforcement in prior studies (Aarde et al., 2015; Creehan et al., 2015; Marusich et al., 2019a; Marusich et al., 2019b; Nguyen et al., 2017a; Nguyen et al., 2016; Vandewater et al., 2015), and were at the peak of the dose-effect curves in most past studies (Aarde et al., 2015; Gannon et al., 2017; Nguyen et al., 2016). The naïve groups provided a control for the prior saline self-administration groups (Marusich et al., 2019a; Marusich et al., 2019b) that were never exposed to an operant contingency or exposed to surgical procedures. Rats in the naïve group were not exposed to any experimental conditions, were not implanted with jugular catheters, and were not exposed to any drugs or saline. These rats were food restricted, weighed daily, and housed in the colony room for 14-15 days to match the duration of time that rats in prior autoshaping-only groups were in the colony room (Marusich et al., 2019a; Marusich et al., 2019b). Thus, the naïve groups captured the neurotransmitter levels of adult Sprague-Dawley rats following housing in our colony room.
Rats in the α-PVP and 4MMC groups were first trained to self-administer through an autoshaping procedure for 7 days (Carroll and Lac, 1993; Marusich et al., 2010). This procedure, which was used in prior groups (Marusich et al., 2019a; Marusich et al., 2019b), ensured that all rats received a consistent, limited amount of drug exposure, regardless of how quickly they acquired self-administration. During autoshaping sessions, active lever extension was paired with an infusion (0.1 ml) based on a random time 60 s schedule. Fifteen seconds of lever extension, or a lever press resulted in an infusion (5.9 s) and initiated a 20-s timeout, which was signaled by illumination of both stimulus lights. Throughout training and self-administration sessions, the inactive lever was extended, and presses on this lever were recorded, but had no programmed consequence. Autoshaping sessions delivered 15 infusions within the first 30 min of the session. Rats then remained in the operant conditioning chamber for 15 min with only the inactive lever present and no drug infusions available, which provided additional exposure to the lack of programmed consequences associated with the inactive lever.
Following autoshaping, α-PVP and 4MMC rats continued to self-administer on a fixed ratio 1 schedule of reinforcement (FR1) for an additional 21 days during daily 6 h sessions (long access; LgA). Self-administration under LgA conditions typically leads to increased stimulant intake across sessions (Ahmed and Koob, 1998, 1999; Koob and Le Moal, 1997). Our previous self-administration groups that were exposed to ShA conditions (21 days of daily 1 h sessions) displayed few neurochemical effects that aligned with hypothesized stages of drug abuse (Marusich et al., 2019a; Marusich et al., 2019b). Therefore, we hypothesized that the LgA groups in the present study would show neurochemical effects associated with several stages of drug abuse (Koob, 2010; Koob and Le Moal, 2005, 2008; Koob and Volkow, 2010). Data from our previous studies in which male and female adult Sprague-Dawley rats self-administered α-PVP (0.1 mg/kg/infusion), 4MMC (0.5 mg/kg/infusion), or saline during daily 1 h sessions (ShA) (n=7-8/group/sex) are included in the results section for comparison (Marusich et al., 2019a; Marusich et al., 2019b), including analyses of sex differences which were not included in the prior publications.
2.6. Brain Sample Collection
Rats were euthanized by rapid decapitation approximately 24 h after their last self-administration session in order to avoid direct drug effects (Marusich et al., 2019a; Marusich et al., 2019b; Wee et al., 2007b). The hypothalamus was removed from the ventral side of the brain and divided into halves. The brain was then cut down the mid line and each cortical half was opened, and the hippocampus was removed (Spijker, 2011). Next, each half was cut into three coronal slices using midline anatomical markers moving rostral to caudal. The first cut was made at the beginning of the corpus callosum, the second at the fornix, and the third cut at the end of the corpus callosum. PFC was taken from the first section. Striatum was removed from the second slice. Finally, thalamus and amygdala were removed from the third section (Chiu et al., 2007; Honkanen, 1999). All tissue samples were placed in aluminum foil, inserted into a cryovial, flash frozen in liquid nitrogen, and then stored at −80°C.
2.7. Neurotransmitter Quantitation
Brain tissue samples were weighed and placed in cryovials containing stainless steel grinding balls. A tissue buffer solution (pH 3) consisting of 0.05 M Na2HPO4, 0.03 M citric acid, 2 mM ascorbic acid, and an Internal Standard (IS) of 200 ng/ml 3,4-dihydroxybenzylamine (DHBA) was added to each brain tissue sample at a ratio of 20 μl/mg of tissue while samples were homogenated by two 30-s cycles on the geno/grinder (SPEX SamplePrep, Metuchen, NJ), and then centrifuged at 16000 g for 2 min. Supernatant was then transferred to a 96 well plate for analysis by electrochemical detection (ECD).
For the GLU analysis, an additional 10 μl aliquot of supernatant was transferred to a 700 μl deep, 96 well plate, and diluted with 490 μl of 5 mM ammonium acetate and 0.1% formic acid, aqueous (LC-MS/MS mobile phase A). The plate was mixed at 1000 RPM for 4 min on an Eppendorf MixMate orbital shaker (Enfield, CT). Then 100 μl was transferred to a new 700 μl deep, 96 well plate and diluted with an additional 400 μl of LC-MS/MS mobile phase A (total dilution of 250 fold). A 50 μl aliquot of 10 μg/ml L-glutamic −2,3,3,4,4-d5 acid (d5-GLU) in water was added. The plate was sealed and mixed again at 1000 RPM for 4 min on an Eppendorf MixMate orbital shaker prior to analysis.
2.7.1. Electrochemical methods.
Ultra-high pressure liquid chromatography (UPLC) coupled with ECD was used to simultaneously measure DA, dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 5-HT, 5-hydroxy-3-acetic acid (5-HIAA), and NE. A 10-μl aliquot was injected onto a Luna Omega 2.1 x 150 mm column (Phenomenex, Torrance, CA) coupled to a LPG-3400RS pump, WPS-3000TBRS autosampler, and a CoulArray electrochemical detector. The column was heated to 30°C. The mobile phase consisted of 50 mM sodium phosphate, 47 mM citric acid, 0.14 mM EDTA, 0.64 mM octanesulfonic acid, and 5% methanol, with a flow rate of 0.4 ml/min. The detector was set to sequentially deliver potentials of −150 mV, 150 mV, 400 mV, and 600 mV.
Standards for ECD were prepared by weighing approximately 1 mg of analytes DA (Sigma-Aldrich, Buchs, Switzerland), DOPAC (Sigma-Aldrich, Buchs, Switzerland), HVA (Sigma-Aldrich, Buchs, Switzerland), 5-HT (Sigma-Aldrich, St. Louis, MO), 5-HIAA (Sigma-Aldrich, St. Louis, MO), and NE (Sigma-Aldrich, St. Louis, MO). Each standard was transferred to a volumetric flask and diluted to volume with tissue buffer to create stock solutions. A stock solution containing approximately 10 μg/ml of analyte was then diluted to encompass a concentration range from 1000 to 0.5 ng/ml.
2.7.2. LC-MS/MS methods.
GLU was analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) using an API 4000 Triple Quadrupole mass spectrometer with a Turbo Ion Spray source (Applied Biosystems/MDS Sciex, Foster City, CA) in positive ion mode, coupled with Agilent 1100 HPLC system (Santa Clara, CA). A XBridge HILIC 4.6 x 150 mm column (Waters, Ireland) was used for analyte separation. Mobile phase A consisted of 5 mM ammonium acetate and 0.1% formic acid (aqueous) and mobile phase B consisted of acetonitrile Mobile phase A was held at 50% for 1 min, then increased linearly to 90% for 1.5 min, and held for 2.5 min before returning to initial conditions. The flow rate was 0.5 ml/min. Ion source temperature and spray voltage were 650 °C and 4000 V, respectively. Transitions monitored for quantitation were: GLU, 148.0→ 83.9; GLUd5 (IS), 152.9→ 134.8.
Standards for GLU analysis were prepared by weighing approximately 25 mg of GLU (Alfa Aesar, Ward Hill, MA). The standard was transferred to a volumetric flask and diluted to volume with mobile phase A (5 mM ammonium acetate and 0.1% formic acid, aqueous) and labeled as stock solution. The stock solution containing approximately 2.5 mg/ml of GLU was then diluted to encompass a concentration range from 2500 to 10 ng/ml.
2.8. Data Analysis
Statistical analyses were conducted using NCSS (Number Cruncher Statistical Systems, Kaysville, Utah, USA). For all analyses, α-PVP and 4MMC were analyzed separately. In most instances, data from naïve and LgA groups (present study) were statistically analyzed separately from ShA groups (Marusich et al., 2019a; Marusich et al., 2019b) because the neurotransmitter assays were conducted at different times for these studies. One male α-PVP ShA rat lost catheter patency. Additionally, one male 4MMC ShA and saline ShA rat were exposed to the incorrect infusion volume. All self-administration and neurotransmitter data for these three rats were excluded from graphs and analyses.
For self-administration data, autoshaping data were analyzed with separate mixed factors ANOVAs (day x lever x sex) to compare active and inactive lever presses, with day and lever as within-subjects factors, and sex as a between-subjects factor. The additional 21 days of self-administration were also analyzed with separate mixed factors ANOVAs (day x lever x sex), which compared responses on the active and inactive levers. All analyses of self-administration data were examined for circularity using Mauchly’s test. If the assumption of circularity was violated, the Geisser-Greenhouse Adjustment was employed.
Neurotransmitter and metabolite data from LgA and naïve groups were analyzed with between-factors condition (LgA vs naïve) x sex ANOVAs. Neurotransmitter and metabolite data from ShA groups were analyzed with between-factors drug (cathinone vs saline) x sex ANOVAs. In select instances, neurotransmitter and metabolite data from ShA and LgA groups were compared with between-factors condition (ShA saline vs ShA drug vs LgA drug) x sex ANOVAs. Each brain region and neurotransmitter (or metabolite) combination was analyzed separately. All tests were considered significant at p<0.05 and were followed with Tukey’s post hoc tests as appropriate.
There were two scenarios in which neurotransmitter data were excluded from graphs and analyses. First, there were a few cases in which amygdala samples had very elevated levels of DA, DOPAC, and HVA. Because of the closeness of amygdala and striatum in the brain, it is likely that a small amount of striatum was inadvertently included as part of the amygdala sample. Second, an unknown peak interfered with the integration of the DOPAC or NE peak in select samples of PFC and hypothalamus. Additionally, in some samples of thalamus, DOPAC levels were below the level of quantitation. Instead of omitting these values from graphs and analyses, we substituted a value of 0.005 ng/mg, which is halfway between zero and the level of quantitation of 0.01 ng/mg.
3.0. Results
3.1. Self-Administration
Self-administration data from the autoshaping phase for LgA groups are shown in the Supplementary Material (Fig. S1). There were no sex differences in responding for either drug, no sex x lever interactions, and no sex x day interactions (P>0.05). Rats in the α-PVP groups responded more on the active than inactive lever during autoshaping [main effect of lever α-PVP: F(1, 14)=4.67, P<0.05]. Rats in the α-PVP group responded more on days 3-7 than on day 1 [main effect of day: F(6, 84)=3.52, P<0.05].
Autoshaping data for ShA groups, described in detail in our previous studies (Marusich et al., 2019a; Marusich et al., 2019b), are shown the Supplementary Material (Fig. S2). There were no significant main effects of sex or lever for α-PVP (P>0.05). Males responded more on the active lever for α-PVP than females during autoshaping [sex x lever interaction: F(1, 13)=8.07, P<0.05]. For 4MMC and saline, there were no significant effects of lever, day, or sex, nor were there any significant interactions (P>0.05) (Fig. S2). Results suggest that rats in all LgA and ShA groups were administered most infusions noncontingently during autoshaping.
Self-administration data from the FR1 phase for LgA rats are shown in Fig. 1. Rats responding for α-PVP and 4MMC responded more on the active than inactive lever [main effect of lever α-PVP: F(1, 14)=407.77, P<0.05; 4MMC: F(1, 14)=171.87, P<0.05], and neither drug showed a main effect of sex or sex x lever interaction on responses (P>0.05). α-PVP rats increased their responding from Day 1 on Days 3, 9-21 [main effect of day: F(20, 280)=7.81, P<0.05], and increased active responses from Day 1 on Days 3, 7-21 [day x lever interaction: F(20, 280)=7.69, P<0.05]. When responses were collapsed across active and inactive levers, males responded more for α-PVP on Days 12, 15-16, 18-21 than Day 1, and females responded more on Days 3, 18-21 than Day 1. Females responded more than males on Day 3 [day x sex interaction: F(20, 280)=3.23, P<0.05]. The reason for this spike in responding on the active lever on Day 3 is unknown. All rats of one sex began the study on the same day, therefore, data for this aberrant point were collected on the same date for all female rats. Males completed the study before females began the study, and, therefore, males did not have a session on this date. The most plausible explanation for this sex difference is that an environmental disturbance occurred on that date for females; however, no disturbances were detected by the experimenters. For 4MMC, LgA rats showed increased responding on Days 3-21 relative to Day 1 [main effect of day: F(20, 280)=19.21, P<0.05], and responded more on the active lever on Days 2-21 than on Day 1 [day x lever interaction: F(20, 280)=24.40, P<0.05]. There was no significant interaction between sex and day for 4MMC (P<0.05).
Fig. 1.

Mean responses on the active (filled symbols) and inactive (open symbols) levers as a function of session for LgA rats self-administering α-PVP (top panel) or 4MMC (bottom panel) on an FR1 schedule of reinforcement during 6 h sessions. $ indicates a significant difference between the inactive and active levers (main effect of lever), # indicates a significant difference from Day 1 (main effect of day), and * indicates significant difference from male (day x sex interaction) (P < 0.05). M = male; F = female; n=8/sex/group.
Self-administration data from the FR1 phase for ShA groups, described in detail in our previous studies (Marusich et al., 2019a; Marusich et al., 2019b), are shown in Fig. 2. Results in this manuscript provide information on potential sex differences that were not analyzed in our previous publications. Rats in both cathinone groups and the saline group responded more on the active than inactive lever [main effect of lever α-PVP: F(1, 13)=456.73, p<0.05; 4MMC: F(1, 13)=227.34, P<0.05; saline: F(1, 13)= 12.81, P<0.05], and no ShA groups showed a main effect of sex or sex x lever interaction on responses (P>0.05). α-PVP rats showed greater responding on Days 7, 15-16, and 19-21 compared to Day 1, and greater active responses on Days 3-21 than on Day 1 [main effect of day: F(20, 260)=1.99, P<0.05; day x lever interaction: F(20, 260)=8.37, P<0.05]. Similarly, 4MMC rats showed greater responding on Days 4-21 compared to Day 1, and greater active responses on Days 2-21 than on Day 1 [main effect of day: F(20, 260)=9.19, P<0.05; day x lever interaction: F(20, 260)=8.97, P<0.05]. For the saline groups, males responded more than females on Days 6-7 [day x sex interaction: F(20, 260)=1.67, P<0.05]. There was no day x sex interaction for α-PVP or 4MMC groups, and no main effect of day or day x lever interaction for saline groups (P>0.05).
Fig. 2.

Mean responses on the active (filled symbols) and inactive (open symbols) levers as a function of session for ShA rats self-administering α-PVP (top panel), 4MMC (middle panel), or saline (bottom panel) on an FR1 schedule of reinforcement during 1 h sessions. $ indicates a significant difference between the inactive and active levers (main effect of lever), # indicates a significant difference from Day 1 (main effect of day), and * indicates significant difference from male (day x sex interaction) (P < 0.05). M = male; F = female; n=7-8/sex/group.
3.2. Neurotransmitters
3.2.1. Effects of LgA vs naïve condition.
Neurotransmitter and metabolite concentrations for all brain regions and all LgA and naïve rat groups are shown in Fig. 3. There were differences between condition (LgA vs naïve), between males and females, and there were interactions between condition and sex. All statistically significant effects of condition (LgA vs naïve) on neurotransmitters, and interactions between condition and sex are shown in Table 1 and Fig. 3. Interestingly, the effects of cathinone self-administration on 5-HT varied by drug. Self-administration of α-PVP during LgA conditions had no effect on 5-HT in any brain region but increased 5-HIAA in all brain regions compared to naïve. In contrast, self-administration of 4MMC during LgA conditions decreased 5-HT in all brain regions except thalamus and decreased 5-HIAA in most brain regions compared to naïve (Table 1 and Fig. 3). Small, but significant changes in DA levels for LgA groups compared to naïve were found in select brain regions where α-PVP decreased DA levels in thalamus, whereas 4MMC increased DA levels in hypothalamus and thalamus. The most striking effects of condition were large elevations of DOPAC and HVA levels in striatum that were noted following LgA self-administration of both α-PVP and 4MMC. DOPAC level was also elevated in hypothalamus for LgA groups compared to naïve (Table 1 and Fig. 3). Self-administration of either synthetic cathinone also increased NE levels in several brain regions, with the biggest increases shown in hypothalamus. Synthetic cathinone self-administration also altered GLU levels compared to naïve in select brain regions, but the magnitude and direction of effects varied by synthetic cathinone.
Fig. 3.

Mean neurotransmitter and metabolite concentrations as a function of brain region for rats in LgA or naïve groups. Neurotransmitter units are ng/mg tissue for all analytes except for GLU which is shown in μg/mg tissue. Note different y axis scale for hypothalamus and striatum. $ indicates a significant difference from naïve for the same sex (main effect or interaction), and * indicates a significant difference from male for the same drug/naïve (main effect or interaction) (P < 0.05). M = male; F = female; n=4-7/group for HVA in amygdala; n=4-7/group for NE in PFC; n=6-8/group for DOPAC in thalamus; n=7-8/group for all other neurotransmitters and metabolites.
Table 1.
F values for significant main effects of condition (LgA vs naïve), and significant condition by sex interactions on neurotransmitter concentrations (P < 0.05). Arrows indicate the direction of effects of condition compared to the naïve group. Cells containing M or F indicate interactions that were only significant for males or females, respectively. Cells without M or F indicate main effects. Degrees of freedom are 1, 28 for all comparisons except for 5-HIAA for 4MMC and NE for α-PVP in hippocampus, which have 1, 27 degrees of freedom, and NE in PFC for 4MMC and α-PVP, which have 1, 19, and 1, 15 degrees of freedom, respectively.
| Brain Region | 5-HT | 5-HIAA | DA | DOPAC | HVA | NE | GLU |
|---|---|---|---|---|---|---|---|
| α-PVP | |||||||
| Amygdala | ↑ 8.95 | ↑ 11.15 | ↑ 12.44 | ||||
| Hippocampus | ↑ 6.52 | ↑ 6.37 | |||||
| Hypothalamus | ↑ 12.96 | ↑ 21.27 | ↑ 29.66 | ||||
| ↑ F 6.70 | |||||||
| Striatum | ↑ 21.53 | ↑ 96.05 | ↑ 77.81 | ↑ 4.62 | |||
| Thalamus | ↑ 6.42 | ↓ 10.12 | |||||
| ↑ F 4.46 | |||||||
| 4MMC | |||||||
| Amygdala | ↓ 10.74 | ↓ 6.11 | ↑ 4.36 | ||||
| Hippocampus | ↓ 29.18 | ↓ 40.11 | ↓ 9.54 | ||||
| ↓ F 7.27 | |||||||
| Hypothalamus | ↑ 9.20 | ↑ 5.66 | ↑ 21.35 | ↓ 9.88 | |||
| PFC | ↓ 20.76 | ↓ 9.28 | ↑ 5.89 | ||||
| Striatum | ↓ 9.00 | ↑ 38.77 | ↑ 17.60 | ↑ 8.69 | |||
| ↑ M 21.06 | |||||||
| Thalamus | ↑ 6.60 | ||||||
3.2.2. Effects of synthetic cathinone ShA vs saline ShA.
All statistically significant effects of drug (synthetic cathinone vs saline) on neurotransmitters in ShA groups, and interactions between drug and sex are shown in Table 2 and Fig. 4. Self-administration of 4MMC produced widespread decreases in 5-HT and 5-HIAA levels compared to saline self-administration, whereas self-administration of α-PVP had little effect on serotonergic levels compared to saline. ShA self-administration of α-PVP and 4MMC did not alter DA levels from those of saline groups (Table 2 and Fig. 4). Synthetic cathinones altered DA metabolite levels in a few select locations in the brain compared to saline. Both synthetic cathinones lowered NE levels in hippocampus and altered GLU levels in some brain regions compared to saline, but the effects on GLU occurred primarily for males (Table 2 and Fig. 4).
Table 2.
F values for significant main effects of drug (cathinone vs saline), and significant drug by sex interactions on neurotransmitter concentrations for ShA groups (P < 0.05). Arrows indicate the direction of effects of condition compared to the saline group. Cells containing M or F indicate interactions that were only significant for males or females, respectively, and cells containing C indicate interactions that were only significant for the cathinone (not saline). Cells without M, F, or C indicate main effects. Degrees of freedom are 1, 26 for all comparisons except the following: amygdala DA, DOPAC, and HVA were 1, 22 for α-PVP, and 1, 24 for 4MMC; degrees of freedom are 1, 25 for 5-HIAA in PFC and NE in striatum for 4MMC; degrees of freedom are 1, 25 for 5-HT, 5-HIAA, and NE in hippocampus, 5-HT, 5-HIAA, DA, DOPAC, and NE in hypothalamus, 5-HT and 5-HIAA in PFC, and NE in striatum for α-PVP.
| Brain Region | 5-HT | 5-HIAA | DA | DOPAC | HVA | NE | GLU |
|---|---|---|---|---|---|---|---|
| α-PVP | |||||||
| Amygdala | ↑ 8.55 | ↑ 7.71 | ↓ 5.23 | ||||
| ↓ M 6.69 | |||||||
| Hippocampus | ↓ 11.67 | ||||||
| Hypothalamus | ↓ 4.52 | ||||||
| PFC | ↓ M 7.47 | ||||||
| Striatum | ↑ 5.12 | ↓ C 6.02 | |||||
| 4MMC | |||||||
| Amygdala | ↓ 9.87 | ||||||
| Hippocampus | ↓ 12.35 | ↓ 13.94 | ↓ 15.66 | ||||
| Hypothalamus | ↓ 5.11 | ↓ 9.40 | |||||
| ↓ M 5.64 | |||||||
| PFC | ↓ 32.04 | ↓ 15.59 | |||||
| ↓ F 4.50 | |||||||
| Striatum | ↓ 11.01 | ↓ 9.43 | ↓ F 8.52 | ||||
| Thalamus | ↓ 5.00 | ↓ 4.28 | ↓ M 8.68 | ||||
| ↓ F 4.65 | |||||||
Fig. 4.

Mean neurotransmitter and metabolite concentrations as a function of brain region for rats in ShA groups. Neurotransmitter units are ng/mg tissue for all analytes except for GLU which is shown in μg/mg tissue. Note different y axis scale for hypothalamus and striatum. * indicates a significant difference from male for the same drug/control (main effect or interaction), and # indicates a significant difference from saline for the same sex (main effect or interaction) (P < 0.05). Sal = saline; M = male; F = female. n=5-8/group for DA, DOPAC, and HVA in amygdala; n=3-8/group for DOPAC in PFC; n=7-8/group for all other neurotransmitters and metabolites.
3.2.3. Effects of sex.
All statistically significant effects of sex on neurotransmitters are shown in Table 3 and Fig. 3 for LgA and naïve groups, and in Table 4 and Fig. 4 for ShA groups. ShA groups showed considerably more instances of sex differences than LgA and naïve groups. There were sex differences in 5-HT levels for ShA rats, with both synthetic cathinones producing greater 5-HT levels for females than males in amygdala and PFC (Table 4 and Fig. 4). There were also sex differences in 5-HT levels for hippocampus, hypothalamus, and striatum for 4MMC ShA groups, but the direction of the effects was region dependent. 5-HIAA levels were higher for females than males for rats in most self-administration conditions and in most brain regions, effects that were observed for both synthetic cathinones. LgA and naïve groups did not show any sex differences in DA or DOPAC levels. In contrast, female ShA rats had lower DA levels than males in PFC, higher DOPAC levels than males in amygdala, hypothalamus, and striatum, and higher HVA levels than males in amygdala and striatum. LgA females showed lower NE levels than males in hypothalamus, whereas ShA rats showed sex differences in NE levels in the other brain regions. Females had lower NE levels than males in amygdala and thalamus for ShA rats, the latter of which only occurred for 4MMC groups, and higher NE levels than ShA males in hippocampus, PFC, and striatum. There were a few sex differences in GLU levels, but the affected brain region and direction of the effects varied by synthetic cathinone and by self-administration condition (Fig. 3–4).
Table 3.
F values for significant main effects of sex on neurotransmitter concentrations for LgA and naïve groups (P < 0.05). Arrows indicate the direction of effects of sex compared to males. Degrees of freedom are 1, 28 for all comparisons except for 5-HIAA for 4MMC and NE for α-PVP in hippocampus, which have 1, 27 degrees of freedom, and NE in PFC for 4MMC and α-PVP, which have 1, 19, and 1, 15 degrees of freedom, respectively.
| Brain Region | 5-HT | 5-HIAA | NE | GLU |
|---|---|---|---|---|
| α-PVP | ||||
| Amygdala | ↑ 15.96 | ↑ 4.46 | ||
| Hippocampus | ↑ 24.71 | |||
| Hypothalamus | ↑ 11.94 | ↓ 5.27 | ||
| PFC | ↑ 5.03 | |||
| Striatum | ↑ 9.22 | ↑ 5.85 | ||
| 4MMC | ||||
| Amygdala | ↑ 10.34 | |||
| Hippocampus | ↓ 17.67 | ↓ 5.99 | ||
| Hypothalamus | ↓ 5.69 | ↓ 4.28 | ||
| PFC | ↑ 8.68 | |||
| Striatum | ↑ 9.22 | |||
Table 4.
F values for significant main effects of sex on neurotransmitter concentrations for ShA groups (P < 0.05). Arrows indicate the direction of effects of sex compared to males. Degrees of freedom are 1, 26 for all comparisons except the following: amygdala DA, DOPAC, and HVA were 1, 22 for α-PVP, and 1, 24 for 4MMC; degrees of freedom are 1, 25 for 5-HIAA in PFC and NE in striatum for 4MMC; degrees of freedom are 1, 25 for 5-HT, 5-HIAA, and NE in hippocampus, 5-HT, 5-HIAA, DA, DOPAC, and NE in hypothalamus, 5-HT and 5-HIAA in PFC, and NE in striatum for α-PVP.
| Brain Region | 5-HT | 5-HIAA | DA | DOPAC | HVA | NE | GLU |
|---|---|---|---|---|---|---|---|
| α-PVP | |||||||
| Amygdala | ↑ 6.37 | ↑ 6.88 | ↑ 8.16 | ↑ 13.76 | ↓ 41.46 | ||
| Hippocampus | ↑ 19.95 | ||||||
| Hypothalamus | ↑ 6.24 | ↑ 6.84 | |||||
| PFC | ↑ 8.77 | ↑ 10.70 | ↓ 8.48 | ↑ 27.59 | ↑ 49.79 | ||
| Striatum | ↑ 33.11 | ↑ 13.66 | ↑ 5.15 | ↑ 10.86 | |||
| Thalamus | ↑ 29.44 | ||||||
| 4MMC | |||||||
| Amygdala | ↑ 6.21 | ↓ 9.28 | ↑ 16.25 | ↑ 13.10 | ↓ 38.79 | ||
| Hippocampus | ↑ 5.08 | ↑ 27.28 | |||||
| Hypothalamus | ↓ 7.73 | ↑ 15.51 | |||||
| PFC | ↑ 10.17 | ↓ 59.14 | ↑ 30.00 | ↑ 67.07 | |||
| Striatum | ↑ 25.27 | ↑ 33.67 | ↑ 18.25 | ↑ 5.91 | ↑ 6.37 | ||
| Thalamus | ↑ 16.32 | ↓ 7.37 | ↑ 21.04 | ||||
4.0. Discussion
Rats acquired self-administration of α-PVP and 4MMC, consistent with past studies (Aarde et al., 2015; Nguyen et al., 2017a; Nguyen et al., 2016; Vandewater et al., 2015). LgA rats showed greater escalation of drug intake over time than ShA groups, as expected. Interestingly, ShA drug self-administration also led to escalation of intake, particularly for 4MMC. This finding suggests that dysregulated drug intake is not limited to LgA conditions for α-PVP and 4MMC, similar to a past study showing that LgA and ShA cocaine self-administration both led to escalation of intake (Beckmann et al., 2012). To our knowledge, this is the first study to statistically compare male and female rodents for 4MMC and α-PVP self-administration, and the resulting neurochemical changes. Sex differences in self-administration were largely absent. Sex differences for α-PVP during autoshaping (Fig. S2) did not lead to sex differences in α-PVP self-administration (Fig. 2). LgA α-PVP groups showed sex differences in self-administration on one day (Fig. 1), but this effect did not persist into other days of the study. Thus, the similarity in α-PVP intake across sexes was akin to that found in previous studies (Aarde et al., 2015; Javadi-Paydar et al., 2018). The similarity in 4MMC self-administration for both sexes is also consistent with past studies, which showed no sex difference in acquisition of 4MMC self-administration (Creehan et al., 2015; Vandewater et al., 2015). Sex differences in ShA saline self-administration were noted (Fig. 2) but were only statistically different for two days.
In contrast to the minor sex differences in self-administration behavior, sex differences in neurochemical changes were more widespread. Notably, sex differences in neurochemistry were more abundant for ShA than LgA groups, and the cause of this is unknown. Prominent sex differences emerged for NE levels in amygdala, hippocampus, PFC, and striatum for ShA groups (Fig. 4). There were also large sex differences in GLU levels in PFC, and 5-HIAA levels in striatum and thalamus for ShA groups (Fig. 4). Sex differences in neurochemistry in LgA groups were largely confined to 5-HIAA levels (Fig. 3). Future studies are needed to determine why there were vast sex differences in the neurochemistry of ShA groups that seemed to dissipate in the LgA groups.
The present study sought to determine if stimulant-induced neurochemical changes spread as drug use persists (cf. Koob and Le Moal, 2005, 2008; Koob and Volkow, 2010). Neurotransmitter data from groups that self-administered under ShA or LgA conditions are compared in Fig. 5–7 to assess how neuronal signaling changed based on duration of drug exposure. Changes in DA levels in striatum and thalamus are hypothesized to occur during the binge and intoxication stage because activation of the mesolimbic DA system produces acute reinforcing properties of psychostimulants (Koob and Volkow, 2010). Furthermore, the dorsal striatum is involved in escalation of drug taking and compulsive behaviors (Clark et al., 2013; Willuhn et al., 2012). LgA, but not ShA, synthetic cathinone self-administration elevated DOPAC and HVA levels in striatum compared to saline and synthetic cathinone ShA levels (Fig. 5), encompassing the binge and intoxication stage of Koob and Volkow’s model. Interestingly, while the metabolites were altered, DA levels only changed in striatum and thalamus for 4MMC and α-PVP LgA groups, respectively, compared to saline ShA. These findings are consistent with a past study showing that ShA 4MMC self-administration did not alter DA, DOPAC, or HVA in striatum (Motbey et al., 2013). Thus, LgA synthetic cathinone self-administration for 21 days models most neurochemical changes predicted by the Koob and Volkow hypothesis for the binge and intoxication stage.
Fig. 5.

Mean concentrations of DA and DA metabolites in striatum and thalamus for rats in LgA or ShA groups. $ indicates a significant difference from Sal ShA for the same sex, # indicates a significant difference from drug ShA for the same sex, and * indicates a significant difference from male for the same condition. [effect of condition on striatum DA 4MMC: F(2, 40)=4.04, P<0.05]; [effect of condition on striatum DOPAC α-PVP: F(2, 40)=23.01; 4MMC: F(2, 40)=9.20, P<0.05]; [effect of sex on striatum DOPAC α-PVP: F(1, 40)=11.86; 4MMC: F(1, 40)=18.06, P<0.05]; [effect of condition on striatum HVA α-PVP: F(2, 40)=123.67; 4MMC: F(2, 40)=118.34; P<0.05]; [effect of condition on thalamus DA α-PVP: F(2, 40)=31.10, P<0.05]. M = male; F = female; n=7-8/group.
Fig. 7.

Mean NE concentrations as a function of brain region for rats in LgA or ShA groups. $ indicates a significant difference from Sal ShA for the same sex, # indicates a significant difference from drug ShA for the same sex, and * indicates a significant difference from male for the same condition. [effect of condition on amygdala NE α-PVP: F(2, 40)=6.58, P<0.05]; [effect of sex on amygdala NE α-PVP: F(1,40)=35.29; 4MMC: F(1,40)=35.14, P<0.05]; [condition x sex interaction for amygdala NE α-PVP: F(2, 40)=12.54; 4MMC: F(2, 40)=5.77, P<0.05]; [effect of condition on hypothalamus NE α-PVP: F(2, 39)=7.78, P<0.05]. M = male; F = female; n=6-8/group.
GLU is thought to play a pivotal role in the anticipation and preoccupation stage of drug abuse because increased GLU neurotransmission is associated with behavioral sensitization to psychostimulants (Pierce et al., 1996), drug seeking, and reinstatement (Koob and Volkow, 2010). LgA self-administration for 21 days modeled the anticipation and preoccupation stage as demonstrated by increased levels of GLU in hippocampus, PFC, and striatum for LgA compared to saline and ShA synthetic cathinone levels (Fig. 6), which are predicted by the model (Koob and Volkow, 2010). The only exception was that 4MMC LgA females showed similar GLU levels as ShA groups in striatum.
Fig. 6.
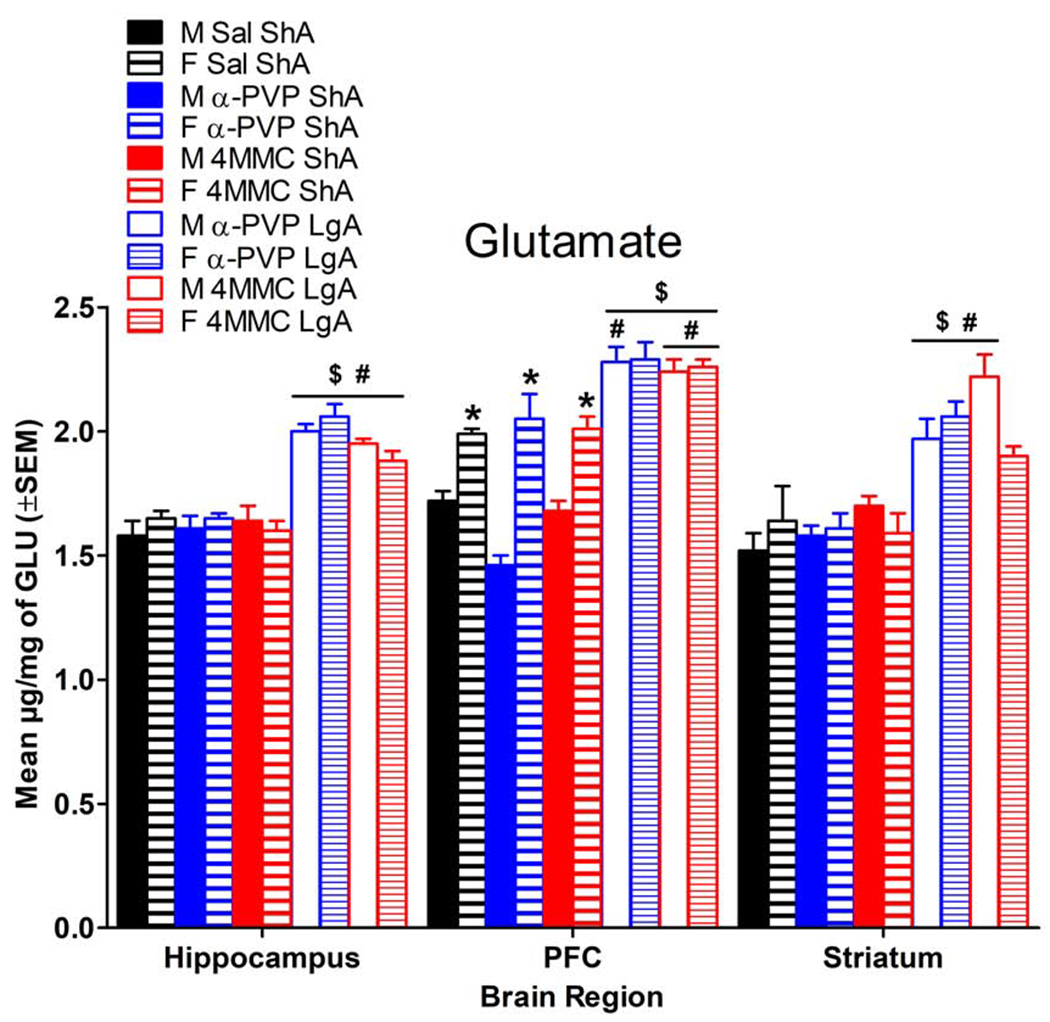
Mean GLU concentrations as a function of brain region for rats in LgA or ShA groups. $ indicates a significant difference from Sal ShA for the same sex, # indicates a significant difference from drug ShA for the same sex, and * indicates a significant difference from male for the same condition. [effect of condition on hippocampal GLU α-PVP: F(2, 40)=69.91; 4MMC: F(2, 40)=36.67, P<0.05]; [effect of condition on PFC GLU α-PVP: F(2, 40)=39.08; 4MMC: F(2, 40)=72.46, P<0.05]; [effect of sex on PFC GLU α-PVP: F(1, 40)=31.54; 4MMC: F(1, 40)=42.79, P<0.05]; [condition x sex interaction for PFC GLU α-PVP: F(2, 40)=10.55; 4MMC: F(2, 40)=8.70, P<0.05]; [effect of condition on striatum GLU α-PVP: F(2, 40)=17.77; 4MMC: F(2, 40)=18.65, P<0.05]; [condition x sex interaction for striatum GLU 4MMC: F(2, 40)=3.35]. M = male; F = female; n=7-8/group.
Because drug dependence increases NE levels in amygdala (Koob, 2009), NE is the primary neurotransmitter associated with the negative reinforcement and withdrawal stage of drug abuse. We observed minimal noradrenergic changes thought to accompany the negative reinforcement and withdrawal stage. NE levels decreased in amygdala for male LgA rats compared to ShA rats, but no changes occurred in amygdala for females, and minimal noradrenergic changes occurred in hypothalamus (Fig. 7) (Koob and Volkow, 2010). Notably, this study did not measure levels of corticotropin-releasing factor or dynorphin, which are also associated with the negative reinforcement and withdrawal stage of the Koob hypothesis (Koob and Volkow, 2010). Thus, future studies should examine these additional neurochemicals associated with the withdrawal stage to more fully understand if the self-administration methods employed here modeled the neurochemistry of the withdrawal stage of drug abuse.
Overall, these data suggest that 21 days of LgA, but not ShA, self-administration of synthetic cathinones produces most of the early-stage neurochemical changes predicted by Koob and Volkow (Koob and Volkow, 2010). Self-administration of α-PVP and 4MMC induced plasticity in neural circuitry that may be driving compulsive drug taking or producing a deficit state for normal reward, thereby increasing motivation to continue self-administration (Koob and Le Moal, 2005). Furthermore, the increased GLU levels observed for LgA groups may be enhancing drug seeking (Koob, 2010), although drug seeking was not directly measured in the present study. Cocaine and amphetamine, which have similar mechanisms of action as α-PVP and 4MMC, respectively, induce synaptic plasticity within the DA system and DA receptive neurons. This plasticity hijacks normal learning mechanisms to create habits that persist despite adverse consequences and contribute to the deterioration of cognitive performance (Koob and Volkow, 2010). Thus, it is possible that the same processes caused dysfunctional learning or deterioration of cognitive performance for rats self-administering α-PVP and 4MMC in the present study. Given that the present study primarily observed early-stage neurochemical changes, experimental paradigms using greater than 21 sessions of self-administration may be necessary to reach the negative reinforcement and withdrawal stage of the Koob and Volkow model. It is also possible that Koob and Volkow’s hypothesized neurochemical changes are dependent on drug contingency, dose, or other methodological considerations.
Notably, the mechanism of synthetic cathinone action had little effect on neurochemistry. Both 4MMC and α-PVP produce robust functional changes in the DA system, albeit by different mechanisms of action, releasing DA, or blocking DA transport, respectively (Baumann et al., 2012; Cameron et al., 2013; Glennon and Young, 2016; Marusich et al., 2014; Simmler et al., 2013). Overall, there were few neurochemical differences following self-administration of a DA uptake inhibitor versus a DA releaser, and the magnitude of the differences between effects of these synthetic cathinones was small (Fig. 3 and 4). This suggests that synthetic cathinones may lead to similar neuroplasticity, regardless of the mechanism of action. In contrast, the amount of drug access during self-administration (ShA vs. LgA) is a pivotal factor in the neurochemical effects of synthetic cathinones.
Interestingly, synthetic cathinone self-administration had little effect on total DA. ShA synthetic cathinone self-administration did not alter total DA in any measured brain region. LgA self-administration only affected DA levels in hypothalamus and thalamus compared to naïve, and in striatum and thalamus compared to saline ShA, and these changes were small in magnitude. Surprisingly, only LgA 4MMC affected total DA in striatum when compared to saline ShA, a region in which LgA exposure increased DA metabolites (Fig. 3 and 5). Thus, while α-PVP and 4MMC are known to elevate DA (Baumann et al., 2012; Baumann et al., 2017; Kehr et al., 2011; Marusich et al., 2014), this affect is fleeting, and was not present one day after final drug exposure when brain samples were collected. Meanwhile, synthetic cathinone-induced DA metabolism in striatum appears to occur later or has a longer duration of effect. The levels of DA metabolites in striatum following ShA 4MMC or ShA saline self-administration were consistent with that of a previous study, however, the present study produced approximately 60% higher levels of DA in striatum compared to the previous study (Motbey et al., 2013). Due to methodological differences in the self-administration doses used across studies, it is unclear why the self-administration regimen used in the present study led to greater DA levels.
While effects on DA were largely absent in this study, self-administration of either synthetic cathinone altered 5-HT and 5-HIAA levels. The serotonergic effects of 4MMC were expected because it releases 5-HT (Baumann et al., 2012). α-PVP does not have functional serotoninergic effects (Marusich et al., 2014), but α-PVP surprisingly increased 5-HIAA levels in several brain regions under LgA conditions. It is unclear if these unexpected serotonergic effects are a result of α-PVP binding to 5-HT1A (Rickli et al., 2015). Furthermore, it was noteworthy that 4MMC decreased 5-HT and 5-HIAA levels, whereas α-PVP increased 5-HIAA levels. The decreased 5-HIAA levels in striatum for 4MMC ShA groups compared to saline ShA groups is consistent with a past study; however, the present study produced slightly higher levels of 5-HT and 5-HIAA in striatum for both 4MMC and saline ShA groups than the past study (Motbey et al., 2013).
In addition to the observed changes in neurotransmitter levels, there were several interesting changes in neurotransmitter to metabolite ratios based on duration of drug exposure. DOPAC/DA and 5-HIAA/5-HT ratios are shown in Table S1 in the Supplementary Material. For both synthetic cathinones, LgA self-administration enhanced DOPAC/DA compared to ShA synthetic cathinone or ShA saline self-administration in both amygdala and striatum. One of the largest differences in DOPAC/DA was observed in amygdala where females showed greater change from DA to DOPAC than males, regardless of the self-administration condition. This intriguing sex difference was particularly evident for saline groups. For 5-HIAA/5-HT ratios, LgA self-administration of both synthetic cathinones lowered 5-HIAA/5-HT compared to ShA groups. Furthermore, α-PVP LgA, but not 4MMC LgA, elevated 5-HIAA/5-HT in PFC compared to α-PVP ShA and saline ShA. While an in-depth discussion of the significance of these ratios is beyond the scope of this manuscript, it is notable that the duration of synthetic cathinone self-administration not only alters levels of neurotransmitters but possibly affects the rate of neurotransmitter metabolism or clearance. It should be noted these are whole tissue measurements and do not reflect changes at the synapse.
Methodological inconsistencies across studies complicates comparison of results. Many studies measure the neurochemical effects of stimulants of abuse during direct drug effects (Ahmed et al., 2004; Mantsch et al., 2004), which does not provide information on lasting brain changes. In contrast, other studies measured neurochemical effects several days after the last drug exposure (Briand et al., 2008; Hadlock et al., 2011; Schwendt et al., 2009), which may be during withdrawal. Brain tissue was collected approximately 24 h after the last drug exposure in this study and the past companion studies (Marusich et al., 2019a; Marusich et al., 2019b). Prior studies have only examined the presence of synthetic cathinone withdrawal at two or more days of drug abstinence (Atehortua-Martinez et al., 2019; Bullock et al., 2019; Gregg et al., 2016; Hicks et al., 2017; Kolesnikova et al., 2019; Li et al., 2019; Lisek et al., 2012; Philogene-Khalid et al., 2017; Xu et al., 2016; Yoon et al., 2019), thus, it is unclear if the animals in this study were in acute withdrawal.
A few limitations of this study should be noted. One limitation is that the neurotransmitter data for LgA and naïve were analyzed at a different time than those for ShA. This difference in analyses is a potential reason for the neurochemical differences between LgA and ShA groups. Accordingly, much of the GLU data from all groups were reanalyzed simultaneously. GLU levels were similar to the original analyses (Supplementary Material Table S2), and the differences between LgA and ShA were still evident in the reanalysis. Furthermore, data for several neurotransmitter levels were similar across groups, despite the analyses being conducted at different times. Thus, it is unlikely that the timing of the analyses caused differences in neurotransmitter levels. Another limitation was that only one self-administration dose of α-PVP and 4MMC was included. Although the doses were located at similar points on the dose-effect curves (Aarde et al., 2015; Gannon et al., 2017; Nguyen et al., 2016), it is unknown if the results of this study will generalize across doses.
5.0. Conclusion
The present study suggests that 21 days of LgA self-administration models the neurochemistry of the beginning stages of the Koob and Volkow model of dysregulated drug intake (Koob and Le Moal, 2005, 2008; Koob and Volkow, 2010). Most past research using the escalation model of self-administration has used 21 sessions (Anker et al., 2010; Gipson et al., 2011; Kitamura et al., 2006; Marusich et al., 2010; Wee et al., 2007a), or fewer. The identified studies that used more than 21 sessions of self-administration (Ahmed and Koob, 1999; Belin et al., 2009; Greenwell et al., 2009a; Greenwell et al., 2009b) provided very little information on the neurochemical changes that occur beyond 21 sessions. Thus, future research should extend the duration of self-administration past 21 sessions to examine what neurochemical changes transpire and if increasing neurochemical dysregulation occurs with continued drug use as hypothesized.
Supplementary Material
Acknowledgements
Declarations of interest: none. The authors thank Daniel Barrus, Ricardo Cortes, Kimberly Custer, Tony Landavazo, Timothy Lefever, Nikita Pulley, Shanequa Taylor, Jenny Wiley, and Joseph Wilson for technical assistance. Funding: This research was supported by National Institute of Health [grant numbers DA039315 and DA012970]. The funding source had no other role other than financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA, 2015. In vivo potency and efficacy of the novel cathinone alpha-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology (Berl.) 232, 3045–3055. 10.1007/s00213-015-3944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF, 1998. Transition from moderate to excessive drug intake: change in hedonic set point. Science 282, 298–300. 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF, 1999. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl.) 146, 303–312. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Lin D, Koob GF, Parsons LH, 2004. Escalation of cocaine self-administration does not depend on altered cocaine-induced nucleus accumbens dopamine levels. J. Neurochem. 86, 102–113. 10.1046/j.1471-4159.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Zlebnik NE, Carroll ME, 2010. Differential effects of allopregnanolone on the escalation of cocaine self-administration and sucrose intake in female rats. Psychopharmacology (Berl.) 212, 419–429. 10.1007/s00213-010-1968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atehortua-Martinez LA, Masniere C, Campolongo P, Chasseigneaux S, Callebert J, Zwergel C, Mai A, Laplanche JL, Chen H, Etheve-Quelquejeu M, Mégarbane B, Benturquia N, 2019. Acute and chronic neurobehavioral effects of the designer drug and bath salt constituent 3,4-methylenedioxypyrovalerone in the rat. Journal of psychopharmacology (Oxford, England) 33, 392–405. 10.1177/0269881118822151. [DOI] [PubMed] [Google Scholar]
- Baumann MH, 2014. Awash in a sea of ‘bath salts’: implications for biomedical research and public health. Addiction 109, 1577–1579. 10.1111/add.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA Jr., Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV, 2012. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 37, 1192–1203. 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Bukhari MO, Lehner KR, Anizan S, Rice KC, Concheiro M, Huestis MA, 2017. Neuropharmacology of 3,4-Methylenedioxypyrovalerone (MDPV), Its Metabolites, and Related Analogs. Curr. Top. Behav. Neurosci. 32, 93–117. 10.1007/7854_2016_53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Gipson CD, Marusich JA, Bardo MT, 2012. Escalation of cocaine intake with extended access in rats: dysregulated addiction or regulated acquisition? Psychopharmacology (Berl.) 222, 257–267. 10.1007/s00213-012-2641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Balado E, Piazza PV, Deroche-Gamonet V, 2009. Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biol. Psychiatry 65, 863–868. 10.1016/j.biopsych.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, Robinson TE, 2008. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology 33, 2969–2980. 10.1038/npp.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock TA, Berquist MD, Baker LE, 2019. Locomotor sensitization in male Sprague-Dawley rats following repeated concurrent treatment with 4-methylmethcathinone and 3,4-methylenedioxymethamphetamine. Behav. Pharmacol. 30, 566–573. 10.1097/fbp.0000000000000491. [DOI] [PubMed] [Google Scholar]
- Cameron K, Kolanos R, Vekariya R, De Felice L, Glennon RA, 2013. Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of “bath salts,” produce opposite effects at the human dopamine transporter. Psychopharmacology (Berl.) 227, 493–499. 10.1007/s00213-013-2967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, 1993. Autoshaping i.v. cocaine self-administration in rats: effects of nondrug alternative reinforcers on acquisition. Psychopharmacology (Berl.) 110, 5–12. [DOI] [PubMed] [Google Scholar]
- Chiu K, Lau WM, Lau HT, So KF, Chang RC, 2007. Micro-dissection of rat brain for RNA or protein extraction from specific brain region. Journal of visualized experiments : JoVE, 269. 10.3791/269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JJ, Collins AL, Sanford CA, Phillips PEM, 2013. Dopamine Encoding of Pavlovian Incentive Stimuli Diminishes with Extended Training. The Journal of Neuroscience 33, 3526–3532. 10.1523/jneurosci.5119-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creehan KM, Vandewater SA, Taffe MA, 2015. Intravenous self-administration of mephedrone, methylone and MDMA in female rats. Neuropharmacology 92, 90–97. 10.1016/j.neuropharm.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEA, 2017. 2017 National Drug Threat Assessment. U.S. Drug Enforcement Administration, Springfield, VA. [Google Scholar]
- DEA, 2019. National Forensic Laboratory Information System: NFLIS-Drug Midyear Report 2018, in: Division DC. (Ed.). U. S. Drug Enforcement Administration, Springfield, VA. [Google Scholar]
- Gannon BM, Galindo KI, Mesmin MP, Sulima A, Rice KC, Collins GT, 2018. Relative reinforcing effects of second-generation synthetic cathinones: Acquisition of self-administration and fixed ratio dose-response curves in rats. Neuropharmacology 134, 28–35. 10.1016/j.neuropharm.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Rice KC, Collins GT, 2017. Reinforcing effects of abused ‘bath salts’ constituents 3,4-methylenedioxypyrovalerone and alpha-pyrrolidinopentiophenone and their enantiomers. Behav. Pharmacol. 28, 578–581. 10.1097/FBP.0000000000000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ, 2015. Comparative Behavioral Pharmacology of Three Pyrrolidine-Containing Synthetic Cathinone Derivatives. J. Pharmacol. Exp. Ther. 354, 103–110. 10.1124/jpet.115.223586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT, 2011. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology (Berl.) 214, 557–566. 10.1007/s00213-010-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Young R, 2016. Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and alpha-pyrrolidinovalerophenone (alpha-PVP). Brain Res. Bull. 126, 111–126. 10.1016/j.brainresbull.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice KC, Zorrilla EP, Koob GF, 2009a. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long- but not short-access rats. Addict. Biol. 14, 130–143. 10.1111/j.1369-1600.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell TN, Walker BM, Cottone P, Zorrilla EP, Koob GF, 2009b. The alpha1 adrenergic receptor antagonist prazosin reduces heroin self-administration in rats with extended access to heroin administration. Pharmacol. Biochem. Behav. 91,295–302. 10.1016/j.pbb.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Hicks C, Nayak SU, Tallarida CS, Nucero P, Smith GR, Reitz AB, Rawls SM, 2016. Synthetic cathinone MDPV downregulates glutamate transporter subtype I (GLT-1) and produces rewarding and locomotor-activating effects that are reduced by a GLT-1 activator. Neuropharmacology 108, 111–119. 10.1016/j.neuropharm.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Rawls SM, 2014. Behavioral pharmacology of designer cathinones: a review of the preclinical literature. Life Sci. 97, 27–30. 10.1016/j.lfs.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE, 2011.4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J. Pharmacol. Exp. Ther. 339, 530–536. 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks C, Gregg RA, Nayak SU, Cannella LA, Schena GJ, Tallarida CS, Reitz AB, Smith GR, Rawls SM, 2017. Glutamate carboxypeptidase II (GCPII) inhibitor 2-PMPA reduces rewarding effects of the synthetic cathinone MDPV in rats: a role for N-acetylaspartylglutamate (NAAG). Psychopharmacology (Berl.) 234, 1671–1681. 10.1007/s00213-017-4568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkanen A, 1999. Modulation of Brain Dopaminergic Neurotransmission in Alcohol-Preferring Rats by Alcohol and Opioids, Department of Pharmacy. University of Helsinki. [Google Scholar]
- Javadi-Paydar M, Harvey EL, Grant Y, Vandewater SA, Creehan KM, Nguyen JD, Dickerson TJ, Taffe MA, 2018. Binge-like acquisition of alpha-pyrrolidinopentiophenone (alpha-PVP) self-administration in female rats. Psychopharmacology (Berl.) 235, 2447–2457. 10.1007/s00213-018-4943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T, 2011. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br. J. Pharmacol. 164, 1949–1958. 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L, 2006. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl.) 186, 48–53. 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Kolesnikova TO, Khatsko SL, Eltsov OS, Shevyrin VA, Kalueff AV, 2019. When fish take a bath: Psychopharmacological characterization of the effects of a synthetic cathinone bath salt ‘flakka’ on adult zebrafish. Neurotoxicol. Teratol. 73, 15–21. 10.1016/j.ntt.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Koob GF, 2009. Brain stress systems in the amygdala and addiction. Brain Res. 1293, 61–75. 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, 2010. Drug addiction. The Corsini Encyclopedia of Psychology, 1–4. [Google Scholar]
- Koob GF, Le Moal M, 1997. Drug abuse: hedonic homeostatic dysregulation. Science 278, 52–58. 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M, 2005. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat. Neurosci. 8, 1442–1444. 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M, 2008. Addiction and the brain antireward system. Annu. Rev. Psychol. 59, 29–53. 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238. 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lin Z, Tao X, Huang Z, Zhang Y, Zheng S, Wang H, Rao Y, 2019. Effects of N-ethylpentylone on locomotor activity and anxiety-like behavior in rats. Behav. Pharmacol. 30, 500–505. 10.1097/fbp.0000000000000484. [DOI] [PubMed] [Google Scholar]
- Lisek R, Xu W, Yuvasheva E, Chiu YT, Reitz AB, Liu-Chen LY, Rawls SM, 2012. Mephedrone (‘bath salt’) elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend. 126, 257–262. 10.1016/j.drugalcdep.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madras BK, 2017. The Growing Problem of New Psychoactive Substances (NPS), in: Baumann MH, Glennon RA, Wiley JL. (Eds.), Neuropharmacology of New Psychoactive Substances (NPS), 2016/08/31 ed. Springer, Cham, Switzerland, pp. 1–18. [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ, 2004. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl.) 175, 26–36. 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH, 2014. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV). Neuropharmacology 87, 206–213. 10.1016/j.neuropharm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Beckmann JS, Gipson CD, Bardo MT, 2010. Methylphenidate as a reinforcer for rats: contingent delivery and intake escalation. Exp. Clin. Psychopharmacol. 18, 257–266. 10.1037/a0019814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Gay EA, Blough BE, 2019a. Analysis of neurotransmitter levels in addiction-related brain regions during synthetic cathinone self-administration in male Sprague-Dawley rats. Psychopharmacology (Berl.) 236, 903–914. 10.1007/s00213-018-5011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Gay EA, Watson SL, Blough BE, 2019b. Synthetic cathinone self-administration in female rats modulates neurotransmitter levels in addiction-related brain regions. Behav. Brain Res. 376, 112211. 10.1016/j.bbr.2019.112211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL, 2012. Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. Neurotoxicology 33, 1305–1313. 10.1016/j.neuro.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Lefever TW, Blough BE, Thomas BF, Wiley JL, 2016. Pharmacological effects of methamphetamine and alpha-PVP vapor and injection. Neurotoxicology 55, 83–91. 10.1016/j.neuro.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Lefever TW, Novak SP, Blough BE, Wiley JL, 2013. Prediction and Prevention of Prescription Drug Abuse: Role of Preclinical Assessment of Substance Abuse Liability. Methods Rep RTI Press, 1–14. 10.3768/rtipress.2013.op.0014.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motbey CP, Clemens KJ, Apetz N, Winstock AR, Ramsey J, Li KM, Wyatt N, Callaghan PD, Bowen MT, Cornish JL, McGregor IS, 2013. High levels of intravenous mephedrone (4-methylmethcathinone) self-administration in rats: neural consequences and comparison with methamphetamine. Journal of psychopharmacology (Oxford, England) 27, 823–836. 10.1177/0269881113490325. [DOI] [PubMed] [Google Scholar]
- National Research Council, 2011. Guide for the Care and Use of Laboratory Animals, 8th ed. National Academies Press (US), Washington, D.C. [Google Scholar]
- NDEWS, 2015. National Drug Early Warning System (NDEWS) Sentinel Community Site Profile 2015: Southeastern Florida. National Drug Early Warning System. [Google Scholar]
- NDEWS, 2018a. National Drug Early Warning System (NDEWS): Southeastern Florida (Miami Area) Sentinel Community Site (SCS) Drug Use Patterns and Trends, 2018. National Drug Early Warning System. [Google Scholar]
- NDEWS, 2018b. National Drug Early Warning System (NDEWS): Texas Sentinel Community Site (SCS) Drug Use Patterns and Trends, 2018. National Drug Early Warning System. [Google Scholar]
- NDEWS, 2019. National Drug Early Warning System (NDEWS): Chicago Metro Sentinel Community Site Drug Use Patterns and Trends, 2019. [Google Scholar]
- Nguyen JD, Bremer PT, Ducime A, Creehan KM, Kisby BR, Taffe MA, Janda KD, 2017a. Active vaccination attenuates the psychostimulant effects of alpha-PVP and MDPV in rats. Neuropharmacology 116, 1–8. 10.1016/j.neuropharm.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Grant Y, Creehan KM, Vandewater SA, Taffe MA, 2016. Escalation of intravenous self-administration of methylone and mephedrone under extended access conditions. Addict. Biol. 10.1111/adb.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Grant Y, Creehan KM, Vandewater SA, Taffe MA, 2017b. Escalation of intravenous self-administration of methylone and mephedrone under extended access conditions. Addict. Biol. 22, 1160–1168. 10.1111/adb.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver CF, Palamar JJ, Salomone A, Simmons SJ, Philogene-Khalid HL, Stokes-McCloskey N, Rawls SM, 2019. Synthetic cathinone adulteration of illegal drugs. Psychopharmacology (Berl.) 236, 869–879. 10.1007/s00213-018-5066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, Rutherford C, Keyes KM, 2019. “Flakka” use among high school seniors in the United States. Drug Alcohol Depend. 196, 86–90. 10.1016/j.drugalcdep.2018.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philogene-Khalid HL, Hicks C, Reitz AB, Liu-Chen LY, Rawls SM, 2017. Synthetic cathinones and stereochemistry: S enantiomer of mephedrone reduces anxiety- and depressant-like effects in cocaine- or MDPV-abstinent rats. Drug Alcohol Depend. 178, 119–125. 10.1016/j.drugalcdep.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW, 1996. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J. Neurosci. 16, 1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickli A, Hoener MC, Liechti ME, 2015. Monoamine transporter and receptor interaction profiles of novel psychoactive substances: para-halogenated amphetamines and pyrovalerone cathinones. Eur. Neuropsychopharmacol. 25, 365–376. 10.1016/j.euroneuro.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW, 2009. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J. Pharmacol. Exp. Ther. 331,555–562. 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME, 2013. Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol. 168, 458–470. 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijker S, 2011. Dissection of Rodent Brain Regions, in: Li KW (Ed.), Neuroproteomics. Humana Press, Totowa, NJ, pp. 13–26. [Google Scholar]
- Vandewater SA, Creehan KM, Taffe MA, 2015. Intravenous self-administration of entactogen-class stimulants in male rats. Neuropharmacology 99, 538–545. 10.1016/j.neuropharm.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF, 2014. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Addict. Biol. 19, 165–174. 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Specio SE, Koob GF, 2007a. Effects of dose and session duration on cocaine self-administration in rats. J. Pharmacol. Exp. Ther. 320, 1134–1143. 10.1124/jpet.106.113340. [DOI] [PubMed] [Google Scholar]
- Wee S, Wang Z, Woolverton WL, Pulvirenti L, Koob GF, 2007b. Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacology 32, 2238–2247. 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Everitt BJ, Phillips PE, 2012. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proc. Natl. Acad. Sci. U. S. A 109, 20703–20708. 10.1073/pnas.1213460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Qiu Y, Zhang Y, Bai Y, Xu P, Liu Y, Kim JH, Shen HW, 2016. The Effects of 4-Methylethcathinone on Conditioned Place Preference, Locomotor Sensitization, and Anxiety-Like Behavior: A Comparison with Methamphetamine. Int. J. Neuropsychopharmacol. 19. 10.1093/ijnp/pyv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HS, Ku MJ, Cai WT, Kim JH, 2019. A novel synthetic cathinone, α-pyrrolidinopentiothiophenone (PVT), produces locomotor sensitization in rat: Implications for GSK3β connections in the nucleus accumbens core. Neurochem. Int. 124, 25–30. 10.1016/j.neuint.2018.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


