Abstract
Aging is characterized by a decline in neuronal function in all animal species investigated so far. Functional changes are accompanied by, and may be in part caused by, structurally visible degenerative changes in neurons. In the mammalian brain, normal aging shows abnormalities in dendrites and axons, as well as ultrastructural changes in synapses, rather than global neuron loss. The analysis of the structural features of aging neurons, as well as their causal link to molecular mechanisms on the one hand, and the functional decline on the other hand, is crucial in order to understand the aging process in the brain. Invertebrate model organisms like Drosophila and C. elegans offer the opportunity to apply a forward genetic approach to the analysis of aging. In the present review we aim to summarize findings concerning abnormalities in morphology and ultrastructure in invertebrate brains during normal aging and compare them to what is known for the mammalian brain. It becomes clear that despite of their considerably shorter life span, invertebrates display several age-related changes very similar to the mammalian condition, including the retraction of dendritic and axonal branches at specific locations, changes in synaptic density, and increased accumulation of presynaptic protein complexes. We anticipate that continued research efforts in invertebrate systems will significantly contribute to reveal (and possibly manipulate) the molecular/cellular pathways leading to neuronal aging in the mammalian brain.
Keywords: invertebrates, normal aging, neurons, dendrites, axons, synapses
Introduction
Mammalian brains range from tens of millions (rodents) to up to 100 billion or more (primates, elephants, whales) neurons and about the same number of glial cells. Neurons are large and long-lived cells (more than 100 years in some cases), composed of a cell body with multiple, branched dendrites, and a long, myelinated axon. A given neuron forms thousands of synaptic contacts with its upstream and downstream partners. Brain aging is characterized by structural alterations in neuronal morphology and synaptic connections, accompanied by deterioration in behavioral skills. The mechanisms underlying brain aging remain poorly understood. Advanced technologies and the neurobiology of model organisms promises to help shed light on the processes underlying normal and pathological brain aging.
Invertebrate organisms offer numerous advantages in the analysis of age-related changes in neural structure and function. Compared to mammals, most invertebrates have a short life cycle and are easy to maintain. In mammals, the onset of senescence occurs after several months (e.g., mouse: 6–10) to many years (around 40 in humans; Flurkey et al. 2007). By contrast, senescence sets in after days in invertebrate models (Drosophila: 5–7 days; Dudas and Arking 1995; Rera et al. 2012; Carbone et al., 2016; Honey bee: 10–30 days; Remolina et al., 2007; C. elegans: 3 days; reviewed in Son et al. 2018). Invertebrates have a simple nervous system, often formed by sets of uniquely identifiable cells. Single neurons can be targeted through molecular genetic approaches allowing for the investigation of cellular and molecular basis of behavior at a high level of resolution. In genetic model systems such as Drosophila and C. elegans, access to well curated genomes and the availability of a plethora of molecular tools, has made the connections between gene expression and cellular development, structure and function experimentally tractable in the past decades. Since most of the molecular pathways involved in neural differentiation are highly conserved among animals, these genetic model systems have been widely used to study the molecular root causes of neurodegenerative diseases for many years (Bonini and Fortini 2003; Iijima et al. 2004; Troulinaki and Tavernarakis 2005; Beckingham et al. 2005; Kretzschmar 2005; Iijima and Iijima-Ando 2008; Ling and Salvaterra 2009; Wentzell and Kretzschmar 2010; White et al. 2010; Johnson et al. 2010; Ling et al. 2014; McGurk et al. 2015; Vayndorf et al. 2016; Goodman and Bonini 2020). The analysis of cellular mechanisms underlying normal aging has generally not attracted as much attention in the past, but picked up in pace in recent years (Driver et al. 2004; Ling and Salvaterra 2011; Li et al. 2013; Haddadi et al. 2014; Farnsworth et al. 2015; Kounatidis et al. 2017; Liao et al. 2017; Davie et al. 2018; Hussain et al. 2018; Nash et al. 2019). In this context, careful documentation of structural changes characteristic of aging neurons is an important prerequisite to undertaking molecular-genetic experimental studies on the mechanism of aging. The aim of this review is to survey pertinent data published on the structure of aging neurons in several invertebrate systems, including insects, nematodes, and molluscs, and compare them with analogous findings in the mammalian brain.
Structural changes in the aging mammalian brain
Classical electron microscopic studies of neuropathological phenomena in aging mammalian brains distinguished between intracellular neurofibrillary tangles, intracellular granulo-vacuolar degeneration, and extracellular neuritic (or senile) plaques (Tomlinson et al. 1968; Wisniewski and Terry 1973). Neurofibrillary tangles consist of deposits of the microtubule-binding protein tau (Bancher et al. 1987; Grundke-Iqbal et al. 1988). Granulo-vacuolar degeneration entails electron-dense, typically membrane bound vesicles containing oxidized lipids and proteins (Hendy 1971; Double et al. 2008; Moreno-García et al. 2018). These vesicles, also termed lipofuscin granules, occur in many cell types of aged animals, and can be interpreted as terminal stages (“residual bodies”) of endo-lysosomes (Brunk and Brun 1972; Gilissen and Staneva-Dobrovski 2013). Senile plaques are relatively large (up to 50 microns) deposits in the neuropil. Plaques contain (i) parts of degenerating neurites, often filled with neurofibrillary tangles or lipofuscin granules, (ii) glial cells (microglia, astrocytes), and (iii) extracellular deposits of the ß-amyloid protein. It was initially thought that many aging neurons, as a result of the above described degenerative ultrastructural changes, undergo cell death. Thus, in 1955 Brody et al. claimed a reduction in neuron number in all cortical layers in the human cerebral cortex as a feature of the aging brain (Brody 1955). Other studies supported this conclusion by demonstrating a decrease in cortical and subcortical neuron density (Ball 1977; Coleman and Flood 1987) in aging humans and nonhuman primates (Brizzee and Knox 1980). However, later studies based on improved stereological methods (Freeman et al. 2008) showed that neuron loss is not significant in normal aging, and that the earlier numbers were likely skewed due to differential shrinking artefacts and/or the inclusion of pathological specimens in the studies (humans: West et al. 1994; Pakkenberg and Gundersen 1997; non-human primates: Peters 1994; Gazzaley et al. 1997; Merrill et al. 2000; Keuker et al. 2003; rats: Rapp and Gallagher 1996; Rasmussen et al. 1996; Mohammed and Santer 2001; Merrill et al. 2001). By contrast, brains of patients suffering from neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases, show all the above discussed structural ultrastructural changes typical for aging, but in addition suffer from severe neuron loss (Niikura et al. 2006; Donev et al. 2009).
Rather than neuron loss, anatomically detectable changes observed in aging brains include alterations in number of synaptic connections, the dendritic trees, axonal collaterals and myelination (Table 1; Fig. 1a). These changes are typically confined to specific brain compartments and neuron types (Morrison and Hof 1997; Burke and Barnes 2006; Bishop et al. 2010; Petralia et al. 2014). Studies focusing on dendritic spines (short dendritic processes each of which possesses multiple synaptic sites), generally noted a decrease in length and/or density of spines in various types of neurons in the neocortex and hippocampus (for neocortex: Uemura and Ireland 1985; Masliah et al. 1993; Dumitriu et al. 2010; for hippocampus: von Bohlen and Halbach et al. 2006; Fig. 1c, d). Frequently, in the same brain region, one type of synapse may be affected, whereas another is not. For example, Peters et al., who investigated individual synaptic sites electron microscopically, report a 30 % decrease in excitatory and inhibitory synapses in the superficial layers of the primate prefrontal cortex, whereas synapse loss is much less dramatic, and affects only excitatory synapses, in deep layers of the same region (Peters et al. 2008). In the olfactory bulb, aged mice show decreased synaptic numbers in the glomerular layer (the site of interaction between olfactory receptor terminals and projection neurons/local interneurons), but not in the external plexiform layer (Richard et al. 2010).
Table 1.
Structural changes discussed in this review for different animal species at the level of synapses, dendrites and axons. Types of structural changes are written in bold font; brain region or neuron type for which these changes were observed are in regular font. Literature cited is provided to the right of each column.
| Synapse | Dendrite | Axon | ||||
|---|---|---|---|---|---|---|
| Mammal | Decreased number | Decreased spine density | Loss of axon collaterals | |||
| Neocortex (primate) | Peters et al. 2008 | Neocortex (rabbit) | Uemura and Ireland 1985 | Neocortex (primate) | Peters and Sethares 2002 | |
| Olfactory bulb (mouse) | Richard et al. 2010 | (human) | Masliah et al. 1993 | (primate) | Peters and Rosene 2003 | |
| (primate) | Dumitriu et al. 2010 | (primate) | Bowley et al. 2011 | |||
| Increased size | (mouse) | Mostany et al. 2013 | (review) | Pannese 2011 | ||
| Neocortex (mouse) | Cali et al. 2018 | Decreased dendritic length | ||||
| Neocortex (rat) | Vaughan 1977 | |||||
| (primate) | Cupp and Uemura 1980 | |||||
| (human) | Nakamura et al. 1985 | |||||
| (primate) | Page et al. 2002 | |||||
| (mouse) | Eavri et al. 2018 | |||||
| Fruit fly Drosophila melanogaster | Decreased number | Ectopic branching | Loss of axonal branches | |||
| Mushroom body | Haddadi et al. 2014 | Central complex | Koch and Hartenstei n unpub | Motorneurons | Beramen di et al. 2007 | |
| Antennal lobe | Hussain et al. 2018 | Sensory afferents | Corfas and Dudai 1991 | |||
| Neuromuscul ar junction | Beramen di et al. 2007 | |||||
| Increased size | ||||||
| Mushroom body | Gupta et al. 2016 | |||||
| Neuromuscul ar junction | Beramen di et al. 2007 | |||||
| Increased mitochondri al size | ||||||
| Mushroom body | Haddadi et al. 2014 | |||||
| Increased presynaptic proteins | ||||||
| Mushroom body | Gupta et al. 2016 | |||||
|
Honey bee Apis mellifera |
Increased presynaptic proteins | |||||
| Mushroom body | Gehring et al. 2017 | |||||
|
Cricket Teleogryllus oceanicus |
Loss of axonal branches | |||||
| Ascending interneurons | Atkins and Pollack 1986 | |||||
|
Cockroach Periplaneta americana |
Increased mitochondri al size | Increased spine density | Defasciculatio n | |||
| Mushroom body | Brown and Strausfel d 2009 | Mushroom body | Brown and Strausfeld 2009 | Mushroom body | Brown and Strausfel d 2009 | |
|
Nematode Caenorhabdit is elegans |
Delayed vesicle docking Decreased quantal release | Ectopic branching | Defasciculation | |||
| Mechanorecepto rs | Toth etal. 2012 | Mechanorecepto rs | Pan et al. 2011 | |||
| Motorneurons | Liu et al. 2013 | Hess etal. 2019 | Tank et al. 2011 | |||
|
Pond snail Lymnea stagnalis |
Decreased number | Loss of axon branches | ||||
| Neurosecreto ry neurons | Janse et al. 1996, 1999 | Neurosecretory neurons | Janse et al. 1996, 1999 | |||
|
Sea hare Aplysia californica |
Increased size | |||||
| Motorneurons | Peretz et al. 1984 |
Fig. 1.
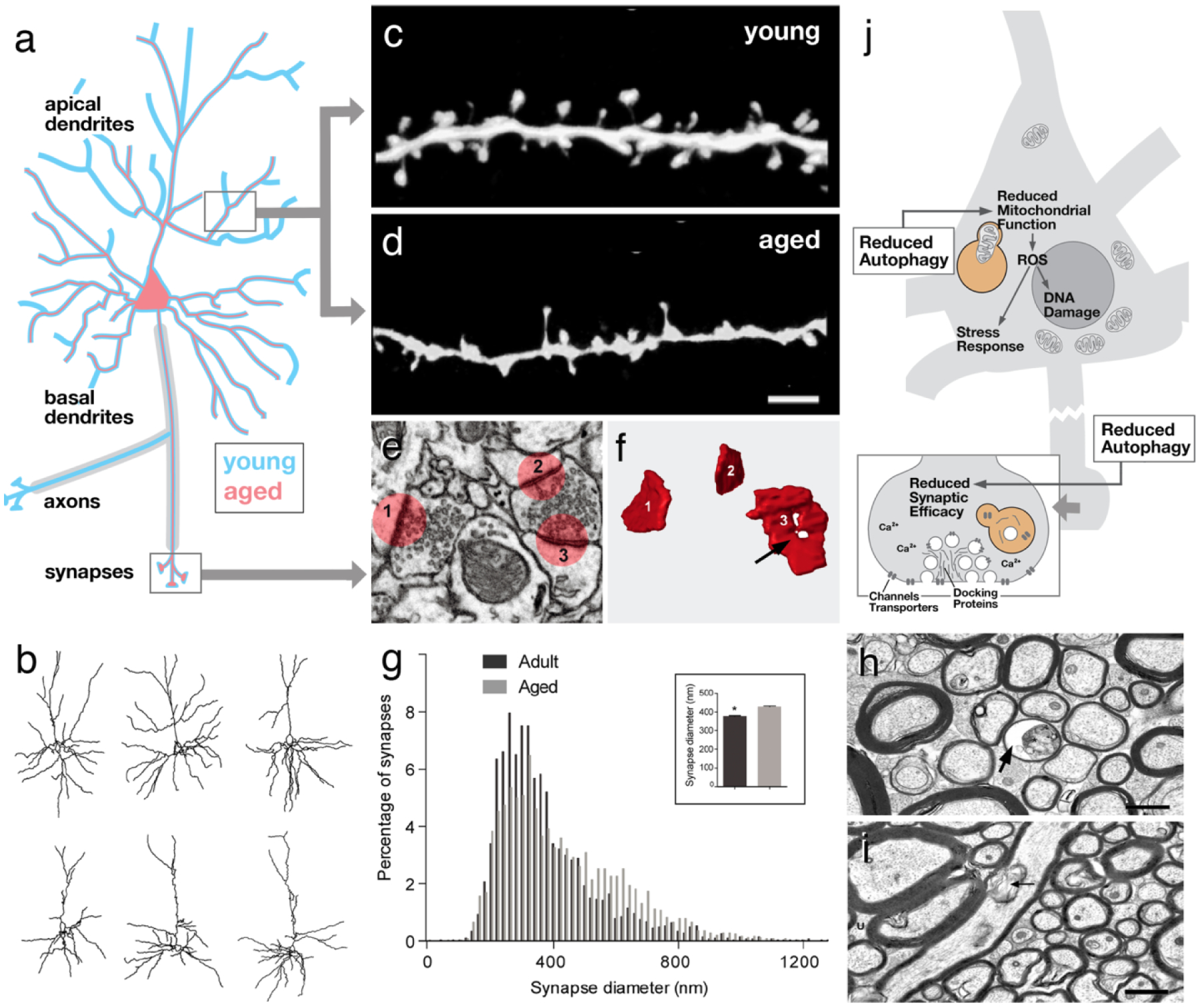
Structural aspects of aging in vertebrate neurons. a Schematic representation of cortical pyramidal neuron. Aged neuron (red outline), in a region-specific and cell type-specific manner, exhibits a reduction in terminal dendrites, often restricted to specific (e.g., apical) parts of their arbor. Loss of myelinated axons has also been recorded. b Three representative superficial pyramidal cells of mouse prefrontal cortex at the age of 8 months (top) and 28 months (bottom; from Grill and Riddle 2002, with permission). c, d At the synaptic level, postsynaptic dendritic spines frequently show a reduction in size and/or density with age (from Dickstein et al. 2013, with permission). e, f, g Whereas spines maybe decreased in size, individual presynaptic sites (i.e., active zones) can be increased (g) and show ultrastructural abnormalities, such as an enhanced fraction of ring-shaped zones (arrow in F; from Calì et al. 2018, with permission). h, i Myelinated axons of the cingulate bundle in 24-year-old macaque (h) are reduced in number and density compared to 9-year-old animal (i; from Bowley et al. 2010, with permission). Some aged fibers show ballooning of myelin sheath (arrow). j Schematic representation of neuron (inset: magnified view of synapse) summarizing cellular/molecular motifs associated with aging. Scale bars: 2 microns (c, d); 2 microns (h, i)
Modern techniques that allow tracking individual neurons over time under different experimental conditions, generally support the conclusion that spine size and spine stabilization decreases with age (for rodent somatosensory cortex: Mostany et al. 2013). On the other hand, the size of individual synaptic contacts appears to increase. Thus, a given dendritic spine carries multiple synaptic contacts, a single contact being defined by its pre- and postsynaptic density. In their dense 3D EM reconstruction of layer 1 of mouse somatosensory cortex, Cali et al. find that the size of synaptic contacts increases by approximately 12 % (Fig. 1e–g), whilst the overall number of synaptic contacts decreases (16.5 % for asymmetric, presumably excitatory synapses; more than 40 % for symmetric, inhibitory synapses). This study points at the importance of assessing morphological changes in synaptic connectivity by looking at multiple parameters; some parameters (e.g., spine size or spine number), may be affected differently from others (e.g., synapse size) (Calì et al. 2018).
Along with changes in the number and structure of synapses, the size and shape of the neuronal trees also varies between young and old animals, depending on the brain region and type of neuron investigated. In the hippocampus, the dendritic length of granule cells showed no substantial change in aged rats (Flood and Coleman 1993). In some hippocampal neuron types, such as the CA1 pyramidal neurons in rats, an increase in dendritic length and distal branching may even occur with aging (Pyapali and Turner 1996). However, more generally, a reduction in dendritic complexity has been demonstrated for several neuron types. For the neocortex it was reported that the terminal branches of the dendritic arbor decrease in number and/or length with age; this was the case in superficial (but not deep) pyramidal neurons of the rat prefrontal cortex (Grill and Riddle 2002; Fig. 1b). Often the reduction in dendritic complexity is confined to a specific domain of the dendritic arbor of pyramidal neurons, which can be apical (Cupp and Uemura 1980; Page et al. 2002) or basal (Vaughan 1977; Nakamura et al. 1985). Changes are clearly neuron specific; for example, in the mouse neocortex, Eavri et al. found simplified arbors of inhibitory interneurons, whereas pyramidal neurons are not affected (Eavri et al. 2018).
Aside from dendritic changes, aging neurons of the mammalian brain also show alterations in their axonal plexus. Cortical pyramidal neurons form branched, myelinated axons that project to different cortical and subcortical targets. Numerous counts of myelinated fibers at defined locations have demonstrated that axons are lost. Heavily affected are, for example, anterior callosal fiber systems and association tracts that interconnect the prefrontal cortex (Peters and Sethares 2002; Bowley et al. 2010; Pannese 2011; Fig. 1h, i). Axon loss is correlated with an overall volume decrease of the white matter in this region (Marner et al. 2003; Yankner et al. 2008; Vernooij et al. 2008). The fact that, according to current consensus, aging does not involve major neuronal death implies that neurons maintain some of their axonal branches while losing others (Peters and Rosene 2003; Bowley et al. 2010).
Cellular and biochemical changes in aging neurons: a unified hypothesis of aging?
Conserved cellular and biochemical pathways are affected by the aging process and may be causally related to the structural neuronal changes and decline in brain functions summarized in the foregoing section. Comprehensive reviews have appeared in recent years that focus on various molecular aspects of the aging process (Muller et al. 2007; Bishop et al. 2010; Salvadores et al. 2017; Vijayan and Verstreken 2017; Daniele et al. 2018; Liang and Sigrist 2018; Castelli et al. 2019; Foster 2019) and we will not duplicate these efforts here. However, briefly, many studies show that a reduced mitochondrial turnover during aging leads to a lack of energy and creates pathological concentrations of ROS, which, in turn, causes further mitochondrial damage, as well as DNA damage (Bishop et al. 2010; Salvadores et al. 2017). DNA damage evokes a stress-response, designed to counteract the propagation of faulty cellular components. Age-related downregulation of genes involved in mitochondrial energy metabolism, and upregulation of genes involved in the stress-response have been observed in genome-wide gene expression studies of aging brains conducted for several systems, including mouse, rat, chimpanzee, human, and the invertebrates Drosophila and C. elegans (Blalock et al. 2003; Lu et al. 2004; Zahn et al. 2007; Loerch et al. 2008; Yankner et al. 2008; Bishop et al. 2010; Davie et al. 2018). Trigger for the decreased mitochondrial function could be a downturn in autophagy, a cellular process by which damaged mitochondria, but also cytotoxic protein complexes, are degraded. This idea is supported by the finding that the expression of BECN1 and several other genes of the autophagic pathway is reduced in the aging human brain (Shibata et al. 2006; Lipinski et al. 2010; Rubinsztein et al. 2011). It is known that reduced autophagy and mitochondrial function, and heightened stress-response, stimulate the induction of neuronal death. However, how these changes can account for the restructuring of dendrites and axons that is characteristic of normal aging remains to be demonstrated.
Synaptic changes, including altered size and density of spines and individual synaptic active zones, could also be related to age-dependent reduction in autophagy. Numerous studies confirm that autophagosomes are concentrated at presynaptic sites where they play an important role in the recycling of proteins involved in the trafficking of vesicles and neurotransmitter metabolism (Vijayan and Verstreken 2017; Liang and Sigrist 2018). Recent findings in Drosophila (summarized below) link defects in autophagy to abnormalities in synapse ultrastructure (Gupta et al. 2016; Bhukel et al. 2019), which can be plausibly related to functional decline. Another feature of aging synapses (which may or may not be related to reduced autophagy) concerns changes in calcium homeostasis. Synaptic plasticity underlying learning and memory relies upon the regulation of calcium fluxes and calcium-mediated signaling pathways. For example, the after-hyperpolarizing potential (AHP) following bursts of action potentials in hippocampal neurons is triggered by a rise in intracellular calcium levels. Ca2+ levels are increased in aging brains, due to a combination of expression changes of genes encoding Ca2+ channels and Ca2+ buffers (Lu et al. 2004; Thibault et al. 2007; Nikoletopoulou and Tavernarakis 2012). These alterations, causing a decline in synaptic plasticity, might be causally related to the functional deterioration of some aging brain structures, like the hippocampus and prefrontal cortex. Again, how they relate to the structural changes typical for aging neurons also remains to be seen.
In summary, many molecular changes are correlated with age. A number of larger “motifs” are evident, involving the processes of autophagy, mitochondrial efficacy, ROS production, stress response, and synaptic calcium homeostasis (Fig. 1j). How these motifs are causally related, and whether there is always a single initial motif that subsequently sets in motion the other observed alterations, is not yet known.
The aging brain in insects (Fruit fly, Honeybee, Cricket, Cockroach)
Like vertebrates, insects and other arthropods have relatively complex brains that can be anatomically divided into specific centers with defined functions. Regarding neuronal architecture, insect and vertebrate brains show two major differences. Contrary to the vertebrate brain, the somata of insect neurons reside in a cortex (rind) surrounding the neuropil, to which they contribute neurites (Fig. 2a). Lacking the somatic dendrites which create the major site of synaptic integration in vertebrate neurons, invertebrate synaptic connection occurs within the neuropil. Furthermore, insect neurons lack myelin.
Fig. 2.
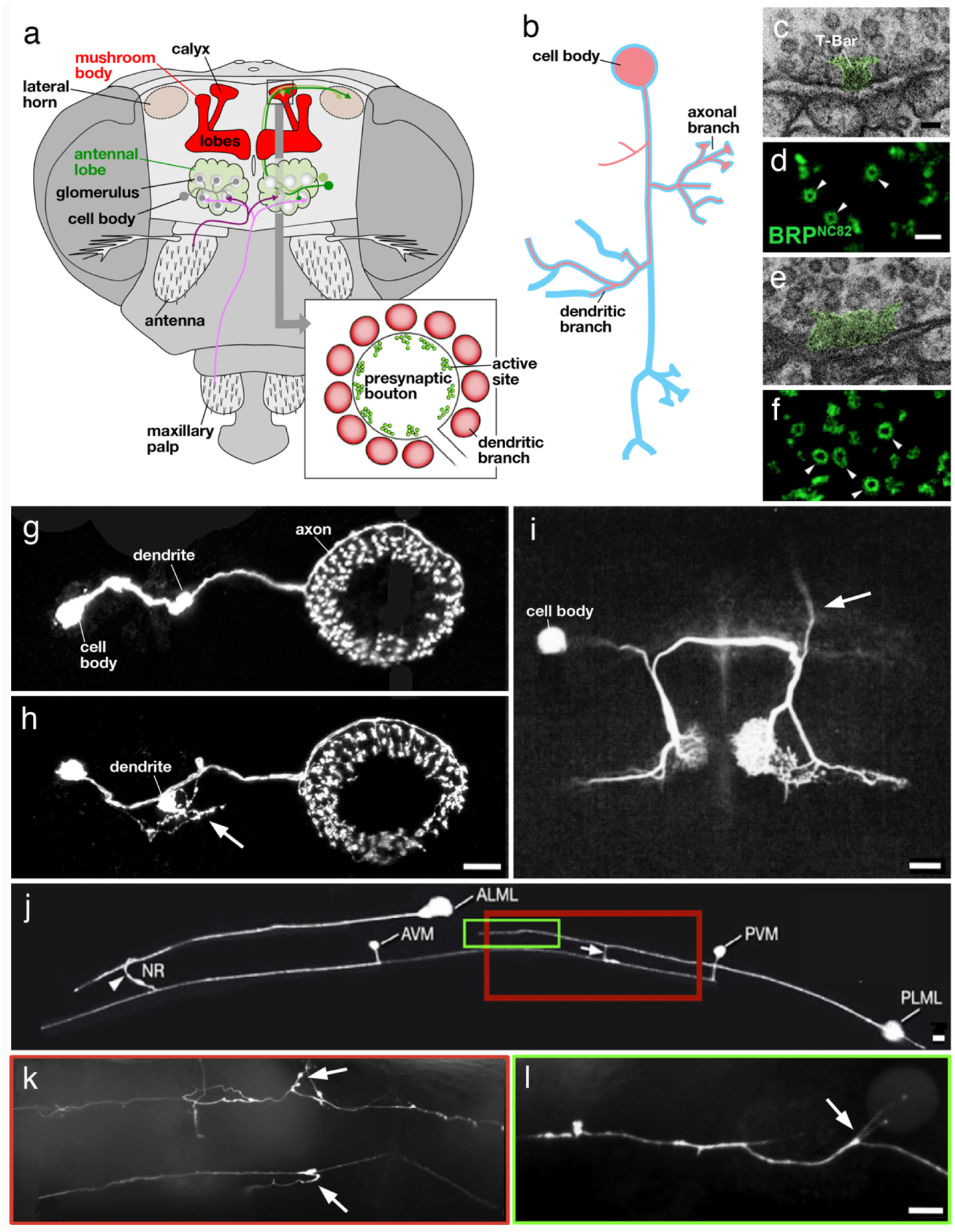
Structural aspects of aging in invertebrate neurons. a Olfactory pathway in Drosophila. Olfactory receptor neurons (magenta) project to the antennal lobe (green); from here olfactory projection neurons (green) relay the signal to the calyx of the mushroom body (red). Bulbous presynaptic boutons of projection neurons (see inset) contact dendritic branches of intrinsic mushroom body neurons (Kenyon cells; after Perisse et al. 2013, with permission). b Schematic representation of insect neuron, showing cell body, dendritic branches and axonal branches. Aged neuron (red outline) frequently lack dendritic and/or axonal branches; another characteristic is the occurrence of new branches at ectopic positions. c-f Ultrastructure of mushroom body calycal synapse in young adult fly (c, d) and aged fly (e, f). Note widening of T-bar (green shading), an ultrastructurally distinct feature of the active zone, in aged synapse (e). (d, f) show increased diameter of active zone, labeled by an antibody against the presynaptic protein BRP, in aged fly (f) compared to young fly (d; from Gupta et al. 2016, with permission). g, h GFP labeled ring neurons of the Drosophila ellipsoid body in young fly (g) and aged fly (h). Note ectopic fine branches sprouting from short bulbous dendrite (arrow). i Fluorescently labeled acoustic interneuron in cricket. Arrow points at ascending branch that is found in majority of young animals but absent from most aged animals (from Atkins and Pollack 1986; with permission). j-l Fluorescently labeled ALM and PLM touch receptor neurons in young C. elegans worm (j) and aged animal (k, l). Boxed regions in (j) corresponds to parts of fibers shown, for an aged animal, in (k) and (l). Note ectopic branches (arrows) occurring in aged animal (From Toth et al. 2012, with permission). Scale bars: 50 nm (c, e); 500 nm (d, f); 10 microns (g, h, j, k, l); 100 microns (i)
Classical studies comparing ultrastructural features of young vs old insect neuropils concluded that, similar to what had been established for the mammalian brain (see above), aged brains showed an increase in “vacuolated areas”, “swollen neurites” and intracytoplasmic, electron-dense bodies (Herman et al. 1971). Dense bodies resemble and could etiologically correspond to lipofuscin granules seen in aged vertebrate neurons (and other cells). Aside from dense bodies, other types of inclusions noted in electron microscopic investigations of aging fly brains are autophagosomes (“autophagy–lysosomal vesicles”) containing multilamellar structures (Ling and Salvaterra 2011). Vacuoles were interpreted as extracellular spaces left behind by degenerating neurites. Swollen neurites could represent earlier stages in the process of degeneration. However, degenerating neurites were not accompanied by microglia accumulations (insects do not possess microglia) or amyloid deposits. Small amyloid plaques could be induced only experimentally in the retina and optic lobe by overexpressing mutant forms of amyloid precursor protein and other Alzheimer’s related proteins (Greeve et al. 2004).
Most recent studies concerning aging have focused on age-related neuronal changes in a few of the brain compartments, notably those responsible for olfaction, vision, and learning/memory. The insect olfactory system includes olfactory receptor neurons (ORNs) whose cell bodies and dendrites are located in sensory hairs (sensilla) located on the antenna and several other appendages (Fig. 2a). ORN axons project to the antennal lobe of the central brain. Research in Drosophila has shown that each ORN expresses one odorant receptor gene out of approximately 50. Similar to the relationship between specific olfactory receptors and their central targets (olfactory bulb) in vertebrates, Drosophila ORNs which share a given receptor converge onto a single target (glomerulus) in the antennal lobe (Rössler 2013; Wilson 2013). ORNs target several populations of local interneurons and projection neurons, which relay olfactory information to higher brain centers, the mushroom body and the lateral horn (Fig. 2a). The mushroom body (MB) of the insect brain is a critical structure for learning and memory, from simple associative learning to processing of information related to complex stimuli. The scaffolding of the MB is provided by the axons and dendrites of a large number (1000s to 100,000s) of neurons called Kenyon cells, which receive input from olfactory projection neurons, and neurons conveying other modalities, on their dendritic branches which form a compartment called calyx (Fig. 2a). The input fibers form large synapses with Kenyon cells, called microglomeruli, whereby short postsynaptic branches of Kenyon cells surround, and are partially enwrapped in, the bulbous presynaptic endings of projection neurons (Fig. 2a).
Fruit fly (Drosophila melanogaster)
Fruit flies undergo rapid development, spending less than a day as embryos in the egg, followed by a 4-day period of larval life during which they undergo three molts. During a 4–5-day pupal period Drosophila metamorphoses into an adult fly, with a life span of approximately 50 days (Fig. 3). Note that the life span of insects and many other poikilothermic invertebrates can be dramatically lengthened by raising animals at lower temperatures. Drosophila raised at 18°C live twice as long as those raised at 25°C.
Fig. 3.
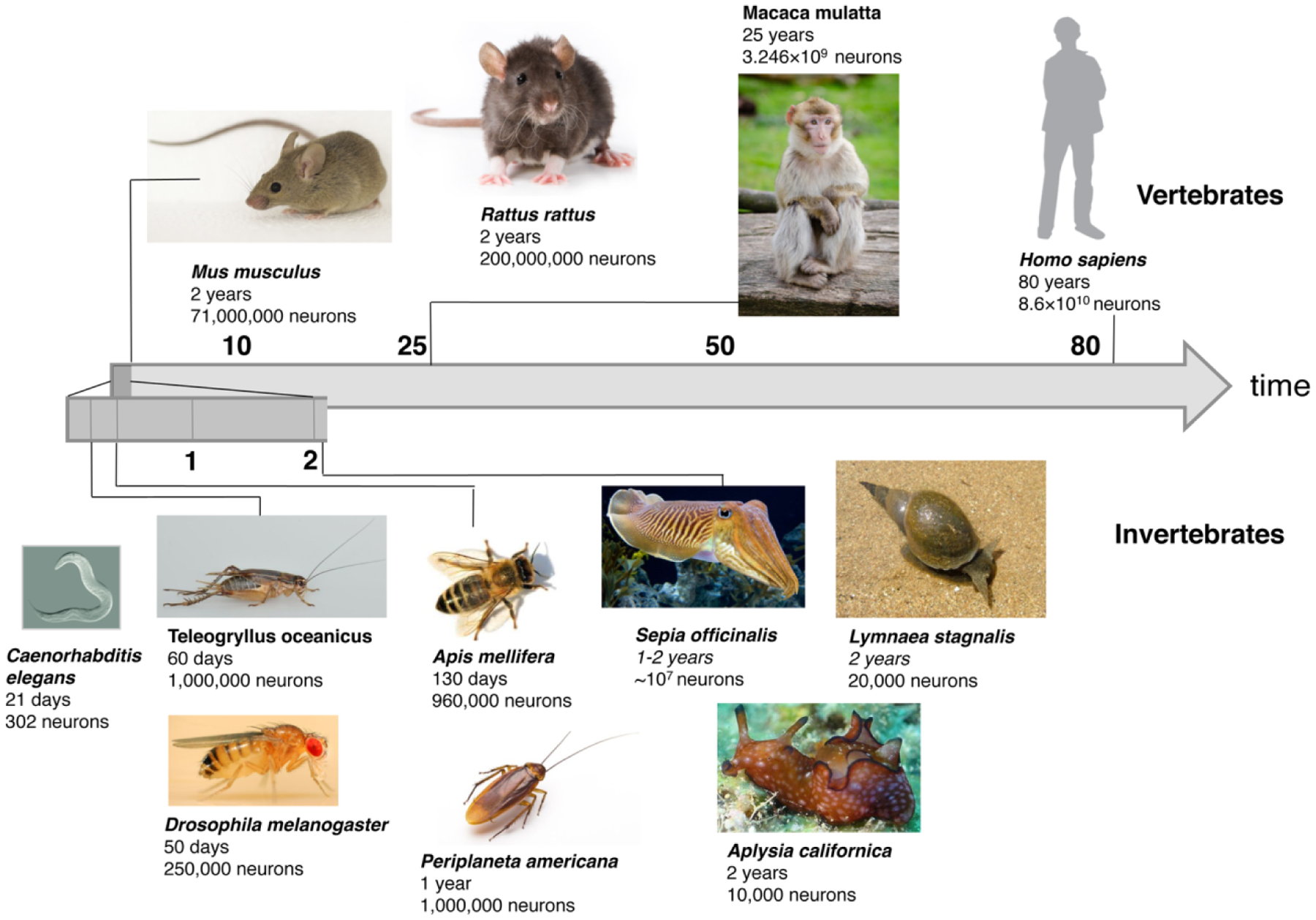
Overview of life span and neuron numbers of reviewed vertebrates and invertebrates. Vertebrates: mouse (Mus musculus), rat (Rattus rattus), monkey (Macaca mulatta) and human (Homo sapiens). Invertebrates: worm (Caenorhabditis elegans), cricket (Teleogryllus oceanicus), fruit fly (Drosophila melanogaster), honeybee (Apis mellifera), cockroach (Periplaneta Americana), cuttlefish (Sepia officinalis), sea hare (Aplysia caliornica) and pond snail (Lymnea stagnalis)
Numerous studies have addressed functional changes in the aging Drosophila brain. They demonstrated that with age flies display age-related changes in learning and memory, olfaction, vision, orientation as well as circadian rhythm and sleep (e.g. Tamura et al. 2003; Haddadi et al. 2014; Guven-Ozkan and Davis 2014). Functional studies (White et al. 2010) and gene expression studies in aging flies (Hall et al. 2017; Davie et al. 2018) have also been done. How are the physiological and behavioral deficits, or the genetic changes, related to structural aspects of neurological aging in the fly brain? The types of neuronal alterations that have been observed are in many ways similar to what has been summarized for the mammalian brain in the previous section (Fig. 2b). In general, there does not seem to be a significant loss of entire neurons in aged flies (but see Hussain et al., 2018, below). For example, the study by White et al. (White et al. 2010) which focused on the dopaminergic neurons of 60-day-old flies shows no decline in these cells despite locomotor degeneration. Instead of neuron loss, age-related changes consist of alterations in the number and structure of synaptic contacts, as well as the dendritic and axonal arbor.
Haddadi et al. (Haddadi et al. 2014) report a decrease in the number of synapses in the mushroom body (MB; Table 1). Furthermore, mitochondria in the MBs of old flies are enlarged and show an irregular shape. These changes could be due to mitochondrial damage caused by increased cellular oxidative stress in older cells. Light microscopic observations indicated vacuolation of neuropil and cortex, which might suggest an increase in neuronal apoptosis. However, in view of the fact that the widespread initial assumption of age-related cell death in mammalian brains has been generally refuted by more careful quantitative studies (see above), the question of whether cell death really occurs in aging brains of insects and other invertebrates needs further confirmation.
The study by Gupta et al. (2016) addressed the relationship between synaptic changes and the reduced ability of forming new memories with age (Gupta et al. 2016). Brains of old flies release significantly more synaptic vesicles and are characterized by an increased size of the presynaptic active zone leading to enhanced synaptic transmission (Table 1; Fig. 2c–f). This was determined by an increase in the levels of Bruchpilot (BRP; homolog of ELKS/CAST) in older flies. Using transmission electron microscopy authors imaged a whole calyx cross-section and used the small clear core synaptic vesicle, localized in projection neuron boutons, as quantifiable markers. Older animals show a significantly enlarged average size of presynaptic specializations (e.g., synaptic ribbons or T-bars) compared to young flies. Immuno-EM demonstrated increased levels of BRP in synapses of aged flies. These changes, which are detrimental to memory formation, were ameliorated by feeding spermidine, a compound known to activate autophagy. The author speculated that reduced autophagy could account for synaptic changes and impaired memory formation in old flies. This hypothesis could be confirmed in a follow-up study (Bhukel et al. 2019), in which targeted suppression of autophagy increased BRP in synaptic terminals, causing similar changes as those observed previously in aging brains.
Similar structural synaptic changes have been reported for the olfactory (antennal) lobe. Hussain et al. (Hussain et al. 2018) show that a decline in olfaction in aged Drosophila can be attributed to oxidative stress, caused by an age-related decline in the expression of ROS scavengers like SOD1 (superoxide dismutase 1), in the cholinergic projection neurons. These neurons show a decrease in odor response, accompanied by loss of synaptic boutons and mitochondria (which are localized in synaptic boutons) in the MB calyx. By comparing 7-day to 50-day-old flies the authors also see a mild but significant reduction of the average number of reporter-labeled PNs in aged flies, and a slight decrease in cell body size of these neurons. To analyze synaptic integrity of PNs transgenic reporter constructs were used to generate fluorescently labeled postsynaptic proteins. With age a substantial signal reduction of these proteins occurred in the antennal lobe.
Degenerative synaptic changes are also prominent in the peripheral nervous system, notably the neuromuscular junctions (NMJ). It should be mentioned that the muscular system of Drosophila, as well as that of C. elegans and other invertebrates, exhibit a much more dramatic structural and functional decline with age than the nervous system. Changes in the musculature include nuclear fragmentation and apoptosis, proceeded by abnormally shaped mitochondria, loss of organelles, and changes in myofilament structure (Augustin and Partridge 2009). Accompanying these degenerative events in the muscle cells are changes in NMJ structure: Beramendi et al. described a decline in motor axonal branch length and branch number, a decrease in synaptic sites and concomitant increase in presynaptic bouton diameter, and enlarged synaptic vesicles as well as signs of impaired endocytosis (Beramendi et al. 2007).
Studies documenting changes in the overall neuronal shape in aged fly brains are rare. Corfas et al. investigated the morphological characteristics of the antero-notopleural (ANP) mechanosensory neuron in young and old flies (Corfas and Dudai 1991). The ANP projects to the prothoracic ganglion of the central nervous system. One can discern a proximal (incoming) branch that subsequently splits into an anterior and posterior branch. All three branches give off terminal secondary branches. Interestingly, flies carrying mutations in genes involved in learning and memory (dunce, rutabaga) exhibited a significant increase in secondary branches emanating from the incoming branch, but not the other two branches. Aged flies show decay in the number of side branches and varicosities in the ANP incoming branch (Table 1).
A recent study analyzing the morphogenesis and age-related deterioration of individually labeled ring (R) neurons of the ellipsoid body, a compartment centrally involved in spatial memory and orientation (Neuser et al. 2008; Omoto et al. 2018), was able to show the outgrowth of ectopic processes as one hallmark of aging (Koch and Hartenstein unpublished data). Cell bodies of R-neurons of the freshly eclosed adult fly send a single ring-shaped process terminating in the ellipsoid body (Fig.2g, h). In addition, the R-neuron gives off of a single, short dendrite with a large terminal thickening (“microglomerulus”) that carries multiple postsynaptic sites. In aged flies, the overall number of R-neurons [as assayed by expression of the driver line R59B10; (Jenett et al. 2012)] remains unchanged. However, ectopic, irregularly shaped processes appear at cell bodies and, particularly, at the dendritic microglomeruli (Table 1; Fig. 2g, h). Interestingly, the ectopic branches of R-neurons of aged flies are reminiscent of the widespread filopodial extensions that can be seen before these neurons differentiate (Koch and Hartenstein unpublished data).
Honeybee (Apis mellifera)
Aging is generally associated with decay in the performance of a number of behaviors. In honeybees and other social insects, brain senescence is confounded by the fact that the animal adopts different roles (“castes”) at different life stages, brought about by changes in brain structure and function (Seehuus et al. 2006). Bee colonies have a single reproductive bee, the fecund queen, in addition to 10,000 to 40,000 sterile female workers and several hundreds of males (drones). While the life span of the fecund queen may be several years, sterile female workers may live anywhere between a few weeks up to a year. After eclosion, young females spend 2.5–4-weeks as “workers” (also called “nurses”) in the hive, caring for the larvae and pupae. This is followed by a 1–2-week period spent as foragers, which involves gathering nectar (carbohydrates), pollen (protein and lipids), and propolis (antimicrobial material, and water). The transition from nurse to forager depends on a neuro-hormonal mechanism, and can be delayed or prevented, depending on changes in the population and overall health of the colony (Behrends et al. 2007). The maximum life span of foragers is usually about two weeks, although it can range from 5 to over 200 days. The longest life span in workers is in winter, or ‘diutinus’ bees, which mature in autumn, when the brood is dormant. These winter bees can live nearly a year (Münch et al. 2013).
The honeybee brain, compared to Drosophila and most other insects, is large, containing in the order of 1,000,000 neurons (Wolschin et al. 2009) (Fig. 3). With the well-developed MB of bees, the relationship between brain structure and behavior can be analyzed. The cast-dependent behaviors are reflected in significant changes in the MB. For example, queens have significantly fewer microglomeruli than workers or foragers, and this difference depends on the pupal rearing temperature within the range of naturally occurring temperatures [32–36°C; (Groh et al. 2004)]. Workers and foragers show similar numbers of microglomeruli; however, during the transition from worker to forager, triggered by a rise in juvenile hormone (JH) associated with changes in the TOR and insulin signaling cascades (Amdam 2011), glomeruli expand in size. Foragers exhibit spines with markedly larger profile areas and shorter stems than those in workers (Coss et al. 1980). The increase in synaptic size is accompanied by a greater volume of different divisions of the calyx. It has been speculated that synaptic changes may be due to a more complex sensory environment and the need for complex spatial memories in foragers (Groh et al. 2012).
Structural changes in the central brain that occur in the transition from worker to forager are accompanied by changes in the periphery. For example, changes in expression levels of several gustatory receptors in the sensilla of the mouthparts, or proteins of the visual pathway (opsin, arrestin) in the compound eye were reported (Sasagawa et al. 2003; Simcock et al. 2017). Whitfield et al. found that the transition from hive work to foraging is also associated with changes in mRNA quantities in the brain for approximately 39 % of 5,500 genes tested (Whitfield et al. 2003). When experimental manipulation separated behavior and age, the mRNA changes were clearly associated with behavior only. In sum, many structural, physiological and biochemical changes are dependent on the foraging experience and behavior independent of age (Withers et al. 1993).
Only a few studies in bees explicitly address the question of neuronal change related to age, rather than cast/behavior, in honeybees. A decline in learning ability has been observed for older foragers (Münch et al. 2013). This reduced ability consists in the aging bees’ loss or extinction of spatial memory, that is, the bees having no memory of the location of an abandoned nest. These learning and extinction deficits are important as they mirror functional decline in mammals. Wolschin et al. likewise reported a decline in learning ability in old foragers. The levels of kinases and other neuronal proteins involved in the growth of synapses and neurons showed an overall drop in the central brain (Wolschin et al. 2009). The analysis of presynaptic protein expression (synapsin, Bruchpilot) in the MB calyx revealed a gradual increase with age, independent of worker vs foraging activity (Gehring et al. 2017), which matches the findings in Drosophila (Table 1). Similarly, the calycal expression levels of dopamine receptor (AmDop2) rise with increasing age and experience (Humphries et al. 2003).
Cricket (Teleogryllus oceanicus) and Cockroach (Periplaneta americana)
Crickets and cockroaches belong to the hemimetabolous insects which lack a pupal phase. Crickets, following a 2-week embryonic period, enter the nymphal stage, which lasts about two to three months, and involves 8–10 molts. Adult crickets live up to 60 days. Cockroaches also have a 2–3-week embryonic period, and a nymphal phase lasting up to 2 years. Adults live up to one year. The brain of crickets and cockroaches contains in the order of 1.000.000 neurons. (Fig. 3)
By using intracellular staining methods to visualize auditory interneurons, the so-called omega neurons 1 (ON1s), Atkins and Pollack show that the occurrence of the ascending axon in the ON1 is age-dependent in female crickets. Compared to young animals where 75 % of ON1s possess the ascending axons, only 30 % of old animals have those (28 days; Table 1; Fig. 2i). Surprisingly, the axon loss here is not connected to auditory dysfunction (Atkins and Pollack 1986).
Brown and Strausfeld investigated the effect of aging on place memory in cockroaches. They analyzed brain morphology changes in 50-week-old specimens and observed neuronal modification associated with memory loss. Although the gross morphology of the brain is maintained as individuals age, some ultrastructural changes are particularly evident in structures such as the mushroom body. Using light microscopy (Golgi impregnation) combined with electron microscopy, the authors investigated the lobes of the MB, a domain where output synapses (which are supposedly modulated by learning), as well as reciprocal synapses among MB neurons, are located (Fig. 2a). Output synapses are located on boutons, also called varicosities, which occur at variable intervals along the axon; input synapses are located on short side branches (“spines”) of the axons. Old cockroaches have a higher density of spines and varicosities than young cockroaches. While young cockroaches show spines with a uniform shape and size, old animals show an irregular shape and size. Electron microscopy revealed that MB axons of young animals are organized strictly parallel, whereas old animals show a more irregular arrangement (Brown and Strausfeld 2009). The most significant aging effect in individual synapses (varicosities) was an increased diameter of mitochondria.
In summary, insect neurons and their synaptic contacts show age-related structural changes that largely parallel those observed in mammals. Numbers of synapses are decreased for certain neuropils, yet individual synaptic contacts, as well as the expression of certain presynaptic proteins, may be increased in size and expression, respectively. Some neurons lose (large) branches. The number of short, dendritic branches (spines) can be increased, and spines adopt irregular shapes. Irregularly shaped processes are also formed at ectopic positions, as on the soma and the dendritic domain.
Neuronal aging in other invertebrate systems
Nematoda (Caenorhabditis elegans)
The robust genetics, simple anatomy, and short life span of C. elegans make it an ideal model organism to study a molecular network of genes that regulates the structure and function of neurons during aging. The life cycle of the nematode C. elegans consists of a brief embryonic stage (9 hours), four larval stages (L1-L4, about 10–12 hours each) and adulthood, which lasts up to 20 days. Dauer larva is an alternative developmental stage (alternative L3 stage) of C. elegans, whereby the larva goes into a type of stasis and can survive harsh conditions. C. elegans has 302 neurons born in a fixed lineage pattern (Fig. 3). Neurons are concentrated in a nerve ring (“brain”) surrounding the pharynx, and several nerve cords (ventral, lateral, dorsal) extending along the body. Individual neurons are small and almost branchless, and most do not have glial cells. Synapses do not form spines, and are aligned along the neuronal fiber, whereby input and output synapses are intermingled. Even though most neurons in C. elegans lack action potentials (Goodman et al. 1998; Lockery and Goodman 2009), potential spikes with traits of action potentials have been observed in populations of sensory neurons (Liu et al. 2018). Despite these physiological differences, molecular pathways involved in neuronal development, synapse formation and synaptic transmission identified in C. elegans are evolutionarily conserved (Shaye and Greenwald 2011; Chen et al. 2013).
The use of C. elegans as an invertebrate model for the study of neurodegenerative diseases, including Alzheimer’s, Parkinson’s, Amyotrophic Lateral Sclerosis, has also rapidly expanded over the past decade (Calahorro and Ruiz-Rubio 2011; Li and Le 2013; Lublin and Link 2013; Alexander et al. 2014; Therrien and Parker 2014; Kim et al. 2016; Maulik et al. 2017; Pir et al. 2017; Cooper and Van Raamsdonk 2018; Youssef et al. 2019; Gaeta et al. 2019). In addition, important insights into the molecular mechanism of normal aging were gained in this animal (Gao et al. 2018; Blackwell et al. 2019; Dall and Færgeman 2019; Son et al. 2019; Alvarez et al. 2020). As in Drosophila, the documentation of structural changes occurring in aged C. elegans neurons is comparatively limited. Similarly, to vertebrates and insects, the nervous system of the normally aging C elegans does not appear to suffer from cell death (Herndon et al. 2002), but shows discrete morphological changes in specific neurons. These were studied in several neuron populations, notably the touch receptor neurons which signal through interneurons to motor neurons to initiate movement. Touch receptor neurons extend long fibers that span either the anterior or posterior half of the nematode. Toth et al. reported that the aging process in touch receptors (ALM, AVM, PLM, PVM) of C. elegans is characterized by several morphological aspects: (1) novel outgrowths from the soma and fiber (Fig. 2j–l); (2) changes in the shape of the fiber, and (3) changes in synaptic ultrastructure (Table 1). Neuronal processes show a wavy appearance instead of being straight like in young animals and have a beaded (varicose) appearance (Toth et al. 2012). Mitochondria tend to relocalize with age and are found in many of the varicosities and at branch sites. The synaptic neuropil is reduced in size, reflecting a depletion of synaptic vesicles as well as a decrease in presynaptic densities. Interestingly, age-induced abnormalities differed in magnitude across different neurons, even though all neurons investigated share comparable structural and functional properties. Similar changes were noted by Pan et al. and Tank et al. in the touch receptors, as well as several other neuron types, including GABAergic motor neurons. These authors demonstrated axon beading and defasciculation (a loosening of bundles of neurons) with age. Structural changes in touch receptors are associated with reduced reaction to light touch and decreased mobility (Pan et al. 2011; Tank et al. 2011). Genes that influence the aging process also affect neuronal structure. For example, ptl-1, homolog to the human tau gene, is able to elicit the above described structural changes in touch receptor neurons when expressed at lower, but also higher levels (Chew et al. 2013). Furthermore, inhibiting genes controlling proteostasis (e.g., genes encoding proteasomal subunits, lysosomal genes) resulted in an enhanced formation of ectopic branches in aging touch receptors (Vayndorf et al. 2016).
Using confocal microscopy and generating a three-dimensional representation of the neurons Hess et al. investigated the age-dependent morphological changes of sensory neurons in 1-day-old worms compared to 8-day-old worms. They could validate the work of Toth et al., Tank et al. and Pan et al. They were also able to quantify additional morphological features, such as the volume of the touch receptor somata, which decreases with age (Hess et al. 2019). Neuronal aging in C. elegans also includes age-dependent decline in presynaptic release function (Chen et al. 2013). In synapses of motor neurons, a delay in vesicle fusion (reduced docking and priming of vesicles) and a diminished quantal vesicle size is observed. Motor function can be improved in aging animals through the increase of synaptic transmission in motor neurons by pharmacological means (Liu et al. 2013).
Mollusca (Lymnea stagnalis, Aplysia californica, Sepia officinalis)
Gastropods like the pond snail (Lymnea stagnalis) and the sea hare (Aplysia californica) have a life expectancy of 1–2 years. After an embryonic period lasting for several weeks larvae or juveniles hatch; juveniles of Lymnea take 2–4 weeks to reach sexual maturity, those of Aplysia 3–4 months. Gastropods and other molluscs have large, in part uniquely identifiable neurons with complex neurite trees. Neuron numbers are comparable to those in insects, ranging from a few tens of thousands (Lymnea) to many millions (Sepia officinalis, Fig. 3)
Classical EM analyses demonstrated that age-associated structural changes in molluscan neurons resembled those in mammals. These include loss of mitochondrial cristae, elevated occurrence of autophagosomes, and electron-dense cytoplasmic deposits (residual bodies; corresponding to lipofuscin granules; Frolkis et al. 1984). On the other hand, one observes cytological features that could be interpreted as compensatory changes, like the hypertrophy of mitochondria and hyperplasia of the Golgi.
Studies focusing on degenerative changes in identified neurons revealed that aging can cause a simplification of neuron morphology, characterized by a general retraction process and a loss of synaptic efficacy. A study of neurosecretory cells involved in the control of reproductive behavior shows that reduced egg laying in aged snails is correlated with a decrease in axonal branching and synapse numbers (Janse et al. 1996, 1999; Table 1).
Aplysia is a favorable experimental model to study neural development and function. A substantial literature on aging phenomena in the Aplysia nervous system has accumulated in recent years, focusing mostly on physiological and biochemical changes in aging neurons. For example, Akhmedov et al. detected a decreased response to acetylcholine (ACh) during aging (Akhmedov et al. 2013). A single neuron (R15) showed lower burst number and duration of action potentials induced by ACh in old animals compared to young animals. Other authors observed that the tail withdrawal reflex shows a significant reflex time increase, a reflex amplitude decrease, and an abolition of its short-term sensitization in aged Aplysia (Kempsell and Fieber 2014, 2015). In contrast to physiological studies of aging, detailed descriptions of structural changes in aging neurons are sparse. In a classical study dating back several decades, Peretz et al. examined the physiological and morphometrical properties of two Aplysia motor neurons involved in the gill withdrawal (Peretz et al. 1984). For one of these, L7, the junctional contact length between presynapse and muscle membrane increases, and the width of the synaptic cleft decreases, with age, leading to a reduced muscle contraction. By contrast, the second neuron, LDG1, which synapses on efferent vessel musculature, did not show these age-related changes, reminiscent of the situation in the mammalian brain where different compartments/neuron populations react to age in different ways.
Cephalopods possess a central nervous system that approaches that of mammals in cell number and complexity (Budelmann 1995). Chichery and Chichery describe the regression in visual and motor coordination as well as long-term memory, leading to a perturbed predatory behavior in aging cuttlefish. Using Fink-Heimer’s technique to stain the brains, they showed that the central nervous system of the cuttlefish shows extensive synaptic terminal degeneration in aged animals, with most obvious signs of deterioration in the areas of the nervous system with multimodal inputs. Brains of two-year old animals show an impregnation of a large number of more or less spheroidal particles representing degenerating terminal axon ramifications often referred to as ‘terminal degeneration’ and irregular shapes of fragmented fibers and a significant rise in argyrophilia of the affected fibers and compared it to one-year-old animals. Additionally, the neuropil areas of the optic lobe present degeneration granules and the beading of some fibers in old animals (Chichery and Chichery 1992). More recent studies confirm that, at the synaptic level, postsynaptic proteins such as the ACh-receptor decrease with age (Bellanger et al. 2005). However, investigations looking at changes in neuronal size and branch complexity have yet to be performed.
Conclusion
Many studies in vertebrate and invertebrate models have demonstrated age-associated changes in brain structure and function. These changes include loss of synapses, dendrites and dendritic spines, and axonal branches. They generally do not include a loss of entire neurons, despite previous claims (in humans and other mammals) to the contrary. It is important to note that in vertebrates and invertebrates alike, the impact of aging appears to differ significantly when comparing different neuron populations: whereas some show a decline in synapse numbers, synaptic transmitter release, and neuronal complexity (number and length of branches), other neuron populations are not affected. Some of the changes, such as the combination of decreased number/size of synaptic sites, accompanied by an increase in the size of synaptic boutons, could result from a decline in autophagy (Hussain et al. 2018). Loss of synaptic contacts could in turn cause gross structural changes in the neuronal tree, such as the retraction of dendritic and axonal branches. Complex factors causing defects in the myelin sheath, which accounts for much of the decrease in brain volume in mammals (Bowley et al. 2010), may have a secondary effect on the ensheathed axons; this aspect of neuron aging is restricted to vertebrates, since invertebrate neurons (with so far few exceptions) lack myelin (Hartline and Colman 2007; Hartline 2011).
An interesting finding in invertebrate neurons, specifically C. elegans and Drosophila, is the occurrence of ectopic branches at specific positions. It should be noted that ectopic branching might occur in vertebrate systems as well, but was so far not emphasized because of the typically higher complexity of vertebrate neurons. Ectopic branching could result from regressive changes in the molecular machinery controlling cytoskeletal dynamics. Filopodia extensions in developing neurons are widespread; they occur at locations along the nerve fiber that later, in the (young) adult stage, have no neurites, and they often reach into neuropil domains beyond the volume innervated by (young) adult dendrites and axons. This finding implies that neuronal differentiation includes pruning and the restriction of branch points, processes which could require active maintenance mechanisms throughout adult life. Senescence may result in a weakening of these mechanisms, resulting in the (re-) appearance of branches at ectopic positions.
As shown in many of the studies surveyed in this review, an important advantage invertebrate model systems offer is that experimental manipulations can be directed at individual, genetically marked neurons, rather than large populations. This makes it possible to measure effects of genetic mutations or pharmacological treatments with a higher degree of certainty, reducing the possibility of statistical error. It is therefore to be expected that continued research efforts in studying brain aging in invertebrate systems will make significant contributions towards understanding the molecular/cellular pathways involved in neuronal aging generally.
Funding information
NIH Grant NS054814-14 to V.H.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest On behalf of all authors, the corresponding author states that there is no conflict of interest.
Informed consent N/A
Ethical approval This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Akhmedov K, Rizzo V, Kadakkuzha BM, et al. (2013) Decreased Response to Acetylcholine during Aging of Aplysia Neuron R15. PLoS One 8:e84793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AG, Marfil V, Li C (2014) Use of C. elegans as a model to study Alzheimer’s disease and other neurodegenerative diseases. Front Genet 5:1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J, Alvarez-Illera P, García-Casas P, et al. (2020) The Role of Ca2+ Signaling in Aging and Neurodegeneration: Insights from Caenorhabditis elegans Models. Cells 9:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV. (2011) Social context, stress, and plasticity of aging. Aging Cell 10:18–27 [DOI] [PubMed] [Google Scholar]
- Atkins G, Pollack GS (1986) Age-dependent occurrence of an ascending axon on the omega neuron of the cricket, Teleogryllus oceanicus. J Comp Neurol 243:527–534 [DOI] [PubMed] [Google Scholar]
- Augustin H, Partridge L (2009) Invertebrate models of age-related muscle degeneration. Biochim Biophys Acta 1790:1084–1094 [DOI] [PubMed] [Google Scholar]
- Ball MJ (1977) Neuronal loss, neurofibrillary tangles and granulovacuolar degeneration in the hippocampus with ageing and dementia. Acta Neuropathol 37:111–118 [DOI] [PubMed] [Google Scholar]
- Bancher C, Lassmann H, Budka H, et al. (1987) Neurofibrillary tangles in Alzheimer’s disease and progressive supranuclear palsy: antigenic similarities and differences. Acta Neuropathol 74:39–46 [DOI] [PubMed] [Google Scholar]
- Beckingham KM, Texada MJ, Baker DA, et al. (2005) Genetics of Graviperception in Animals. Adv Genet 55:105–145 [DOI] [PubMed] [Google Scholar]
- Behrends A, Scheiner R, Baker N, Amdam GV (2007) Cognitive aging is linked to social role in honey bees (Apis mellifera). Exp Gerontol 42:1146–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellanger C, Halm M-P, Dauphin F, Chichery R (2005) In vitro evidence and age-related changes for nicotinic but not muscarinic acetylcholine receptors in the central nervous system of Sepia officinalis. Neurosci Lett 387:162–167 [DOI] [PubMed] [Google Scholar]
- Beramendi A, Peron S, Casanova G, et al. (2007) Neuromuscular junction in abdominal muscles of Drosophila melanogaster during adulthood and aging. J Comp Neurol 501:498–508 [DOI] [PubMed] [Google Scholar]
- Bhukel A, Beuschel CB, Maglione M, et al. (2019) Autophagy within the mushroom body protects from synapse aging in a non-cell autonomous manner. Nat Commun 10:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop NA, Lu T, Yankner BA (2010) Neural mechanisms of ageing and cognitive decline. Nature 464:529–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell K, Sewell AK, Wu Z, Han M (2019) TOR signaling in caenorhabditis elegans development, metabolism, and aging. Genetics 213:329–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, et al. (2003) Gene microarrays in hippocampal aging: Statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci 23:3807–3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini NM, Fortini ME (2003) Human Neurodegenerative Disease Modeling Using Drosophila. Annu Rev Neurosci 26:627–656 [DOI] [PubMed] [Google Scholar]
- Bowley MP, Cabral H, Rosene DL, Peters A (2010) Age changes in myelinated nerve fibers of the cingulate bundle and corpus callosum in the rhesus monkey. J Comp Neurol 518:3046–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzee KR, Knox C (1980) The aging process in the neuron. Adv Exp Med Biol 129:71–98 [DOI] [PubMed] [Google Scholar]
- Brody H (1955) Organization of the cerebral cortex. III. A study of aging in the human cerebral cortex. J Comp Neurol 102:511–516 [DOI] [PubMed] [Google Scholar]
- Brown S, Strausfeld N (2009) The effect of age on a visual learning task in the American cockroach. Learn Mem 16:210–223 [DOI] [PubMed] [Google Scholar]
- Brunk U, Brun A (1972) The effect of aging on lysosomal permeability in nerve cells of the central nervous system. An enzyme histochemical study in rat. Histochemie 30:315–324 [DOI] [PubMed] [Google Scholar]
- Budelmann BU (1995) The cephalopod nervous system: What evolution has made of the molluscan design. In: The Nervous System of Invertebrates. pp 115–138 [Google Scholar]
- Burke SN, Barnes CA (2006) Neural plasticity in the ageing brain. Nat Rev Neurosci 7:30–40 [DOI] [PubMed] [Google Scholar]
- Calahorro F, Ruiz-Rubio M (2011) Caenorhabditis elegans as an experimental tool for the study of complex neurological diseases: Parkinson’s disease, Alzheimer’s disease and autism spectrum disorder. Invert Neurosci 11:73–83 [DOI] [PubMed] [Google Scholar]
- Calì C, Wawrzyniak M, Becker C, et al. (2018) The effects of aging on neuropil structure in mouse somatosensory cortex - A 3D electron microscopy analysis of layer 1. PLoS One 13:1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone MA, Yamamoto A, Huang W et al. (2016) Genetic architecture of natural variation in visual senescence in Drosophila. Proc Natl Acad Sci U S A 113:E6620–E6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli V, Benedetti E, Antonosante A, et al. (2019) Neuronal cells rearrangement during aging and neurodegenerative disease: Metabolism, oxidative stress and organelles dynamic. Front Mol Neurosci 12:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Chen YC, Jiang HC, et al. (2013) Neuronal aging: Learning from C. elegans. J Mol Signal 8:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew YL, Fan X, Götz J, Nicholas HR (2013) PTL-1 regulates neuronal integrity and lifespan in C. elegans. J Cell Sci 126:2079–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichery MP, Chichery R (1992) Behavioural and neurohistological changes in aging Sepia. Brain Res 574:77–84 [DOI] [PubMed] [Google Scholar]
- Coleman PD, Flood DG (1987) Neuron numbers and dendritic extent in normal aging and Alzheimer’s disease. Neurobiol Aging 8:521–545 [DOI] [PubMed] [Google Scholar]
- Cooper JF, Van Raamsdonk JM (2018) Modeling Parkinson’s disease in C. elegans. J Parkinsons Dis 8:17–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfas G, Dudai Y (1991) Morphology of a sensory neuron in Drosophila is abnormal in memory mutants and changes during aging. Proc Natl Acad Sci U S A 88:7252–7256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss RG, Brandon JG, Globus A (1980) Changes in morphology of dendritic spines on honeybee calycal interneurons associated with cumulative nursing and foraging experiences. Brain Res 192:49–59 [DOI] [PubMed] [Google Scholar]
- Cupp C, Uemura E (1980) Age-related changes in prefrontal cortex of Macaca mulatta: quantitative analysis of dendritic branching patterns. Exp Neurol 69:143–163 [DOI] [PubMed] [Google Scholar]
- Dall KB, Færgeman NJ (2019) Metabolic regulation of lifespan from a C. Elegans perspective. Genes Nutr 14:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele S, Giacomelli C, M C (2018) Brain ageing and neurodegenerative disease: The role of cellular waste management. Biochem Pharmacol 158:207–216 [DOI] [PubMed] [Google Scholar]
- Davie K, Janssens J, Koldere D, et al. (2018) A Single-Cell Transcriptome Atlas of the Aging Drosophila Brain. Cell 174:982–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein D, Weaver C, Luebke J, Hof P (2013) Dendritic spine changes associated with normal aging. Neuroscience 251:21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donev R, Kolev M, Millet B, Thome J (2009) Neuronal death in Alzheimer’s disease and therapeutic opportunities. J Cell Mol Med 13:4329–4348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Double KL, Dedov VN, Fedorow H, et al. (2008) The comparative biology of neuromelanin and lipofuscin in the human brain. Cell Mol Life Sci 65:1669–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver C, Georgiou A, Georgiou G (2004) The Contribution by Mitochondrially Induced Oxidative Damage to Aging in Drosophila Melanogaster. Biogerontology 5:185–192 [DOI] [PubMed] [Google Scholar]
- Dudas SP, Arking R (1995) A coordinate upregulation of antioxidant gene activities is associated with the delayed onset of senescence in a long-lived strain of Drosophila. J Gerontol A Biol Sci Med Sci 50:B117–27 [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, et al. (2010) Selective Changes in Thin Spine Density and Morphology in Monkey Prefrontal Cortex Correlate with Aging-Related Cognitive Impairment. J Neurosci 30:7507–7515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eavri R, Shepherd J, Welsh CA, et al. (2018) Interneuron simplification and loss of structural plasticity as markers of aging-related functional decline. J Neurosci 38:8421–8432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth DR, Bayraktar OA, Doe CQ (2015) Aging neural progenitors lose competence to respond to mitogenic Notch signaling. Curr Biol 25:3058–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood DG, Coleman PD (1993) Dendritic regression dissociated from neuronal death but associated with partial deafferentation in aging rat supraoptic nucleus. Neurobiol Aging 14:575–587 [DOI] [PubMed] [Google Scholar]
- Flurkey, Currer, and Harrison, 2007. “The mouse in biomedical research” in Fox James G. (ed.), American College of Laboratory Animal Medicine series (Elsevier, AP: Amsterdam; Boston: ) [Google Scholar]
- Foster T (2019) Senescent neurophysiology: Ca2+ signaling from the membrane to the nucleus. Neurobiol Learn Mem 164:107064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SH, Kandel R, Cruz L, et al. (2008) Preservation of Neuronal Number Despite Age-Related Cortical Brain Atrophy in Elderly Subjects Without Alzheimer Disease. J Neuropathol Exp Neurol 67:1205–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolkis VV, Stupina AS, Martinenko OA, et al. (1984) Aging of neurons in the mollusc Lymnaea stagnalis. Structure, function and sensitivity to transmitters. Mech Ageing Dev 25:91–102 [DOI] [PubMed] [Google Scholar]
- Gaeta AL, Caldwell KA, Caldwell GA (2019) Found in translation: The utility of C. elegans Alpha-Synuclein models of Parkinson’s disease. Brain Sci 9:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao A, Uit de Bos J, Sterken M, et al. (2018) Forward and reverse genetics approaches to uncover metabolic aging pathways in Caenorhabditis elegans. Biochim Biophys Acta Mol Basis Dis 1864:2697–2706 [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, Thakker MM, Hof PR, Morrison JH (1997) Preserved number of entorhinal cortex layer II neurons in aged macaque monkeys. Neurobiol Aging 18:549–553 [DOI] [PubMed] [Google Scholar]
- Gehring KB, Heufelder K, Depner H, et al. (2017) Age-associated increase of the active zone protein Bruchpilot within the honeybee mushroom body. PLoS One 12:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilissen EP, Staneva-Dobrovski L (2013) Distinct types of lipofuscin pigment in the hippocampus and cerebellum of aged cheirogaleid primates. Anat Rec (Hoboken) 296:1895–1906 [DOI] [PubMed] [Google Scholar]
- Goodman L, Bonini N (2020) New Roles for Canonical Transcription Factors in Repeat Expansion Diseases. Trends Genet 36:81–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M, Hall D, Avery L, Lockery S (1998) Active Currents Regulate Sensitivity and Dynamic Range in C. elegans Neurons. Neuron 20:763–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeve I, Kretzschmar D, Tschäpe J-A, et al. (2004) Age-Dependent Neurodegeneration and Alzheimer-Amyloid Plaque Formation in Transgenic Drosophila. J Neurosci 24:3899–3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill JD, Riddle DR (2002) Age-related and laminar-specific dendritic changes in the medial frontal cortex of the rat. Brain Res 937:8–21 [DOI] [PubMed] [Google Scholar]
- Groh C, Lu Z, Meinertzhagen IA, Rössler W (2012) Age-related plasticity in the synaptic ultrastructure of neurons in the mushroom body calyx of the adult honeybee Apis mellifera. J Comp Neurol 520:3509–3527 [DOI] [PubMed] [Google Scholar]
- Groh C, Tautz J, Rössler W (2004) Synaptic organization in the adult honey bee brain is influenced by brood-temperature control during pupal development. Proc Natl Acad Sci U S A 101:4268–4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Vorbrodt AW, Iqbal K, et al. (1988) Microtubule-associated polypeptides tau are altered in Alzheimer paired helical filaments. Mol Brain Res 4:43–52 [DOI] [PubMed] [Google Scholar]
- Gupta VK, Pech U, Bhukel A, et al. (2016) Spermidine Suppresses Age-Associated Memory Impairment by Preventing Adverse Increase of Presynaptic Active Zone Size and Release. PLoS Biol 14:e1002563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guven-Ozkan T, Davis RL (2014) Functional neuroanatomy of Drosophila olfactory memory formation. Learn Mem 21:519–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddadi M, Jahromi SR, Sagar BKC, et al. (2014) Brain aging, memory impairment and oxidative stress: A study in Drosophila melanogaster. Behav Brain Res 259:60–69 [DOI] [PubMed] [Google Scholar]
- Hall H, Medina P, Cooper DA, et al. (2017) Transcriptome profiling of aging Drosophila photoreceptors reveals gene expression trends that correlate with visual senescence. BMC Genomics 18:894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartline DK (2011) The evolutionary origins of glia. Glia 59:1215–1236 [DOI] [PubMed] [Google Scholar]
- Hartline DK, Colman DR (2007) Rapid conduction and the evolution of giant axons and myelinated fibers. Curr Biol 17:29–35 [DOI] [PubMed] [Google Scholar]
- Hendy R (1971) Electron microscopy of lipofuscin pigment stained by the schmörl and fontana techniques. Histochemie 26:311–318 [DOI] [PubMed] [Google Scholar]
- Herman MM, Miquel J, Johnson M (1971) Insect brain as a model for the study of aging. Acta Neuropathol 19:167–183 [DOI] [PubMed] [Google Scholar]
- Herndon L, Schmeissner P, Dudaronek J, et al. (2002) Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 419:808–814 [DOI] [PubMed] [Google Scholar]
- Hess M, Gomariz A, Goksel O, Ewald CY (2019) In-vivo quantitative image analysis of age-related morphological changes of C. elegans neurons reveals a correlation between neurite bending and novel neurite outgrowths. eNeuro 6:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MA, Mustard JA, Hunter SJ, et al. (2003) Invertebrate D2 type dopamine receptor exhibits age-based plasticity of expression in the mushroom bodies of the honeybee brain. J Neurobiol 55:315–330 [DOI] [PubMed] [Google Scholar]
- Hussain A, Pooryasin A, Zhang M, et al. (2018) Inhibition of oxidative stress in cholinergic projection neurons fully rescues aging-associated olfactory circuit degeneration in drosophila. Elife 7:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima K, Iijima-Ando K (2008) Drosophila models of Alzheimer’s amyloidosis: the challenge of dissecting the complex mechanisms of toxicity of amyloid-beta 42. J Alzheimers Dis 15:523–540 [DOI] [PubMed] [Google Scholar]
- Iijima K, Liu HP, Chiang AS, et al. (2004) Dissecting the pathological effects of human Aβ40 and Aβ42 in Drosophila: A potential model for Alzheimer’s disease. Proc Natl Acad Sci U S A 101:6623–6628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse C, Peretz B, van der Roest M, Dubelaar EJ (1999) Excitability and branching of neuroendocrine cells during reproductive senescence. Neurobiol Aging 20:675–683 [DOI] [PubMed] [Google Scholar]
- Janse C, van der Roest M, Jansen RF, et al. (1996) Atrophy and degeneration of peptidergic neurons and cessation of egg laying in the aging pond snail Lymnaea stagnalis. J Neurobiol 29:202–212 [DOI] [PubMed] [Google Scholar]
- Jenett A, Rubin GM, Ngo TTB, et al. (2012) A GAL4-Driver Line Resource for Drosophila Neurobiology. Cell Rep 2:991–1001. 10.1016/j.celrep.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JR, Jenn RC, Barclay JW, et al. (2010) Caenorhabditis elegans: a useful tool to decipher neurodegenerative pathways. Biochem Soc Trans 38:559–563 [DOI] [PubMed] [Google Scholar]
- Kempsell AT, Fieber LA (2014) Behavioral aging is associated with reduced sensory neuron excitability in Aplysia californica. Front Aging Neurosci 6:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempsell AT, Fieber LA (2015) Aging in Sensory and Motor Neurons Results in Learning Failure in Aplysia californica. PLoS One 10:e0127056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuker JIH, Luiten PGM, Fuchs E (2003) Preservation of hippocampal neuron numbers in aged rhesus monkeys. Neurobiol Aging 24:157–165 [DOI] [PubMed] [Google Scholar]
- Kim D-K, Kim TH, Lee S-J (2016) Mechanisms of aging-related proteinopathies in Caenorhabditis elegans. Exp Mol Med 48:e263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounatidis I, Chtarbanova S, Cao Y, et al. (2017) NF-κB Immunity in the Brain Determines Fly Lifespan in Healthy Aging and Age-Related Neurodegeneration. Cell Rep 19:836–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar D (2005) Neurodegenerative mutants in Drosophila: a means to identify genes and mechanisms involved in human diseases? Invert Neurosci 5:97–109 [DOI] [PubMed] [Google Scholar]
- Li J, Le W (2013) Modeling neurodegenerative diseases in Caenorhabditis elegans. Exp Neurol 250:94–103 [DOI] [PubMed] [Google Scholar]
- Li W, Prazak L, Chatterjee N, et al. (2013) Activation of transposable elements during aging and neuronal decline in Drosophila. Nat Neurosci 16:529–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YT, Sigrist S (2018) Autophagy and proteostasis in the control of synapse aging and disease. Curr Opin Neurobiol 48:113–121 [DOI] [PubMed] [Google Scholar]
- Liao S, Broughton S, Nässel DR (2017) Behavioral senescence and aging-related changes in motor neurons and brain neuromodulator levels are ameliorated by lifespan-extending reproductive dormancy in Drosophila. Front Cell Neurosci 11:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling D, Magallanes M, Salvaterra PM (2014) Accumulation of amyloid-like Aβ1–42 in AEL (autophagy-endosomal-lysosomal) vesicles: Potential implications for plaque biogenesis. ASN Neuro 6:95–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling D, Salvaterra P (2011) Brain aging and Aβ1–42 neurotoxicity converge via deterioration in autophagy-lysosomal system: a conditional Drosophila model linking Alzheimer’s neurodegeneration with aging. Acta Neuropathol 121:183–191 [DOI] [PubMed] [Google Scholar]
- Ling D, Salvaterra PM (2009) A central role for autophagy in Alzheimer-type neurodegeneration. Autophagy 5:738–740 [DOI] [PubMed] [Google Scholar]
- Lipinski M, Bin Z, Lu T, et al. (2010) Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer’s disease. Proc Natl Acad Sci U S A 107:14164–14169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang B, Lei H, et al. (2013) Functional aging in the nervous system contributes to age-dependent motor activity decline in C. elegans. Cell Metab 18:392–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kidd PB, Dobosiewicz M, Bargmann CI (2018) C. elegans AWA Olfactory Neurons Fire Calcium-Mediated All-or-None Action Potentials. Cell 175:57–70.e17 [DOI] [PubMed] [Google Scholar]
- Lockery SR, Goodman MB (2009) The quest for action potentials in C. elegans neurons hits a plateau. Nat Neurosci 12:377–378. 10.1038/nn0409-377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loerch PM, Lu T, Dakin KA, et al. (2008) Evolution of the aging brain transcriptome and synaptic regulation. PLoS One 3:e3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao S, et al. (2004) Gene regulation and DNA damage in the ageing human brain. Nature 429:883–891 [DOI] [PubMed] [Google Scholar]
- Lublin A, Link C (2013) Alzheimer’s disease drug discovery: in vivo screening using Caenorhabditis elegans as a model for β-amyloid peptide-induced toxicity. Drug Discov Today Technol 10:e115–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B (2003) Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol 462:144–152 [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Hansen L, et al. (1993) Quantitative synaptic alterations in the human neocortex during normal aging. Neurology 43:192–197. [DOI] [PubMed] [Google Scholar]
- Maulik M, Mitra S, Bult-Ito A, et al. (2017) Behavioral phenotyping and pathological indicators of Parkinson’s disease in C. elegans models. Front Genet 8:1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk L, Berson A, Bonini N (2015) Drosophila as an In Vivo Model for Human Neurodegenerative Disease. Genetics 201:377–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill DA, Chiba AA, Tuszynski MH (2001) Conservation of neuronal number and size in the entorhinal cortex of behaviorally characterized aged rats. J Comp Neurol 438:445–456 [DOI] [PubMed] [Google Scholar]
- Merrill DA, Roberts JA, Tuszynski MH (2000) Conservation of neuron number and size in entorhinal cortex layers II, III, and V/VI of aged primates. J Comp Neurol 422:396–401 [DOI] [PubMed] [Google Scholar]
- Mohammed HA, Santer RM (2001) Total neuronal numbers of rat lumbosacral primary afferent neurons do not change with age. Neurosci Lett 304:149–152 [DOI] [PubMed] [Google Scholar]
- Moreno-García A, Kun A, Calero O, et al. (2018) An overview of the role of lipofuscin in age-related neurodegeneration. Front Neurosci 12:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Hof PR (1997) Life and death of neurons in the aging brain. Science 278:412–419 [DOI] [PubMed] [Google Scholar]
- Mostany R, Anstey JE, Crump KL, et al. (2013) Altered Synaptic Dynamics during Normal Brain Aging. J Neurosci 33:4094–4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller F, Lustgarten M, Jang Y, et al. (2007) Trends in oxidative aging theories. Free Radic Biol Med 15:477–503 [DOI] [PubMed] [Google Scholar]
- Münch D, Kreibich CD, Amdam GV. (2013) Aging and its modulation in a long-lived worker caste of the honey bee. J Exp Biol 216:1638–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Akiguchi I, Kameyama M, Mizuno N (1985) Age-related changes of pyramidal cell basal dendrites in layers III and V of human motor cortex: a quantitative Golgi study. Acta Neuropathol 65:281–284 [DOI] [PubMed] [Google Scholar]
- Nash TR, Chow ES, Law AD, et al. (2019) Daily blue-light exposure shortens lifespan and causes brain neurodegeneration in Drosophila. npj Aging Mech Dis 5:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuser K, Triphan T, Mronz M, et al. (2008) Analysis of a spatial orientation memory in Drosophila. Nature 453:1244–1247 [DOI] [PubMed] [Google Scholar]
- Niikura T, Tajima H, Kita Y (2006) Neuronal Cell Death in Alzheimers Disease and a Neuroprotective Factor, Humanin. Curr Neuropharmacol 4:139–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoletopoulou V, Tavernarakis N (2012) Calcium homeostasis in aging neurons. Front Genet 3:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoto JJ, Nguyen BCM, Kandimalla P, et al. (2018) Neuronal constituents and putative interactions within the drosophila ellipsoid body neuropil. Front Neural Circuits 12:1–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page T, Einstein M, Duan H, et al. (2002) Morphological alterations in neurons forming corticocortical projections in the neocortex of aged Patas monkeys. Neurosci Lett 317:37–41 [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ (1997) Neocortical neuron number in humans: effect of sex and age. J Comp Neurol 384:312–320. 10.1073/pnas.0803652105 [DOI] [PubMed] [Google Scholar]
- Pan C-L, Peng C-Y, Chen C-H, McIntire S (2011) Genetic analysis of age-dependent defects of the Caenorhabditis elegans touch receptor neurons. Proc Natl Acad Sci 108:9274–9279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannese E (2011) Morphological changes in nerve cells during normal aging. Brain Struct Funct 216:85–89 [DOI] [PubMed] [Google Scholar]
- Peretz B, Romanenko A, Markesbery W (1984) Functional history of two motor neurons and the morphometry of their neuromuscular junctions in the gill of Aplysia: Evidence for differential aging. Proc Natl Acad Sci U S A 81:4232–4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perisse E, Burke C, Huetteroth W, Waddell S (2013) Shocking Revelations and Saccharin Sweetness Review in the Study of Drosophila Olfactory Memory. Curr Biol 23:752–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A (1994) The Organization of the Primary Visual Cortex in the Macaque BT - Primary Visual Cortex in Primates. In: Peters A, Rockland KS (eds). Springer US, Boston, MA, pp 1–35 [Google Scholar]
- Peters A, Rosene DL (2003) In aging, is it gray or white? J Comp Neurol 462:139–143 [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C (2002) Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol 442:277–291 [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Luebke JI (2008) Synapses are lost during aging in the primate prefrontal cortex. Neuroscience 152:970–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Mattson MP, Yao PJ (2014) Communication breakdown: The impact of ageing on synapse structure. Ageing Res Rev 14:31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pir GJ, Choudhary B, Mandelkow E (2017) Caenorhabditis elegans models of tauopathy. FASEB J 31:5137–5148 [DOI] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA (1996) Increased dendritic extent in hippocampal CA1 neurons from aged F344 rats. Neurobiol Aging 17:601–611 [DOI] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M (1996) Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci U S A 93:9926–9930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T, Schliemann T, Sorensen JC, et al. (1996) Memory impaired aged rats: no loss of principal hippocampal and subicular neurons. Neurobiol Aging 17:143–147 [DOI] [PubMed] [Google Scholar]
- Remolina SC, Hafez DM, Robinson GE, Hughes KA (2007) Senescence in the worker honey bee Apis Mellifera. J Insect Physiol 53:1027–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rera M, Clark RI, Walker DW (2012) Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci U S A. 109:21528–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard MB, Taylor SR, Greer CA (2010) Age-induced disruption of selective olfactory bulb synaptic circuits. Proc Natl Acad Sci 107:15613–15618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rössler W, Brill M (2013) Parallel processing in the honeybee olfactory pathway: structure, function, and evolution. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 199:981–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC, Mariño G, Kroemer G (2011) Autophagy and aging. Cell 146:682–695 [DOI] [PubMed] [Google Scholar]
- Salvadores N, Sanhueza M, Manque P, Court FA (2017) Axonal degeneration during aging and its functional role in neurodegenerative disorders. Front Neurosci 11:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasagawa H, Narita R, Kitagawa Y, Kadowaki T (2003) The expression of genes encoding visual components is regulated by a circadian clock, light environment and age in the honeybee (Apis mellifera). Eur J Neurosci 17:963–970 [DOI] [PubMed] [Google Scholar]
- Seehuus S-C, Krekling T, Amdam GV (2006) Cellular senescence in honey bee brain is largely independent of chronological age. Exp Gerontol 41:1117–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaye DD, Greenwald I (2011) OrthoList: a compendium of C. elegans genes with human orthologs. PLoS One 6:e20085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Lu T, Furuya T, et al. (2006) Regulation of intracellular accumulation of mutant huntingtin by beclin 1. J Biol Chem 281:14474–14485 [DOI] [PubMed] [Google Scholar]
- Simcock NK, Wakeling LA, Ford D, Wright GA (2017) Effects of age and nutritional state on the expression of gustatory receptors in the honeybee (Apis mellifera). PLoS One 12:e0175158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son HG, Altintas O, Kim EJE, et al. (2019) Age-dependent changes and biomarkers of aging in Caenorhabditis elegans. Aging Cell 18:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Chiang AS, Ito N, et al. (2003) Aging Specifically Impairs amnesiac-Dependent Memory in Drosophila. Neuron 40:1003–1011 [DOI] [PubMed] [Google Scholar]
- Tank EMH, Rodgers KE, Kenyon C (2011) Spontaneous Age-Related Neurite Branching in Caenorhabditis elegans. J Neurosci 31:9279–9288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therrien M, Parker JA (2014) Worming forward: Amyotrophic lateral sclerosis toxicity mechanisms and genetic interactions in Caenorhabditis elegans. Front Genet 5:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Gant J, Landfield P (2007) Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: Minding the store. Aging Cell 6:307–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson BE, Blessed G, Roth M (1968) Observations on the brains of non-demented old people. J Neurol Sci 7:331–356 [DOI] [PubMed] [Google Scholar]
- Toth ML, Melentijevic I, Shah L, et al. (2012) Neurite sprouting and synapse deterioration in the aging Caenorhabditis elegans nervous system. J Neurosci 32:8778–8790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troulinaki K, Tavernarakis N (2005) Neurodegenerative conditions associated with ageing: a molecular interplay? Mech Ageing Dev 126:23–33 [DOI] [PubMed] [Google Scholar]
- Uemura E, Ireland WP (1985) Dendritic alterations in chronic animals with experimental neurofibrillary changes. Exp Neurol 89:530–542 [DOI] [PubMed] [Google Scholar]
- Vaughan DW (1977) Age-related deterioration of pyramidal cell basal dendrites in rat auditory cortex. J Comp Neurol 171:501–515 [DOI] [PubMed] [Google Scholar]
- Vayndorf EM, Scerbak C, Hunter S, et al. (2016) Morphological remodeling of c. Elegans neurons during aging is modified by compromised protein homeostasis. npj Aging Mech Dis 2: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij MW, de Groot M, van der Lugt A, et al. (2008) White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. Neuroimage 43:470–477 [DOI] [PubMed] [Google Scholar]
- Vijayan V, Verstreken P (2017) Autophagy in the presynaptic compartment in health and disease. J Cell Biol 216:1895–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Zacher C, Gass P, Unsicker K(2006) Age-related alterations in hippocampal spines and deficiencies in spatial memory in mice. J Neurosci Res 83:525–31 [DOI] [PubMed] [Google Scholar]
- Wentzell J, Kretzschmar D (2010) Alzheimer’s Disease and Tauopathy studies in flies and worms. Neurobiol Dis 40:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC (1994) Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet 344:769–772 [DOI] [PubMed] [Google Scholar]
- White KE, Humphrey DM, Hirth F (2010) The dopaminergic system in the aging brain of Drosophila. Front Neurosci 4:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield C, Cziko A, Robinson G (2003) Gene expression profiles in the brain predict behavior in individual honey bees. Science (80- ) 302:296–299 [DOI] [PubMed] [Google Scholar]
- Wilson RI (2013) Early olfactory processing in Drosophila: mechanisms and principles. Annu Rev Neurosci 36:217–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski H, Terry RD (1973) Morphology of the Aging Brain, Human and Animal. Neurobiol Asp Matur Aging 40:167–186 [DOI] [PubMed] [Google Scholar]
- Withers GS, Fahrbach SE, Robinson GE (1993) Selective neuroanatomical plasticity and division of labour in the honeybee. Nature 364:238–240 [DOI] [PubMed] [Google Scholar]
- Wolschin F, Münch D, Amdam GV. (2009) Structural and proteomic analyses reveal regional brain differences during honeybee aging. J Exp Biol 212:4027–4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner BA, Lu T, Loerch P (2008) The aging brain. Annu Rev Pathol 3:41–66 [DOI] [PubMed] [Google Scholar]
- Youssef K, Tandon A, Rezai P (2019) Studying Parkinson’s disease using Caenorhabditis elegans models in microfluidic devices. Integr Biol (Camb) 11:186–207 [DOI] [PubMed] [Google Scholar]
- Zahn JM, Poosala S, Owen AB, et al. (2007) AGEMAP: A gene expression database for aging in mice. PLoS Genet 3:2326–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]


