Abstract
More Americans are using marijuana than in previous decades but there are concerns over its long-term impact on cognitive functioning, especially memory. The literature on marijuana use and cognitive functioning is mixed, with some studies showing recovery of functioning upon abstinence from the drug and others showing long-term effects that persist. The latter seems especially true for individuals who initiate marijuana at a younger age and engage in more chronic patterns of use. The goal of the current study is to use prospectively collected data on young adults from a prenatal cohort to determine if there is an effect of early and/or current marijuana use on young adult memory, controlling for prenatal exposure to marijuana use, childhood memory deficits, and other significant covariates of memory functioning. At the 22-year follow-up phase of the Maternal Health Practices and Child Development (MHPCD) study, 524 young adults (58% Black, 42% White, 52% female) completed the Wechsler Memory Scale-III. Multiple regression analyses and structural equation modeling were used to determine the effect of marijuana exposure during gestation, early adolescence, and young adulthood on young adult memory function. Results indicated that initiating marijuana use before age 15 placed young adults at greater risk of memory deficits, even after controlling for childhood memory and current marijuana use. First trimester marijuana exposure also indirectly predicted young adult memory function via childhood memory deficits and early initiation of marijuana. These findings highlight the risk of prenatal marijuana exposure and early initiation of marijuana for long-term memory function in adulthood.
Keywords: marijuana, cannabis, adolescent, young adult, prenatal marijuana, learning and memory, neuropsychology
1. Introduction
Identifying the risks of marijuana use is of critical importance given current trends that show steady or slightly increasing rates of use among adolescents (Cerda et al., 2019; Johnston et al., 2019) and increasing rates of use in adults (Cerda et al., 2019; Schulenberg et al., 2019) and pregnant women (Agrawal et al., 2019; Volkow et al., 2019). In addition, increased frequency of use is predicted by increased availability of the drug through legalization and/or decriminalization for medicinal and recreational purposes in some jurisdictions, widely accepted use of marijuana, and a decrease in the perceived risk of marijuana use (Lipari, 2013; Parker & Anthony, 2018; Salloum, Krauss, Agrawal, Bierut & Grucza, 2018; Schmidt, Jacobs, Vlahov & Spetz, 2019; Schuermeyer et al., 2014; Wen, Hockenberry & Druss, 2019). The latest data from the Monitoring the Future study indicate that over 40% of college students and college-aged youth have used marijuana in the past year (Schulenberg et al., 2019), highlighting how ubiquitous marijuana use has become among young adults in the US. One potential risk that has been of interest is marijuana’s effect on cognitive functioning.
Using a longitudinal study design, the overall aim of this study is to investigate the complex associations among multiple factors that may directly or indirectly be associated with marijuana use in adolescence and young adulthood and memory function in young adults. Research on the proximal relations between current marijuana use and memory function in young adults is mixed. Some studies show no associations in adults after abstinence for at least 72 hours (Scott et al., 2017; Tait, Mackinnon & Christensen, 2011). Other studies report that impairments in learning and memory are associated with chronic use and depend on factors including shorter abstinence duration (Fried, Watkinson & Gray, 2005; Scott, et al., 2018), a higher dose (Bolla, Brown, Eldreth, Tate & Cadet, 2002), increased frequency and duration of use (Meier et al., 2012), and earlier age of onset (Ganzer, Broening, Kraft, Sax & Thomasius, 2016; Gruber, Sagar, Dahlgren, Racine & Lukas, 2012; Pope et al., 2003). In addition, concurrent alcohol use exacerbates the impact of marijuana use on working memory (Winward, Hanson, Tapert & Brown, 2014). Thus, current alcohol use must also be taken into consideration.
In addition to proximal relations with adult memory, other important distal factors must be considered including 1) early marijuana initiation, 2) memory deficits before the onset of marijuana use, and 3) prenatal exposure to marijuana. Results also vary as a function of the type of memory assessed. One, while some studies report an association between early marijuana initiation and a decline in working memory function (Duperrouzel et al., 2019; Fried et al., 2005; Gorey, Kuhns, Smaragdi, Kroon & Cousijn, 2019) that does not resolve with a period of abstinence (Meier et al., 2012), others report that there were no associations with visuospatial memory (Gruber et al., 2012). In a study of prenatal drug exposures, Barthelemy et al. (2019) did not find an association between early initiation of use and repeated measures of learning and memory from childhood through young adulthood. Two, memory and cognitive deficits in childhood are associated with deficits in adulthood (Cohen, 1997; Wechsler, 1991). Further, there may be earlier cognitive deficits that predispose individuals to use marijuana in adolescence (Fried et al., 2005; Meier, Caspi, Danese, Fisher, Houts, Arseneault & Moffitt, 2018; Sloboda, Glantz & Tarter, 2012). Three, prenatal marijuana exposure (PME) is associated with effects on offspring development across several domains of development, and thus may be part of the pathway to deficits in adulthood. Findings from the Maternal Health Practices and Child Development (MHPCD) Project include detrimental effects of PME on child cognitive development (Day et al.,1994; Goldschmidt, Richardson, Willford & Day, 2008) and learning and memory (Richardson, Ryan, Willford, Day & Goldschmidt, 2002), as well as on early-onset marijuana use (Day, Goldschmidt & Thomas, 2006) and marijuana use in young adulthood (Sonon, Richardson, Cornelius, Kim & Day, 2015). These domains are related to memory functioning in adulthood, which may reflect an indirect effect of PME on adult memory. Some of these findings have also been reported by the Ottawa Prenatal Prospective Study (Fried, 2002; Fried, O’Connell & Watkinson, 1992; Porath & Fried, 2005), the only other prospective study of prenatal marijuana exposure on adult functioning.
The current study used a prospective design to disentangle the relations among marijuana exposures across developmental stages and cognitive ability. The MHPCD cohort has sufficient statistical power to adjust for many covariates assessed at prior phases, leading to more rigorous and reliable results. In addition, the MHPCD study oversampled for PME, ensuring that sufficient numbers of offspring had PME at varying levels to examine the direct and indirect effects of PME on adult memory function. The overall aim will be addressed by answering the following questions: 1) Is early marijuana initiation (< 15 years) related to adult memory? 2) Is current marijuana use related to adult memory? 3) Does any relation between early marijuana use and adult memory persist with control for current marijuana use? 4) Does any relation between early marijuana use and adult memory persist with control for earlier memory deficits (measured at age 10)? 5) What are the direct and indirect effects of PME, childhood cognitive and memory performance, and adolescent and young adult marijuana use on adult memory function?
2. Methods
2.1. Study design
Recruitment for the MHPCD Project occurred during the fourth or fifth month of pregnancy from 1982 to 1985. The women were recruited from the Magee-Womens Hospital prenatal clinic to study the effects of prenatal marijuana and alcohol exposure. The study selected two cohorts of women: The first cohort was created by selecting women who used marijuana at least two times/month, along with the next woman interviewed who used marijuana less than that amount or none at all. The second cohort was created by selecting women who drank at least three alcoholic drinks/week, along with the next woman who drank less than that amount or none at all. Women selected for the study were interviewed again in the seventh month of pregnancy and after delivery. The two cohorts were selected independently and with replacement so that a participant could be in one or both cohorts, with a 48% overlap between the two cohorts (Sonon et al., 2015): The two cohorts were combined for analysis. The combined birth sample was 763 live singleton infants. Women and their children were assessed at birth, 8 and 18 months, 3, 6, 10, 14, 16, and 22 years.
The current study is based on 524 subjects (69% of the birth cohort) who completed a memory assessment at the 22-year follow-up. Missing assessments included: 11 offspring who died; 3 who were adopted; 18 who were serving in the military or were in jail; 15 with handicaps, low cognitive functioning, or behavior that interfered with testing; 29 moved out of the area; 56 could not be located; 31 refused to participate; and 76 were interviewed by phone and therefore could not be tested. The attrition was unrelated to prenatal marijuana, alcohol, or tobacco exposure, maternal education, income, or race/ethnicity. The 22-year assessment was completed between 2006 and 2009.
2.2. Sample characteristics
The pregnant women recruited for this study were 18 years or older (mean age = 23 years, SD = 4). They were of lower socioeconomic status with a mean family income of $446/month (SD = $289) at recruitment. Thirty-one percent were married, 25% worked outside of the home, and on average they had 11.8 years of education (SD = 1.4). At delivery, 49% lived with a husband/boyfriend, 24% lived with parents/other adults, and 27% lived on their own.
The mean age of the young adult offspring was 22.7 years (SD = 0.6, range: 21–26). The sample consisted of 220 White (42%) and 304 Black (58%) participants and 249 men (48%) and 275 women (52%). Fourteen percent had less than 12 years of education, 39% completed high school or a GED, and 47% reported post-high school education; 26% were still attending school; 60% worked; and 4% were in the military. The mean personal income/month among those who worked was $1,113 (range: 88 – 5000). Twenty-seven percent lived with their partners and 36% had at least one child.
2.3. Measures
Prenatal substance use.
Women were interviewed at the end of each trimester of pregnancy and the average daily joints of marijuana and the average daily volume of alcohol use were calculated based on the pattern, quantity, and frequency of use. We have previously reported on the development and reliability of these measures (Robles & Day, 1989). Biological markers were not used to ascertain drug use as they would not have yielded this detailed dose and pattern information. All drugs containing THC (marijuana, hashish, and sinsemilla) were included in the calculation of marijuana use and all types of alcoholic beverages (liquor, beer, wine, beer, and wine coolers) were included in the calculation of alcohol use. Tobacco smoking was assessed as the number of packs smoked per day.
Marijuana age of initiation.
At the 14-, 16-, and 22-year phases, the offspring were asked how old they were when they first tried marijuana or weed during the previous year. The age of initiation was recorded only for those who had not initiated at a previous phase. To minimize recall bias, the earliest reported age of initiation was used in the analyses. Early initiation was defined as initiation prior to age 15.
Current offspring substance use.
Offspring substance use at 22 years was assessed using the questionnaire developed by the MHPCD for the mothers (Day & Robles, 1989). The offspring were asked, how often they smoked marijuana during the previous year, how many joints they usually smoked, how often they smoked more than their usual amount, and how often they smoked less. Parallel questions were used for alcohol use. These questions were used to calculate the average daily joints of marijuana and average daily drinks of alcohol, the variables used in the analyses.
In addition to the interview data, a urine sample was collected and analyzed for substance use. Δ-9-tetrahydrocannabinol (THC) was detected in five subjects (1%) who had reported no current marijuana use. By contrast, 98 cases (19%) with negative urine tests reported marijuana use over the past year. The analyses for current marijuana use were repeated with the above 5 cases excluded, and the results did not change.
A variable was constructed to compare the young adult’s recent marijuana use to their longer-term use over the past year. The dummy variable was defined as follows: positive THC and positive self-report (coded as 2); negative THC and positive self-report (coded as 1); and negative THC and negative self-report (coded as 0).
Stanford-Binet Intelligence Scale - Fourth Edition (SBIS-4, Thorndike, Hagen & Sattler, 1986).
Intellectual ability at 6 years was assessed using the SBIS-4. The Composite Score of the four subtests (Verbal Reasoning, Abstract Visual Reasoning, Quantitative Reasoning, Short-Term Memory) was used in this analysis. The SBIS-4 was administered by trained and reliable examiners who were masked to maternal substance use. The Composite Score is age-adjusted with an internal consistency reliability score of 0.96 (Thorndike et al., 1986).
Wide Range Assessment of Memory and Learning - Screening (WRAML-S, Sheslow & Adams, 1990).
Memory at 10 years was assessed using the WRAML-S. The test was administered by trained and reliable examiners who were masked to maternal substance use. The Summary Index score, which is based on four subscales (Picture Memory, Design Memory, Verbal Learning, Story Memory), is standardized to a mean of 100 and SD of 15 and was used in the analyses. The internal consistency reliability coefficients of the four subscales for 10-year-olds range from 0.78 to 0.88 (Sheslow & Adams, 1990).
Wechsler Memory Scale - Third Edition (WMS-III; Tulsky, Zhu & Ledbetter, 2002)
The WMS was administered at the 22-year follow-up by trained examiners who were masked to maternal substance use. Periodic reliability checks were conducted among examiners. Only the auditory scales were used to assess memory at this phase due to time constraints and our previous findings at 14 years (Willford, Richardson, Leech & Day, 2004). The Auditory Immediate Index, composed of the Logical Memory (LM) I and Verbal Paired Associates (VPA) I subtests, is an indication of short-term memory in the verbal auditory domain. The Auditory Delayed Index is composed of the LM II and VPA II subtests and is an indication of long-term memory in the verbal auditory domain. The Auditory Recognition Delayed Index is based on the recognition scores from the LM II and VPA II subtests. The index scores are age-corrected and standardized to a mean of 100 and a standard deviation of 15. For LM, the subject retells a story that was read to him/her. For VPA, the examiner reads a list of word pairs. The examiner then reads the first word of each pair and the subject is asked to recall the second word. The delayed tests occur approximately 30 minutes after the initial tests. We also administered Letter-Number Sequencing (LNS), a measure of auditory working memory, in which a series of numbers and letters are orally presented to the subject. They are then asked to repeat the numbers in ascending order and then the letters in alphabetical order. The internal consistency reliability coefficients of the Auditory Immediate, Auditory Delayed, and Auditory Recognition Delayed Indexes for 20- to 24-year-olds are 0.92, 0.85, and 0.78, respectively, and for the subtests range from 0.75 to 0.91 (Tulsky, Zhu, & Ledbetter, 2002).
2.4. Statistical Analysis
The distributions of the WMS-III outcome variables were screened to determine appropriate test statistics. Multiple regressions were then applied to test the significance of the relations between early marijuana onset and the outcome variables1 adjusting for covariates, such as prenatal exposures to alcohol, marijuana, and tobacco, maternal education, and offspring sex, race/ethnicity, and current alcohol use (Model 1). Although a detrimental relation was hypothesized with the exposure variables, conservative 2-tail significance levels were reported for the regression models. Covariates were retained in the model to avoid unnecessary inflation of the error if they were significant at an alpha level of ≤ 0.10 (2-tail). In the second step, offspring current marijuana use at 22 years (Model 2) and memory measured at age 10 (WRAML) (Model 3) were each separately added to the model to determine if they affected the relation between early marijuana initiation and young adult memory. Tolerance, a measure of multicollinearity (reciprocal to variance inflation factor), was screened to examine the stability of the regression coefficients because these variables are correlated with early marijuana use. All the tolerances were above 0.8, which indicated that the intercorrelations among the predictors were small and not of concern (Myers,1990). Residual diagnostics were performed to test regression assumptions and identify influential cases. Three influential points were found: one with LNS, one with Auditory Delayed, and one with LM II. The results presented here exclude those cases.
Finally, a structural equation model (SEM) was applied to the Auditory Immediate and Auditory Delayed Indexes to test the indirect impact of first trimester marijuana exposure on memory at 22 years through the SBIS Composite Score at age 6, WRAML-S Index at age 10, and early initiation of marijuana use. These variables were selected as mediators because we have previously shown that they were significantly associated with PME (Day et al., 2006; Goldschmidt et al., 2008; Richardson et al., 2002). For the SEM analyses, PME was dichotomized to 1 or more joints/day during the first trimester versus all others.
3. Results
In this cohort, 40% of the subjects reported using marijuana before the age of 15. As shown in Table 1, early initiators scored significantly lower on WMS Auditory Immediate (4.3 points difference, p < 0.01), Auditory Delayed (2.8 points, p < 0.05), LM I and II (1.0, 0.9 points, respectively, p < 0.001), VPA I (0.5 points, p < 0.05), and LNS (0.6 points, p < 0.01) than those who did not initiate prior to 15 years. Early marijuana initiation was also significantly related to more offspring marijuana use at 22 years, lower SBIS composite score at age 6, lower first trimester maternal education, and higher first trimester marijuana and tobacco exposures (Table 1).
Table 1.
Bivariate associations between WMS memory scales and covariates and early marijuana initiation and offspring 22-year marijuana use
| Variables | Early marijuana initiationa Yes (n=209), No (n=315) |
p value (2-tail) | Offspring 22-year marijuana use Yes (n=271), No (n=253) |
p value (2-tail) | ||
|---|---|---|---|---|---|---|
| 22-year Wechsler Memory Scale-III | ||||||
| Auditory Immediate Indexb | 92.7 (15.1) | 97.0 (14.3) | < 0.01 | 93.7 (13.9) | 97.0 (15.8) | < 0.05 |
| Auditory Delayed Indexb | 97.0 (12.2) | 99.8 (14.5) | < 0.05 | 98.0 (12.8) | 99.5 (14.6) | ns |
| Auditory Recognition Delayed Indexb | 97.9 (14.2) | 99.2 (13.9) | Ns | 97.5 (14.3) | 99.9 (13.6) | < 0.05 |
| Logical Memory Ic | 8.2 (2.8) | 9.2 (3.1) | < 0.001 | 8.4 (2.9) | 9.3 (3.0) | < 0.001 |
| Logical Memory IIc | 8.9 (2.9) | 9.8 (3.2) | < 0.001 | 9.1 (3.0) | 9.8 (3.2) | < 0.01 |
| Verbal Paired Associates Ic | 9.3 (2.9) | 9.8 (3.0) | < 0.05 | 9.5 (2.8) | 9.8 (3.2) | ns |
| Verbal Paired Associates IIc | 10.1 (2.4) | 10.2 (2.7) | Ns | 10.3 (2.3) | 10.1 (2.8) | Ns |
| Letter-Number Sequencingc | 8.9 (2.6) | 9.5 (2.7) | < 0.01 | 9.1 (2.8) | 9.4 (2.6) | Ns |
| Other variables considered | ||||||
| Stanford-Binet Composite Score at 6 yearsb | 89.8 (12.7) | 93.1 (14.6) | < 0.01 | 89.9 (14.2) | 93.9 (13.4) | < 0.01 |
| WRAML Screening Index at 10 yearsb | 87.2 (12.5) | 89.6 (14.7) | < 0.10 | 88.0 (13.6) | 89.4 (14.3) | Ns |
| Offspring race/ethnicity (% White) | 43.1 | 41.3 | ns | 36.5 | 47.8 | < 0.01 |
| Sex (% male) | 49.8 | 46.0 | ns | 51.7 | 43.1 | < 0.05 |
| Early-onset marijuana use (%) | --- | --- | --- | 53.1 | 25.7 | < 0.001 |
| Offspring age at current assessment | 22.8 (0.7) | 22.9 (0.7) | ns | 22.7 (0.6) | 22.8 (0.6) | Ns |
| Offspring 22-year marijuana use (joints/day)d | 1.5 (2.7) | 0.5 (1.5) | < 0.001 | --- | --- | --- |
| Offspring 22-year alcohol use (drinks/day)d | 2.4 (3.0) | 1.3 (2.1) | < 0.001 | 2.3 (2.9) | 1.1 (1.8) | < 0.001 |
| 1st trimester maternal education (years) | 11.6 (1.3) | 12.0 (1.3) | < 0.01 | 11.7 (1.3) | 11.9 (1.4) | < 0.05 |
| 1st trimester marijuana use (joints/day)d | 0.58 (1.2) | 0.29 (0.7) | < 0.05 | 0.50 (1.1) | 0.29 (0.8) | < 0.05 |
| 1st trimester marijuana ≥1 joint/day (% yes) | 19.6 | 9.8 | < 0.01 | 16.6 | 10.7 | < 0.05 |
| 1st trimester alcohol use (drinks/day) | 0.61 (1.1) | 0.54 (1.1) | ns | 0.70 (1.4) | 0.44 (0.8) | < 0.05 |
| 1st trimester cigarette use (cigarettes/day) | 9.8 (12) | 7.4 (11) | < 0.05 | 8.8 (11.9) | 7.8 (11.1) | Ns |
Prior to 15 years of age
Standardized Mean = 100, SD = 15
Standardized Mean = 10, SD = 3
Significance based on Mann-Whitney rank-sum test
By 22 years, 84% of the offspring had used marijuana in their lifetime; 52% reported use within the past year. Among current users, 37% smoked at least 1 joint/day. As shown in Table 1, current marijuana users scored significantly lower on WMS Auditory Immediate (3.3 points difference, p < 0.05), Auditory Recognition Delayed (2.4 points, p < 0.05), and LM I and II (0.9, p < .001; 0.7 points, p < 0.01, respectively) than those who did not currently use. Current marijuana use was also significantly related to early-onset marijuana use, lower SBIS composite score at age 6, being Black race, male sex, lower first trimester maternal education, and higher first trimester marijuana and alcohol exposures (Table 1).
Table 2 shows the correlations among the WMS-III scores and variables used in the regression analyses. Maternal education and offspring race/ethnicity and sex were significantly correlated with WMS-III scores. Early marijuana use was generally more correlated with WMS-III scores than was current marijuana use.
Table 2.
Correlations among 22-year Wechsler Memory Scale-III scores and variables used in regression modelsa (N = 524)
| Auditory Immediate | Auditory Delayed | Auditory Recognition Delayed | Logical Memory I | Logical Memory II | Verbal Paired Associates I | Verbal Paired Associates II | Letter-Number Sequencing | |
|---|---|---|---|---|---|---|---|---|
| 1st trimester alcohol (drinks/day) | −0.05 | −0.07 | −0.01 | −0.07 | −0.06 | −0.03 | −0.04 | −0.01 |
| 1st trimester tobacco (cigarettes/day) | 0.02 | 0.001 | 0.11 | 0.03 | 0.02 | 0.002 | −0.01 | 0.04 |
| 1st trimester marijuana (joints/day) | −0.04 | −0.04 | −0.05 | −0.02 | −0.04 | −0.05 | −0.01 | 0.02 |
| 1st trimester maternal education | 0.13 | 0.12 | 0.01 | 0.09 | 0.09 | 0.12 | 0.09 | 0.11 |
| Offspring race/ethnicityb | 0.22 | 0.19 | 0.21 | 0.19 | 0.17 | 0.19 | 0.14 | 0.16 |
| Sexc | −0.10 | −0.10 | −0.14 | −0.12 | −0.14 | −0.05 | −0.02 | 0.05 |
| Offspring age at current assessment | −0.09 | −0.09 | −0.06 | −0.09 | −0.06 | −0.07 | −0.09 | −0.05 |
| Offspring 22-year alcohol (drinks/day) | 0.04 | 0.04 | 0.03 | 0.03 | 0.02 | 0.04 | 0.05 | 0.17 |
| Offspring 22-year marijuana (joints/day) | −0.13 | −0.08 | −0.07 | −0.15 | −0.10 | −0.07 | −0.03 | −0.01 |
| Early marijuana used | −0.14 | −0.10 | −0.04 | −0.15 | −0.15 | −0.09 | −0.02 | −0.13 |
| Stanford-Binet Composite (6 years) | 0.46 | 0.41 | 0.40 | 0.42 | 0.40 | 0.38 | 0.28 | 0.48 |
| WRAML Screening Index (10 years) | 0.53 | 0.49 | 0.44 | 0.51 | 0.50 | 0.41 | 0.30 | 0.42 |
Correlations indicated in bold are significant at p < .05 (2-tail)
0 = Black; 1 = White
0 = female; 1 = male
0 = not early; 1 = early < 15 years
Regression analyses were applied to test whether the significant relations between early marijuana use and 22-year memory function remained significant controlling for sex, race/ethnicity, maternal education, and prenatal exposure to marijuana, alcohol, and tobacco (Table 3, Model 1). Early marijuana onset was a significant predictor of Auditory Immediate and Auditory Delayed Indexes, LM I and II, and LNS, similar to the bivariate analyses. Among the covariates, race/ethnicity, sex, and maternal education were significantly related to WMS-III scores (Table 3). Prenatal alcohol exposure was significantly related to LM I. Prenatal marijuana and tobacco exposure were not significant predictors of the WMS-III scores, after controlling for other covariates.
Table 3.
Multivariate regression analyses of early marijuana use and memory function at age 22 yearsa (N = 524)
| WMS-III scales | Variables | Regression Coefficientb | Standardized Coefficient | t ratio (coef./SE) | p value (2-tail) | R2 |
|---|---|---|---|---|---|---|
| Auditory Immediate | MODEL 1c | |||||
| Offspring race/ethnicityd | 7.4 | 0.24 | 5.81 | < 0.001 | 0.10 | |
| Maternal education | 1.6 | 0.14 | 3.39 | < 0.001 | ||
| Sexe | −3.8 | −0.13 | −3.02 | < 0.001 | ||
| 1st trimester alcohol (log transformed) | −2.3 | −0.07 | −1.67 | = 0.10 | ||
| Early marijuana use | −3.5 | −0.12 | −2.75 | < 0.01 | ||
| MODEL 2: Adding current use | ||||||
| Early marijuana use | −3.2 | −0.10 | −2.34 | =0.02 | ||
| MODEL 3: Adding memory at 10 | ||||||
| Early marijuana use | −2.5 | −0.08 | −2.14 | =0.03 | ||
| Auditory Delayed | MODEL 1 | |||||
| Race/ethnicity | 6.0 | 0.22 | 5.18 | < 0.001 | 0.08 | |
| Maternal education | 1.4 | 0.14 | 3.29 | < 0.001 | ||
| Sex | −3.2 | −0.12 | −2.78 | < 0.01 | ||
| Early marijuana use | −2.5 | −0.09 | −2.08 | = 0.04 | ||
| MODEL 2: Adding current use | ||||||
| Early marijuana use | −2.5 | −0.09 | −1.96 | = 0.05 | ||
| MODEL 3: Adding memory at 10 | ||||||
| Early marijuana use | −2.0 | −0.07 | −1.79 | =0.07 | ||
| Logical Memory I | MODEL 1 | |||||
| Race/ethnicity | 1.3 | 0.22 | 5.20 | < 0.001 | 0.10 | |
| Sex | −0.9 | −0.15 | −3.42 | < 0.001 | ||
| Maternal education | 0.2 | 0.11 | 2.58 | < 0.01 | ||
| 1st trimester alcohol (log transformed) | −0.6 | −0.08 | −1.97 | = 0.05 | ||
| Early marijuana use | −0.8 | −0.13 | −3.18 | < 0.001 | ||
| MODEL 2: Adding current use | ||||||
| Early marijuana use | −0.7 | −0.11 | −2.70 | < 0.01 | ||
| MODEL 3: Adding memory at 10 | ||||||
| Early marijuana use | −0.6 | −0.10 | −2.62 | < 0.01 | ||
| Logical Memory II | MODEL 1 | |||||
| Race/ethnicity | 1.3 | 0.20 | 4.78 | < 0.001 | 0.09 | |
| Sex | −1.0 | −0.16 | −3.74 | < 0.001 | ||
| Maternal education | 0.3 | 0.13 | 3.02 | < 0.001 | ||
| Early marijuana use | −0.9 | −0.14 | −3.23 | < 0.001 | ||
| MODEL 2: Adding current use | ||||||
| Early marijuana use | −0.8 | −0.13 | −3.04 | < 0.001 | ||
| MODEL 3: Adding memory at 10 | ||||||
| Early marijuana use | −0.7 | −0.11 | −2.67 | < 0.01 | ||
| Verbal Paired Associates I | MODEL 1 | |||||
| Race/ethnicity | 1.2 | 0.20 | 4.56 | < 0.001 | 0.06 | |
| Maternal education | 0.3 | 0.12 | 2.83 | < 0.001 | ||
| Sex | −0.5 | −0.08 | −1.74 | =0.08 | ||
| Early marijuana use | −0.5 | −0.08 | −1.90 | =0.06 | ||
| MODEL 2: Adding current use | ||||||
| Early marijuana use | −0.5 | −0.08 | −1.69 | =0.09 | ||
| MODEL 3: Adding memory at 10 | ||||||
| Early marijuana use | −0.3 | −0.05 | −1.17 | NS=0.2 | ||
| Letter-number sequencing | MODEL 1 | |||||
| Race/ethnicity | 0.9 | 0.17 | 4.00 | < 0.001 | 0.05 | |
| Maternal education | 0.2 | 0.11 | 2.53 | =0.01 | ||
| Early marijuana use | −0.6 | −0.11 | −2.60 | =0.01 | ||
| MODEL 2: Adding current use | ||||||
| Early marijuana use | −0.7 | −0.13 | −2.95 | < 0.001 | ||
| MODEL 3: Adding memory at 10 | ||||||
| Early marijuana use | −0.4 | −0.08 | −1.77 | = 0.08 | ||
Results are presented only for those outcomes where early marijuana use was significant.
Regression coefficient represents the magnitude of relation per unit change.
Model 1 includes prenatal exposure to alcohol, marijuana, and tobacco, maternal education, and offspring sex and race/ethnicity. Model 2 adds offspring 22-year marijuana use (for ease of presentation, only the change in the early marijuana use variable is shown). Model 3 adds WRAML screen at age 10 (for ease of presentation, only the change in the early marijuana use variable is shown).
0 = Black, 1 = White
0 = female, 1 = male
As shown in Table 2, the correlations between early marijuana use and WMS-III scores were generally higher than the correlations between offspring 22-year marijuana use and WMS. Thus, in the regression analyses, early use remained a significant predictor of Auditory Immediate, Auditory Delayed, LM I and II, and LNS, controlling for current offspring marijuana use (Table 3, Model 2). We also re-ran Model 2 with the dummy variable defined previously. There was no significant relation with this categorized variable and WMS scores and the significance of early marijuana use remained the same.
In addition to regression analyses, we applied two-way ANCOVA with offspring current use and early initiation as the grouping factors. The deficit in the WMS scores was associated with early initiation. For example, the adjusted means of the Auditory Immediate Index were 97.4, 95.7, 92.5, and 93.5, for the four groups (no early initiation and no current use; no early initiation and current use; early use and no current use; early use and current use), respectively. The early initiation by current use interaction was not statistically significant. The results were similar with current marijuana use dichotomized to 1 or more joints/day.
When the WRAML-S memory scale at age 10 was added to the model, early marijuana use continued to be significantly related to Auditory Immediate and LM I and II (Table 3, Model 3). For Auditory Delayed and LNS, the relation with early initiation was marginally significant (2-tail p < 0.10).
Forty-one percent of the mothers reported marijuana use during the first trimester of pregnancy, 14% reported daily use. Of the women who used in the first trimester, 23% and 19% continued to use during the second and third trimesters, respectively. Only 12 women reported smoking marijuana later in pregnancy who had not used during the first trimester. The indirect effects of first trimester PME on the WMS are shown in Figure 1. The paths are marked with the standardized coefficients to depict the magnitude of each path compared to others. The parameters were estimated using the Mplus statistical package (Muthén & Muthén, 1998–2017). The significance of each indirect effect was calculated using Sobel’s test. The indirect effect is based on the product of coefficients from PME to the mediator and from the mediator to the outcome variable. For the Auditory Immediate Index, the coefficients of indirect effects of PME were significant via 6-year SBIS-4 Composite Score (−1.4 raw score, 2-tailed p = 0.006), 10-year WRAML-S (−2.2 raw score, 2-tailed p = 0.004), and early marijuana use (−1.0 raw score, 2-tailed p = 0.05). For the Auditory Delayed Index, the indirect effect coefficients of PME were significant or marginally significant via 6-year SBIS-4 Composite Score (−1.2 raw score, 2-tailed p=0.009), 10-year WRAML-S (−1.9 raw score, 2-tailed p=0.005), and early marijuana use (−0.7 raw score, 2-tailed p=0.10), respectively. There was a significant relation between PME and current use when current offspring marijuana use was added to the SEM. However, current marijuana use was not related to the WMS scores, the indirect pathway via current use was not significant, and none of the other indirect pathways changed.
Figure 1:
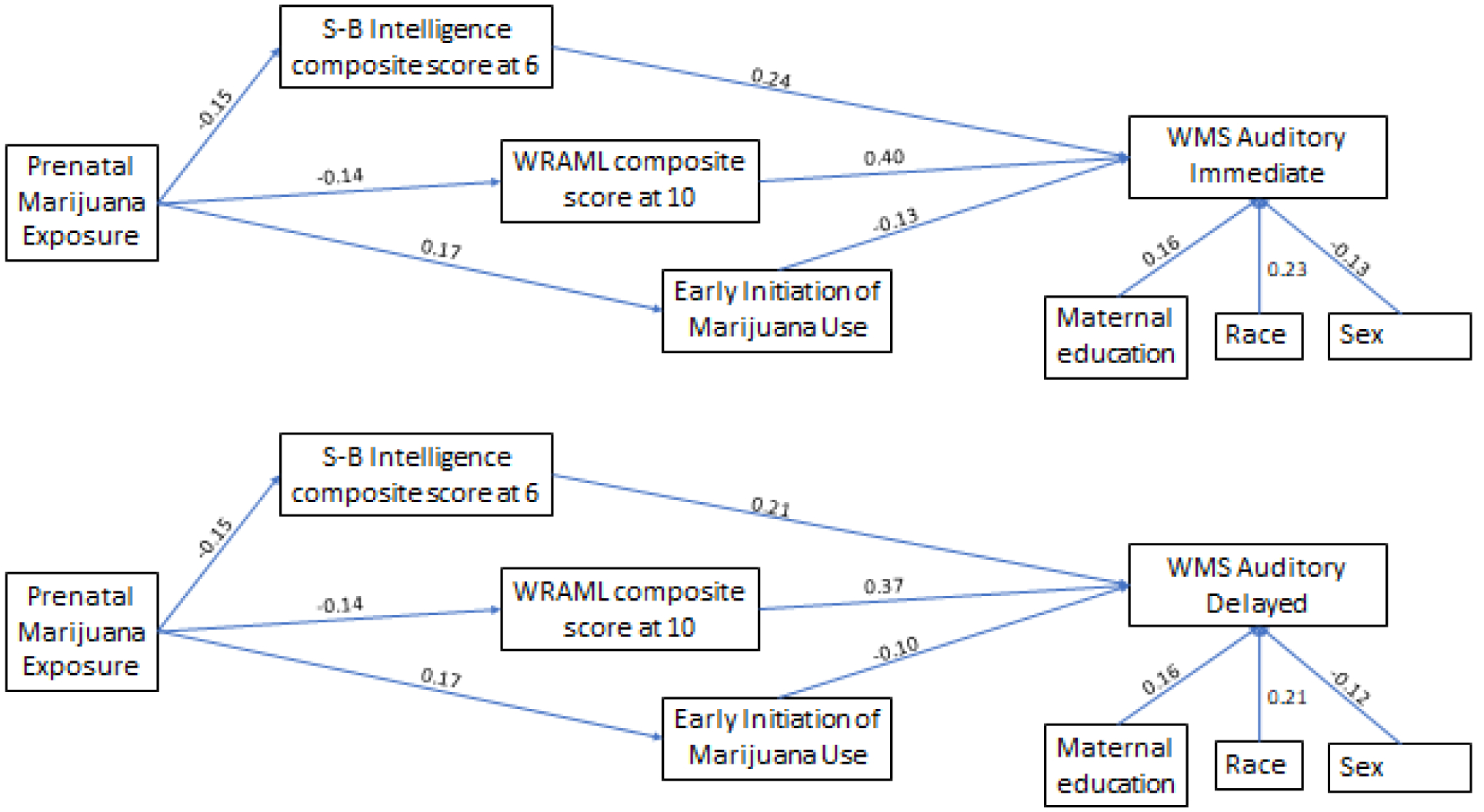
Path analysis model showing indirect association between prenatal marijuana exposure and Wechsler Memory Scale via intelligence score at age 6, memory scale at age 10, and initiation of marijuana by age 15. The significant paths are marked with standardized coefficients (p < .05).
4. Discussion
The purpose of this study was to understand the complex associations between marijuana use in adolescence and young adulthood and memory function in young adults. There were three main findings: 1) early-onset of marijuana use was associated with adult memory, and this relation generally persisted with control for current marijuana use and childhood memory performance; 2) current marijuana use was associated in bivariate analyses with adult memory; however, this relation did not remain significant with control for early marijuana initiation; and 3) there were indirect effects of prenatal marijuana exposure on adult memory through intelligence at age 6, memory at 10 years, and early-onset of marijuana use. We also found that the effects on memory function in young adulthood were consistent across domains of auditory-verbal memory function.
First, early-onset of marijuana use was associated with adult memory function, with control for current marijuana use and childhood memory function, as well as for other predictors of adult memory such as race/ethnicity and sex. We found relations with early marijuana onset and young adult memory for both types of memory tests (logical memory and verbal-paired associates), at both the immediate (short-term) and delayed (long-term, logical memory only) testing phases, and on performance on the Letter-Number Sequencing subtest, a measure of auditory working memory. Further, the results were significant for both of the global indices of memory function, Auditory Immediate and Auditory Delayed. Therefore, there was a consistency in results showing that early initiation of marijuana use is associated with deficits in auditory-verbal memory in young adults.
Multiple studies have reported similar associations between early-onset of marijuana use and adult memory, but the results are mixed due to variations in study methods (Hanson, Winward, Schweinsburg, Medina, Brown & Tapert, 2010; McHale & Hunt, 2008; Schoeler & Bhattacharyya, 2013; Schuster, Hoeppner, Evins & Gilman, 2016; Solowij et al., 2011). Of studies that indicate an association between early initiation of marijuana use and memory function, the memory impairments appear to persist; up to six weeks after cessation in one study (Tapert, Schweinsburg & Brown, 2008) and up to two years with continued use in another (Becker, Collins, Schultz, Urošević, Schmaling & Luciana, 2018). Meier et al. (2012) showed that persistent marijuana use was associated with a neuropsychological decline across domains of function using a longitudinal study design. The impairment among adolescent-onset marijuana users was dose-dependent, and abstinence did not fully restore function.
The effects of marijuana exposure on memory function in early initiators compared to young adult use may, in part, be due to important structural and functional changes that occur in the brain during adolescence. Marijuana use that is initiated in adolescence corresponds with an important stage of neurodevelopment characterized by synaptic pruning of gray matter and increases in white matter density and myelination that parallel development of peak levels of cognitive and executive functions (Casey, Giedd & Thomas, 2000; Luna et al., 2001). Furthermore, the endocannabinoid system is critical to the development of efficient neural circuitry during this stage of brain development through its regulation of the balance between excitatory and inhibitory neurotransmission (Dow-Edwards & Silva, 2017; Meyer, Lee & Gee, 2018). Brain regions including the hippocampus, basal ganglia, and prefrontal cortex, which are implicated in neuropsychological function, are rich in cannabinoid receptors and influenced by their activation (Mackie, 2005). The effect of marijuana on brain development in adolescence may, in part, explain memory deficits that emerge with use during this developmental stage. For example, functional neuroimaging studies have shown decreased performance on complex working memory tasks in adolescents who have used marijuana but are then abstinent, suggesting a long-term effect of the drug exposure (Hanson et al., 2010; Jager, Block, Luijten & Ramsey, 2010). Thus, it is important to consider the impact of marijuana use on neurobehavioral function in adolescence.
The impact of early-onset of marijuana use in adolescence in humans is further clarified and corroborated by animal models. While animal models are limited in their application to understanding complex associations in humans, the results of a recent preclinical primate study found that adolescent exposure to THC affects working memory in rhesus macaque monkeys (Verrico, Mathai, Gu, Sampson & Lewis, 2014). As would be predicted in humans, THC exposure over a 12-month period was associated with slower increases in working memory performance for both simple and complex tasks. Performance was recovered, however; monkeys who continued to train on those cognitive tasks were eventually able to perform them. Importantly, these results showed a reinforcement-associated learning impairment. Human adolescents are not required to remain engaged in the same cognitive tasks until mastery and, therefore, marijuana-induced learning and memory deficits may be more persistent. This has serious implications for educational achievement among adolescents who use marijuana.
Second, we found that current marijuana use was not associated with verbal memory function in young adults when early initiation of marijuana use was controlled statistically. Similarly, previous research has shown that marijuana use in young adults is not consistently associated with memory, regardless of the domain of function (e.g., verbal or visual-spatial) or assessment type (neuropsychological testing or cognitive paradigms). Among adult heavy marijuana users, acute exposure to the drug is associated with neurocognitive impairment for several days, but disappears after a period of abstinence, whereas the deficits are detectable for longer in adolescent users (Tapert et al., 2008). It should be noted, however, that past studies of the long-term effects of marijuana on memory have had difficulty dissociating the chronic effects from the residual effects of an acute dose of marijuana on memory function. It is also possible that, with long-term use, behavioral compensatory mechanisms develop in the context of chronic marijuana exposure. Finally, most studies of marijuana effects on memory in young adults have not considered the potentially important antecedents such as prenatal marijuana exposure, pre-existing memory deficits, and early initiation of marijuana use that are important in understanding the complex associations between marijuana and memory function.
Third, the SEM analyses showed that while there was no direct effect of prenatal marijuana exposure on adult memory, there were indirect associations through childhood intelligence and memory and early-onset of marijuana use. These findings are consistent with earlier reports of associations with prenatal marijuana exposure and childhood cognition and memory, as well as with early marijuana initiation (Day et al., 1994; Day et al., 2006; Fried, 2002; Fried et al.,1992; Goldschmidt et al., 2008; Porath & Fried, 2005; Richardson et al., 2002). Notably, this study did not show a significant association between maternal smoking or alcohol and memory function. The MHPCD has previously reported the effects of prenatal tobacco exposure on verbal learning and design memory in children at age 10 (Cornelius, Ryan, Day, Goldschmidt, & Willford, 2001) and the effects of prenatal alcohol on verbal learning and memory in adolescents at age 14 (Willford et al., 2004). The lack of effect of prenatal tobacco or alcohol on young adult memory in the MHPCD could be explained, in part, by one or more of the following: there is no effect of prenatal tobacco or alcohol exposure on memory function in young adults; an inability to detect subtle changes in memory due to elements of study design or characteristics of the MHPCD cohorts; or “catch up” in the development of cognitive functions in which deficits related to prenatal tobacco or alcohol exposure were reported at younger ages. The premorbid differences in memory function associated with prenatal marijuana exposure may precede the onset of marijuana use and increase the risk for early initiation, exacerbate the effects of marijuana exposure on memory function once use is initiated, or both.
There are important strengths of the current study. The longitudinal study design allowed for an assessment of time, that is, the degree to which earlier developmental effects such as prenatal marijuana exposure, memory function during childhood, and the timing of marijuana initiation were important factors in understanding the association between marijuana use and memory function in young adults. The study also included detailed assessments of marijuana use during each trimester of pregnancy and each additional follow-up phase, a comprehensive neuropsychological evaluation across childhood, adolescence and young adulthood, and control for sociodemographic, environmental, and psychological variables that are associated with memory function, including current alcohol use, sex, race/ethnicity, and IQ. Further, the study sample was large, had a balanced representation of Black and White participants, and follow-up rates across assessment phases were excellent. In addition, early marijuana initiation was defined as < 15 years, whereas others used definitions of < 16 or < 17 years of age. With the trend of using marijuana at a younger age, we are one of the few studies using an earlier age of onset in the analyses. The strengths of this study support the validity of the reported results.
There are study limitations that must be considered. First, this was a low-income sample selected for prenatal drug exposure and thus may not be generalizable to other samples. Second, short- and long-term visual-spatial memory was not assessed due to time constraints. Third, we did not conduct a biological assessment of prenatal drug exposure. However, in another one of our cohorts, we compared our self-report results to those conducted by the hospital. In the cohort analyzed here, we conducted urine screens at 22 years to compare to the interview data. In both cases, we found better detection by self-report than by urine screens (Richardson, Huestis & Day, 2006; Sonon et al., 2015). Lastly, there are potential variables that were not measured (e.g., current IQ) or that were measured but not analyzed (e.g., peer drug use) that could be important predictors of adult memory function.
In summary, we found that early-onset of marijuana exposure was consistently associated with memory impairment in young adults, with control for earlier developmental influences such as intelligence, memory, and sociodemographic characteristics. Current marijuana use in young adults was not associated with deficits in memory function once the early initiation of marijuana was taken into account. We also found indirect effects of prenatal marijuana use on adult memory through pre-existing memory deficits and early initiation of marijuana. These results indicate that the association between marijuana use in young adults and memory function is complex and must be considered in the context of other important developmental variables.
Highlights:
Longitudinal study of the impact of marijuana on adult memory function.
Prenatal, early onset, and young adult marijuana use were examined.
Early onset marijuana use predicted increased memory deficits in young adulthood.
First trimester marijuana exposure indirectly predicted young adult memory.
Prenatal, adolescent, and adult marijuana exposure are each associated with memory.
Acknowledgements
This study was supported by the National Institute on Drug Abuse DA03874 and National Institute on Alcoholism and Alcohol Abuse AA06666 (N. Day, Principal Investigator).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The one exception was the VPA II variable, which was highly skewed. Therefore, this variable was dichotomized to above and below the median score and a logistic regression was used.
References
- Agrawal A, Rogers CE, Lessov-Schlaggar CN, Carter EB, Lenze SN, Grucza RA 2019. Alcohol, cigarette, and cannabis use between 2002 and 2016 in pregnant women from a nationally representative sample. JAMA Pediatr. 173 (1), 95–96. doi: 10.1001/jamapediatrics.2018.3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthelemy OJ, Richardson MA, Heeren TC, Chen CA, Liebschutz JM, Forman LS, … Rose-Jacobs R 2019. Do differences in learning performance precede or follow initiation of marijuana use? J Stud Alcohol Drugs. 80 (1), 5–14. doi: 10.15288/jsad.2019.80.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MP, Collins PF, Schultz A, Urošević S, Schmaling B, Luciana M 2018. Longitudinal changes in cognition in young adult cannabis users. J Clin Exp Neuropsychol. 40 (6), 529–543. doi: 10.1080/13803395.2017.1385729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL 2002. Dose-related neurocognitive effects of marijuana use. Neurology. 59 (9), 1337–1343. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM 2000. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 54 (1–3), 241–257. doi: 10.1016/S0301-051100)00058-2 [DOI] [PubMed] [Google Scholar]
- Cerdá M, Mauro C, Hamilton A, Levy NS, Santaella-Tenorio J, Hasin D, … Martins SS 2020. Association between recreational marijuana legalization in the United States and changes in marijuana use and cannabis use disorder from 2008 to 2016. JAMA Psychiatry. 77 (2), 165–171. doi: 10.1001/jamapsychiatry.2019.3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MJ 1997. Children’s Memory Scale Manual. The Psychological Corporation, Harcourt Brace and Company, San Antonio, TX [Google Scholar]
- Cornelius MD, Ryan CM, Day NL, Goldschmidt L, & Willford JA 2001. Prenatal tobacco effects on neuropsychological outcomes among preadolescents. J Dev Behav Pediatr. 22 (4), 217–225.doi: 10.1097/00004703-200108000-00002 [DOI] [PubMed] [Google Scholar]
- Day NL, Goldschmidt L, Thomas CA 2006. Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction. 101 (9), 1313–1322. doi: 10.1111/j.1360-0443.2006.01523.x [DOI] [PubMed] [Google Scholar]
- Day NL, Richardson GA, Goldschmidt L, Robles NPMT, Taylor PM, Stoffer DS, … Geva D 1994. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol Teratol. 16 (2), 169–175. doi: 10.1016/0892-036294)90114-7 [DOI] [PubMed] [Google Scholar]
- Day N, Robles N 1989. Methodological issues in the measurement of substance use: prenatal abuse of licit and illicit drugs. Ann NY Acad Sci. 562, 8–13. doi: 10.1111/j.1749-6632.1989.tb21002.x [DOI] [PubMed] [Google Scholar]
- Dow-Edwards D, Silva L 2017. Endocannabinoids in brain plasticity: cortical maturation, HPA axis function and behavior. Brain Research. 1654, 157–164. doi: 10.1016/j.brainres.2016.08.037 [DOI] [PubMed] [Google Scholar]
- Duperrouzel JC, Hawes SW, Lopez-Quintero C, Pacheco-Colón I, Coxe S, Hayes T, Gonzalez R 2019. Adolescent cannabis use and its associations with decision-making and episodic memory: Preliminary results from a longitudinal study. Neuropsychology. 3 (5), 701–710. doi: 10.1037/neu0000538 [DOI] [PubMed] [Google Scholar]
- Fried PA 2002. Adolescents prenatally exposed to marijuana: examination of facets of complex behaviors and comparisons with the influence of in utero cigarettes. J Clin Pharmacol. 42 (S1), 97S–102S. doi: 10.1002/j.1552-4604.2002.tb06009.x [DOI] [PubMed] [Google Scholar]
- Fried PA, O’Connell CM, Watkinson B 1992. 60-and 72-month follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol: Cognitive and language assessment. J Dev Behav Pediatr. 13 (6), 383–391.doi: 10.1097/00004703-199212000-00001 [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R 2005. Neurocognitive consequences of marihuana—a comparison with pre-drug performance. Neurotoxicol Teratol. 27 (2), 231–239. doi: 10.1016/j.ntt.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Ganzer F, Broening S, Kraft S, Sack PM, Thomasius R 2016. Weighing the evidence: A systematic review on long-term neurocognitive effects of cannabis use in abstinent adolescents and adults. Neuropsychol Rev. 26 (2), 186–222. doi: 10.1007/s11065-016-9316-2 [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Day NL, Richardson GA 2000. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 22 (3), 325–336. doi: 10.1016/S0892-0362(00)00066-0 [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Richardson GA, Cornelius MD, Day NL 2004. Prenatal marijuana and alcohol exposure and academic achievement at age 10. Neurotoxicol Teratol. 26 (4), 521–532. doi: 10.1016/j.ntt.2004.04.003 [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Richardson GA, Willford J, Day NL 2008. Prenatal marijuana exposure and intelligence test performance at age 6. J Am Acad Child Adolesc Psychiatry. 47 (3), 254–263. doi: 10.1097/CHI.0b013e318160b3f0 [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Richardson GA, Willford JA, Severtson SG, Day NL 2012. School achievement in 14-year-old youths prenatally exposed to marijuana. Neurotoxicol Teratol. 34 (1), 161–167. doi: 10.1016/j.ntt.2011.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorey C, Kuhns L, Smaragdi E, Kroon E, Cousijn J 2019. Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. Eur Arch Psychiatry Clin Neurosci. 269 (1), 37–58. doi: 10.1007/s00406-019-00981-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE 2012. Age of onset of marijuana use and executive function. Psychol Addict Behav. 26 (3), 496–506. doi: 10.1037/a0026269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF 2010. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 35 (11), 970–976. doi: 10.1016/j.addbeh.2010.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G, Block RI, Luijten M, Ramsey NF 2010. Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 49 (6), 561–572. doi: 10.1016/j.jaac.2010.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME 2019. Monitoring the Future National Survey Results on Drug Use, 1975–2018: Overview, Key Findings on Adolescent Drug Use. Institute for Social Research. [Google Scholar]
- Lipari RN 2013. Trends in adolescent substance use and perception of risk from substance use. In the CBHSQ report. Substance Abuse and Mental Health Services Administration US. [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, … Sweeney JA 2001. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 13 (5), 786–793. doi: 10.1006/nimg.2000.0743 [DOI] [PubMed] [Google Scholar]
- Mackie K 2005. Distribution of cannabinoid receptors in the central and peripheral nervous system. In Cannabinoids pp. 299–325. Springer, Berlin, Heidelberg. [DOI] [PubMed] [Google Scholar]
- McHale S, Hunt N 2008. Executive function deficits in short-term abstinent cannabis users. Hum Psychopharm Clin. 23 (5), 409–415. doi: 10.1002/hup.941 [DOI] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, … Moffitt TE 2012. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U. S. A 109 (40), E2657–E2664. doi: 10.1073/pnas.1206820109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Danese A, Fisher HL, Houts R, Arseneault L, Moffitt TE 2018. Associations between adolescent cannabis use and neuropsychological decline: a longitudinal co-twin control study. Addiction. 113 (2), 257–265. doi: 10.1111/add.13946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, Lee FS, Gee DG 2018. The role of the endocannabinoid system and genetic variation in adolescent brain development. Neuropsychopharmacology. 43 (1), 21–33. doi: 10.1038/npp.2017.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO 1998–2017. Mplus User’s Guide. Eighth Edition. Los Angeles, CA: Muthén Muthén. [Google Scholar]
- Myers R 1990. Classical and modern regression with applications 2nd ed. Boston, MA: Duxbury Press. [Google Scholar]
- Parker MA, Anthony JC 2018. A prospective study of newly incident cannabis use and cannabis risk perceptions: Results from the United States Monitoring the Future study, 1976–2013. Drug Alcohol Depend. 187, 351–357. doi: 10.1016/j.drugalcdep.2018.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D 2003. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 69 (3), 303–310. doi: 10.1016/s0376-8716(02)00334-4 [DOI] [PubMed] [Google Scholar]
- Porath AJ, Fried PA 2005. Effects of prenatal cigarette and marijuana exposure on drug use among offspring. Neurotoxicol Teratol. 27 (2), 267–277. doi: 10.1016/j.ntt.2004.12.003 [DOI] [PubMed] [Google Scholar]
- Richardson GA, Huestis MA, Day NL. 2006. Assessing in utero exposure to cannabis and cocaine. In: Bellinger DC (Ed.). Human Developmental Neurotoxicology. Taylor & Francis Group, New York, NY, pp. 287–302. [Google Scholar]
- Richardson GA, Ryan C, Willford J, Day NL, Goldschmidt L 2002. Prenatal alcohol and marijuana exposure: effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol. 24 (3), 309–320. doi: 10.1016/S0892-0362(02)00193-9 [DOI] [PubMed] [Google Scholar]
- Robles N, Day NL 1990. Recall of alcohol consumption during pregnancy. J Stud Alcohol. 51 (5), 403–407. doi: 10.15288/jsa.1990.51.403 [DOI] [PubMed] [Google Scholar]
- Salloum NC, Krauss MJ, Agrawal A, Bierut LJ, Grucza RA 2018. A reciprocal effects analysis of cannabis use and perceptions of risk. Addiction. 113 (6), 1077–1085. doi: 10.1111/add.14174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LA, Jacobs LM, Vlahov D, Spetz J 2019. Impacts of medical marijuana laws on young Americans across the developmental spectrum. Matern Child Health J. 23 (4), 486–495. doi: 10.1007/s10995-018-2656-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeler T, Bhattacharyya S 2013. The effect of cannabis use on memory function: an update. Subst Abuse Rehabil. 4, 11–27. doi: 10.2147/SAR.S25869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuermeyer J, Salomonsen-Sautel S, Price RK, Balan S, Thurstone C, Min SJ, Sakai JT 2014. Temporal trends in marijuana attitudes, availability and use in Colorado compared to non-medical marijuana states: 2003–11. Drug Alcohol Depend. 140, 145–155. Doi: 10.1016/j.drugalcdep.2014.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenberg J, Johnston L, O’Malley P, Bachman J, Miech R, Patrick M 2019. Monitoring the Future national survey results on drug use, 1975–2018: Volume II, college students and adults ages 19–60.
- Schuster RM, Hoeppner SS, Evins AE, Gilman JM 2016. Early onset marijuana use is associated with learning inefficiencies. Neuropsychology, 30 (4), 405. doi: 10.1037/neu0000281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Slomiak ST, Jones JD, Rosen AF, Moore TM, Gur RC 2018. Association of cannabis with cognitive functioning in adolescents and young adults: A systematic review and meta-analysis. JAMA Psychiatry. 75 (6), 585–595. doi: 10.1001/jamapsychiatry.2018.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Wolf DH, Calkins ME, Bach EC, Weidner J, Ruparel K, … Gur RC 2017. Cognitive functioning of adolescent and young adult cannabis users in the Philadelphia Neurodevelopmental Cohort. Psychol Addictive Behav. 31 (4), 423. doi: 10.1037/adb0000268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheslow D, Adams W 1990. Manual for the Wide Range Assessment of Memory and Learning. Jastak Associates, Wilmington, DE. [Google Scholar]
- Sloboda Z, Glantz MD, Tarter RE 2012. Revisiting the concepts of risk and protective factors for understanding the etiology and development of substance use and substance use disorders: Implications for prevention. Subst Use Misuse. 47 (8–9), 944–962. doi: 10.3109/10826084.2012.663280 [DOI] [PubMed] [Google Scholar]
- Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, … Yücel M 2011. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology (Berl). 216 (1), 131–144. doi: 10.1007/s00213-011-2203-x [DOI] [PubMed] [Google Scholar]
- Sonon KE, Richardson GA, Cornelius JR, Kim KH, Day NL 2015. Prenatal marijuana exposure predicts marijuana use in young adulthood. Neurotoxicol Teratol. 47, 10–15. doi: 10.1016/j.ntt.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait RJ, Mackinnon A, Christensen H 2011. Cannabis use and cognitive function: 8-year trajectory in a young adult cohort. Addiction. 106 (12), 2195–2203. Doi: 10.1111/j.1360-0443.2011.03574.x [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Brown SA 2008. The influence of marijuana use on neurocognitive functioning in adolescents. Current Drug Abuse Rev. 1 (1), 99–111. doi: 10.2174/1874473710801010099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike R Hagen E Sattler J 1986. The Stanford–Binet Intelligence Scale, Fourth ed., Riverside Publishing, Chicago, IL. [Google Scholar]
- Tulsky D, Zhu J Ledbetter M 2002. WAIS-III WMS-III Technical Manual. The Psychological Corporation. [Google Scholar]
- Verrico CD, Gu H, Peterson ML, Sampson AR, Lewis DA 2014. Repeated Δ9-tetrahydrocannabinol exposure in adolescent monkeys: persistent effects selective for spatial working memory. Am J Psychiatry. 171 (4), 416–425. doi: 10.1176/appi.ajp.2013.13030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Han B, Compton WM, McCance-Katz EF 2019. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA. 322 (2), 167–169. doi: 10.1001/jama.2019.7982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D 1991. Wechsler Intelligence Scale For Children-III. Psychological Corporation. Harcourt Brace, San Antonio, TX. [Google Scholar]
- Wen H, Hockenberry JM, Druss BG 2019. The effect of medical marijuana laws on marijuana-related attitude and perception among US adolescents and young adults. Prev Sci, 20 (2), 215–223. doi: 10.1007/s11121-018-0903-8 [DOI] [PubMed] [Google Scholar]
- Willford JA, Richardson GA, Leech SL, Day NL 2004. Verbal and visuospatial learning and memory function in children with moderate prenatal alcohol exposure. Alcoholism: Clin Exper Res. 28 (3), 497–507. [DOI] [PubMed] [Google Scholar]
- Winward JL, Hanson KL, Tapert SF, Brown SA 2014. Heavy alcohol use, marijuana use, and concomitant use by adolescents are associated with unique and shared cognitive decrements. J Int Neuropsychol Soc. 20 (8), 784–795. doi: 10.1017/S1355617714000666 [DOI] [PMC free article] [PubMed] [Google Scholar]


