SUMMARY:
We describe a 28-year-old man with presumed VKH syndrome, whose presenting symptoms were bilateral impaired vision and headaches. Orbital MR imaging findings included bilateral choroidal and retrobulbar contrast enhancement, while brain findings included white matter abnormalities on FLAIR and leptomeningeal enhancement. Pachymeningeal enhancement has been described previously; herein, we report a patient with VKH syndrome presenting solely with leptomeningeal enhancement. Thus, MR imaging may detect early CNS involvement by VKH disease before the onset of neurologic symptoms.
VKH syndrome is a rare multiorgan autoimmune disorder, with fewer than 250 cases described in the modern literature. VKH syndrome mainly affects the pigmented tissues of the ocular, auditory, integumentary (skin), and central nervous systems. It is characterized by bilateral uveitis and retinal detachment and is variably associated with patchy vitiligo, alopecia, hearing loss, tinnitus, headaches, and meningismus.1 MR imaging is useful in distinguishing scleral involvement versus choroidal and retinal involvement, in which orbital MR imaging findings typically include diffuse choroidal thickening with scleral sparing and retinal detachment.2 Regarding cerebral involvement on MR imaging, early reports described T2-bright periventricular lesions, while more recent reports have described lesions within the brain stem and peduncle, as well as pachymeningeal enhancement.3–6 We describe a patient with VKH syndrome with a new finding of diffuse leptomeningeal contrast enhancement, along with retrobulbar enhancement and the typical ocular findings of choroidal thickening and retinal detachment.
Case Report
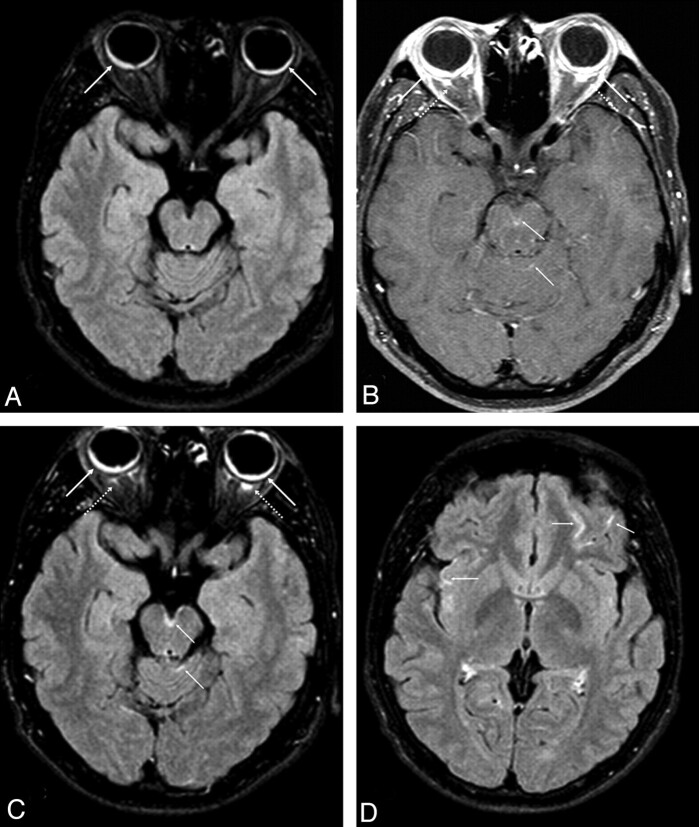
A 28-year-old Hispanic man presented with a 10-day history of red eyes and decreased vision bilaterally, with mild headaches. He had normal vital signs and an unremarkable medical history, and he denied any history of ocular trauma or surgery. The ophthalmologic examination documented bilateral granulomatous uveitis, serous retinal detachment, and disc edema. The patient had a recent history of mild headaches, but the clinical examination failed to reveal integumental (skin) changes, meningeal signs, or neurologic or auditory deficits. B-mode sonography demonstrated bilateral peripheral serous retinal detachment with retinal and choroidal thickening. Fluorescein angiography showed a stippling pattern and mild staining of the disc bilaterally. On orbital and brain MR imaging, there was bilateral abnormal thickening and enhancement of the retina/choroid with retinal detachment, with numerous nonspecific punctate foci on FLAIR images in the subcortical superior frontal white matter intracranially. There was also mild diffuse leptomeningeal enhancement, best visualized on postcontrast FLAIR images (Fig 1).
Fig 1.
Axial unenhanced FLAIR MR image (A) demonstrates symmetric choroidal thickening with retinal detachment (solid arrows), which enhances on postcontrast T1-weighted (B) and postcontrast FLAIR (C and D) images, with bilateral retrobulbar enhancement (dashed arrows). There is multifocal leptomeningeal enhancement within the interpeduncular fossa and cerebellar folia (thin arrows, B and C) and scattered throughout the cerebral sulci on the postcontrast FLAIR image at a higher level (D).
Extensive laboratory and serologic test findings were either negative or within the normal range and did not indicate any evidence of specific infectious, granulomatous, or autoimmune disorders. A lumbar puncture was not performed. The patient was given prednisolone acetate eye drops and oral prednisone, and his vision and headaches gradually improved during the next several weeks. By 6 months, the headaches did not recur and the visual acuity did not decline further.
Discussion
VKH syndrome was first reported in the 10th century by Ali Ibn Isa, an ophthalmologist in medieval Baghdad, who described the characteristic physical appearance of ocular inflammation with a distinct whitening of the hair, eyebrows, and eyelashes.1 The pathophysiology of this disorder has not been fully elucidated but is thought to be related to T-lymphocyte-mediated autoimmunity against melanocyte tyrosinase–related proteins, which are present in the uvea, retina, and the leptomeninges.7 A genetic basis is likely the predisposing factor because there is an increased incidence with darker pigmented skin; however, a particular inheritance pattern has yet to be identified.1 One theory suggests that VKH syndrome has varying clinical manifestations, which depend on the stage of the syndrome at the time of clinical presentation, because 4 different stages have been described.8 For this reason, there has yet to be a consensus regarding a definitive diagnostic test for VKH syndrome, which consequently limits the diagnosis to a combination of clinical and ancillary test findings. Thus, there are 3 distinct categories, classified as complete, incomplete, and probable VKH syndrome based on the following criteria: 1) uveitis without a history of ocular trauma or surgery, 2) uveitis without clinical or laboratory evidence of other ocular disease, 3) bilaterality of the uveitis with retinal detachment, 4) the presence of auditory or other neurologic (nonvisual) findings, and 5) integumentary (skin) findings.9 Complete VKH syndrome manifests all 5 criteria, whereas incomplete VKH syndrome manifests criteria 1–3 along with either 4 or 5. Probable VKH syndrome (as in our patient) represents isolated ocular disease clinically, with criteria 1–3. Thus, the extraocular clinical manifestations of complete VKH syndrome may not be fulfilled until months or years following the initial presentation of ocular disease.10
MR imaging has proved to be a helpful tool in diagnosing VKH syndrome; in addition to the typical bilateral ocular findings, scattered periventricular white matter lesions on T2-weighted imaging/FLAIR have also been described.2,4 Because pachymeningeal enhancement has only recently been described in early 2010, meningeal enhancement and other cerebral findings on MR imaging have not yet been factored in as criteria for VKH.6,11 Our patient with probable VKH syndrome presented with only mild headaches, and the only objective findings were ocular; hence, MR imaging helped ascertain the diagnosis based on extraocular findings. Although it is a systemic condition, 54% of patients with VKH have findings limited solely to ocular inflammation during the early phase of the syndrome, and we note that Hispanic patients have a higher prevalence of probable VKH syndrome (such as in our patient).11–13 Notably, he did not undergo a CSF examination, so we could not determine whether CSF pleocytosis was present, which is 1 criterion for VKH syndrome.13
However, our diagnosis is based on the updated RDC criteria, which are more sensitive and specific, and we note that the RDC criteria do not require the presence of CSF pleocytosis because it is a nonspecific finding that is not present in all patients with VKH.9,14 Thus, given the lack of extraocular findings, our diagnosis is based on ancillary ophthalmic tests such as fluorescein angiography and B-mode sonography; such ancillary tests are not specific for VKH syndrome and can be positive in other infectious, inflammatory, or vasculitic disorders, including sarcoidosis, syphilis, tuberculosis, Lyme disease, toxoplasmosis, Wegener granulomatosis, and sympathetic ophthalmia. However, the MR imaging, clinical findings, and extensive laboratory testing were helpful in excluding these disorders and further pointing to VKH syndrome. The ability of MR imaging to demonstrate meningeal inflammation fits the presumed involvement of melanocyte-rich tissues and could explain the early clinical symptoms, (headaches) other than vision, before the onset of frank neurologic symptoms. Because melanocytes are concentrated in the pia-arachnoid compared with the dura, the leptomeningeal enhancement in our patient may be a marker of early CNS involvement by VKH syndrome. Hence, MR imaging could be an important tool in the early diagnosis of this rare disorder, which could lead to prompt initiation of systemic therapies to minimize the duration and intensity of this often debilitating syndrome.
Abbreviations
- CNS
central nervous system
- FLAIR
fluid-attenuated inversion recovery
- RDC
Revised Diagnostic Criteria
- VKH
Vogt-Koyanagi-Harada
References
- 1. Moorthy RS, Inomata H, Rao NA. Vogt-Koyanagi-Harada syndrome. Surv Opthalmol 1995; 39: 265–92 [DOI] [PubMed] [Google Scholar]
- 2. Ibanez HE, Grand MG, Meredith TA, et al. Magnetic resonance imaging findings in Vogt-Koyanagi-Harada syndrome. Retina 1994; 14: 164–68 [PubMed] [Google Scholar]
- 3. Nitta E, Takamori M. Wallenberg's syndrome in a case of Vogt-Koyanagi-Harada disease. Clin Neurol 1989; 29: 505–08 [PubMed] [Google Scholar]
- 4. Ikeda M, Tsukagoshi H. Vogt-Koyanagi-Harada disease presenting meningoencephalitis. Eur Neurol 1992; 32: 83–85 [DOI] [PubMed] [Google Scholar]
- 5. Hashimoto T, Takizawa H, Yukimura K, et al. Vogt-Koyanagi-Harada disease associated with brainstem encephalitis. J Clin Neurosci 2009; 16: 593–95. Epub 2009 Feb 6 [DOI] [PubMed] [Google Scholar]
- 6. Han HJ, Kim HY, Park JH, et al. Magnetic resonance imaging of pachymeningeal enhancement in Vogt-Koyanagi-Harada disease. Neurol Sci 2010. March 3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7. Rao NA. Mechanisms of inflammatory response in sympathetic ophthalmia and VKH syndrome. Eye 1997; 11: 213–16 [DOI] [PubMed] [Google Scholar]
- 8. Babel J. Syndrome de Vogt-Koyanagi (Uveite bilaterale, poliosis, alopecie, vitiligo et dysacousie). Schweiz Med Wochenschr 1932; 44: 1136–40 [Google Scholar]
- 9. Read RW, Holland GN, Rao NA, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol 2001; 131: 647–52 [DOI] [PubMed] [Google Scholar]
- 10. Rao N, Gupta A, Dustin L, et al. Frequency of distinguishing clinical features in Vogt-Koyanagi-Harada disease. Ophthalmology 2010; 117: 591–99, 599.e1. Epub 2009 Dec 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rao N, Sukavatcharin S, Tsai J. Vogt-Koyanagi-Harada disease diagnostic criteria. Int Ophthalmol 2007; 27: 195–99 [DOI] [PubMed] [Google Scholar]
- 12. Sukavatcharin S, Tsai JH, Rao NA. Vogt-Koyanagi-Harada disease in Hispanic patients. Int Ophthalmol 2007; 27: 143–48 [DOI] [PubMed] [Google Scholar]
- 13. Sugiura S. Vogt-Koyanagi-Harada disease. Jpn J Ophthalmol 1978; 22: 9–35 [Google Scholar]
- 14. Yamaki K, Hara K, Sakuragi S. Application of revised diagnostic criteria for Vogt-Koyanagi-Harada disease in Japanese patients. Jpn J Ophthalmol 2005; 49: 143–48 [DOI] [PubMed] [Google Scholar]