Abstract
BACKGROUND AND PURPOSE:
The artery of Bernasconi and Cassinari is an important infraclinoid branch of the internal cerebral artery. It is of neuroendovascular relevance in view of its supply to complex lesions such as meningiomas and arteriovenous malformations in the tentorial and falcotentorial regions. The present microanatomic study attempts a morphometric elucidation of this slender but important branch of the meningohypophyseal trunk.
MATERIALS AND METHODS:
The origin, course, dimensions, and related variations of the tentorial artery were studied in 10 formalin-fixed human cadaveric sides.
RESULTS:
The tentorial artery originated from the meningohypophyseal trunk in all but 1 specimen, in which it arose directly from the intracavernous internal carotid artery. In 80% of specimens, it took origin as a single branch; as a bifurcation and trifurcation in 1 each. It was usually the terminal branch of the meningohypophyseal trunk (in 90%). In all, 5 distinct microvascular patterns were noted. The mean diameter of this artery was 0.7 mm (range, 0.3–0.8 mm; SD, ±0.1 mm). The mean length was 15.4 mm (range, 9–23 mm; SD, ±4.4 mm). Its mean distance from the origin of the meningohypophyseal trunk was 1.7 mm (range, 1.3–2.3 mm; SD, ±0.4 mm). The mean distance from the free edge of the tentorium was 3.7 mm (range, 3–5 mm; SD, ±0.7 mm).
CONCLUSIONS:
The artery of Bernasconi and Cassinari is an important vascular conduit to myriad neoplasms and vascular malformations in the vicinity of the tentorium cerebelli. In this era of advanced microneurosurgical techniques and superselective endovascular interventions, morphometric knowledge of this artery is important for precise and safe management of these lesions.
The tentorial artery is the most constant branch of the MHT that, in turn, arises from the proximal curvature of the intracavernous ICA.1 It is important in neuroendovascular interventions for an entire spectrum of neoplasms and vascular lesions in the region of the tentorium cerebelli. Bernasconi and Cassinari2 first reported the angiographic visualization of a tentorial artery supplying tentorial meningiomas. Since then, similar angiographic3–5 and anatomic1,6 observations have been published, but no morphometric study specific to the tentorial artery has been conducted. An anatomic study of this type is of utmost importance for imparting greater “precision” to the management of these lesions as well as in preventing inadvertent vascular compromise to normal structures, especially in this era of advanced superselective endovascular interventions. The present microanatomic study is a step to address this issue.
Materials and Methods
Five fresh human cadaveric heads were prepared for bilateral dissection, yielding a total of 10 datasets. The internal carotid arteries, the vertebral arteries, and the internal jugular veins of the specimens were cannulated and injected with colored silicon dye, as described previously.7 The specimens were kept submerged in 10% formalin for the next 48 hours. The prepared specimens were then dissected under variable magnification by using a neurosurgical operating microscope (Carl Zeiss OPMI, Aalen, Germany), a high-speed drill (Midas Rex, Ft. Worth, Texas) and microinstruments, and a suction-irrigation system (Germed USA, New York, New York).
The heads were maintained in a head-holder clamp (Mayfield Skull Clamp, Cincinnati, Ohio) and screws and turned 45° to the opposite side, with the malar prominence being the most superior point. A standard frontotemporal craniotomy was performed along with a zygomatic osteotomy. The temporal lobe was elevated extradurally to expose the floor of the middle fossa. This was performed in an anterior-to-posterior8 direction as the GSPN was separated from the dura. The arcuate eminence initially was identified along the petrous ridge. Extradural elevation was then continued anteromedially to expose the region of the geniculate ganglion and the GSPN, which lay in the major petrosal groove and were covered by a layer of connective tissue. The middle meningeal artery at the foramen spinosum was identified and divided to allow further release of the temporal dura from the middle fossa cranial base. The dura propria could then be elevated off the lateral wall of the cavernous sinus to expose the mandibular division of the trigeminal nerve (V3) as it exited the foramen ovale. Further extradural elevation exposed the posterior cavernous sinus region. A dural incision was then made in the oculomotor triangle immediately medial and parallel to the oculomotor nerve with gentle separation of the cranial nerves from the reticular membrane, exposing the sixth cranial nerve and the horizontal segment of the ICA. The Parkinson triangle was then opened with identification of the vertical ICA segment, proximal curvature, MHT, and its branches.
Measurement Parameters
Focusing on the tentorial artery, morphometric details of the following parameters were studied: 1) diameter and length, 2) distance from the MHT origin, 3) single or multiple, 4) whether arising directly from the cavernous segment ICA, and 5) distance from the free margin of the tentorium cerebelli.
Measurements
The dimensions were measured by using calipers, and the values were compared with a template calibrated to the nearest 0.04 cm (accuracy).
Results
Origin, Course, and Relationship of the Tentorial Artery to Adjacent Structures
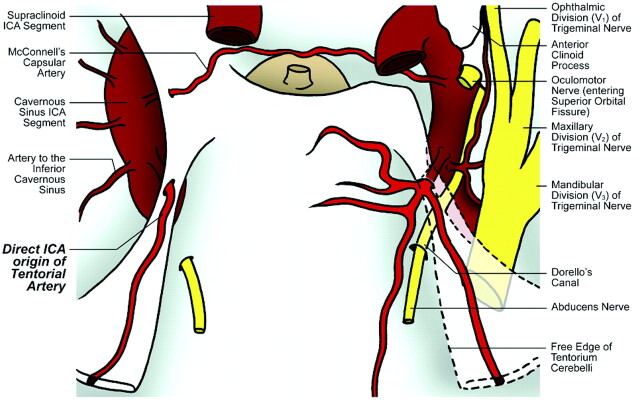
A meticulous dissection of the specimens through the Parkinson triangle disclosed the slender tentorial artery, which arose from the MHT in 9 specimens and directly from the superomedial wall of the medial loop of the cavernous segment of the ICA in 1 specimen (Table). The third and fourth cranial nerves entered the dural roof of the cavernous sinus slightly behind the origin of the tentorial artery. Its origin from the MHT, near the posterior part of the roof of the cavernous sinus, was at a mean distance of 9.7 ± 3.1 mm proximal to the entry point of the fourth cranial nerve into the cavernous sinus. In 80% of specimens, it took origin as a single branch; 7 of these from the MHT and in 1 specimen, directly from the cavernous ICA. In 1 specimen each, it took origin as a bifurcation and a trifurcation, respectively. It passed slightly forward to the roof of the cavernous sinus and then caudally and posterolaterally along the free edge of the tentorium. The artery sent tiny branches to the third and fourth cranial nerves and gasserian ganglion and blended within the 2 leaves of the tentorium caudally, somewhat medial to the free edge. In the 2 specimens where the artery was bifurcated or trifurcated, each branch blended separately with the tentorium. The finer anastomoses with branches from the opposite counterpart were observed in most of our specimens.
Summary of the morphometric analysis of the tentorial artery
| Specimen No. | Diameter (mm) | Length (mm) | Single/Multiple | Distance of MHT from Foramen Lacerum (mm) | Distance from MHT Origin (mm) | Distance from Tentorium Free Margin (mm) | Origin from Cavernous ICA |
|---|---|---|---|---|---|---|---|
| 1. Cadaver 1 (L) | 0.6 | 12.5 | Single | 7.5 | 1.7 | 3.0 | No |
| 2. Cadaver 1 (R) | 0.5 | 17.6 | Single | 5.9 | 1.3 | 5.0 | No |
| 3. Cadaver 2 (L) | 0.8 | 16.1 | Single | 4.0 | Yes | ||
| 4. Cadaver 2 (R) | 0.6 | 16.7 | Single | 11.1 | 1.3 | 4.0 | No |
| 5. Cadaver 3 (L) | 0.3 | 23.0 | Single | 9.0 | 2.0 | 3.0 | No |
| 6. Cadaver 3 (R) | 0.4 (largest branch) | 21.0 (longest branch) | Multiple (3) | 8.2 | 1.3 | 3.5 (most medial branch) | No |
| 7. Cadaver 4 (L) | 0.5 | 13.0 | Single | 13.2 | 1.8 | 3.0 | No |
| 8. Cadaver 4 (R) | 0.7 | 14.5 | Single | 5.3 | 1.6 | 4.7 | No |
| 9. Cadaver 5 (L) | 0.6 | 9.0 | Single | 15.7 | 1.9 | 4.0 | No |
| 10. Cadaver 5 (R) | 0.5 (larger branch) | 10.3 (longer branch) | Multiple (2) | 12.4 | 2.3 | 3.0 (more medial branch) | No |
| Mean | 0.7 ± 0.1 | 15.4 ± 4.4 | 9.8 ±3.5 | 1.7 ± 0.4 | 3.7 ± 0.7 | - |
The mean distance of the MHT origin from the foramen lacerum was 9.8 ± 3.5 mm (range, 5.3–15.7 mm; Table). The MHT was “complete”9 in 7 specimens (divided into 3 branches: the inferior hypophyseal, the dorsal meningeal, and the tentorial arteries, in that order); in 2 specimens, it showed incomplete branching patterns (the tentorial artery and the inferior hypophyseal artery in 1 specimen, and the tentorial artery as well as the dorsal meningeal artery in another specimen). In 1 specimen, it was absent.
Thus, 5 distinct anatomic patterns of the tentorial artery were noted in our study: 1) single origin from a complete MHT; 2) single origin from an incomplete MHT (see On-line Video, demonstrating a specimen of a single trunk tentorial artery, originating from an incomplete variant of the MHT), 3) bifurcated origin from a complete MHT, 4) trifurcated origin from a complete MHT, and 5) direct origin from cavernous ICA segment.
Figures 1–5 provide illustrations of different tentorial artery variants in our study.
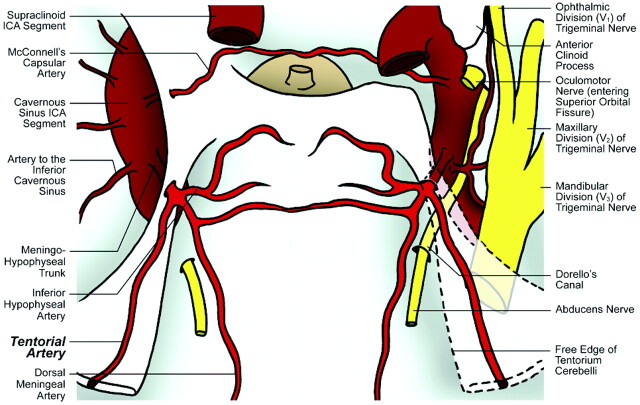
Fig 1.
Illustration of cadaver specimen 1 depicts single origin of the tentorial artery from a complete MHT on both sides.
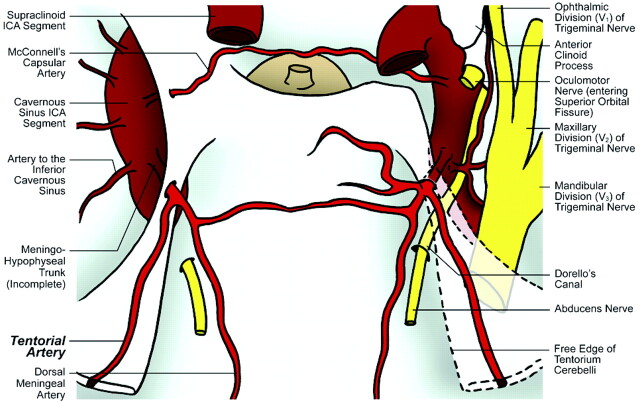
Fig 2.
Illustration of cadaver specimen 2 depicts single origin of the tentorial artery from a complete MHT on the right side and from an incomplete MHT on the left side.
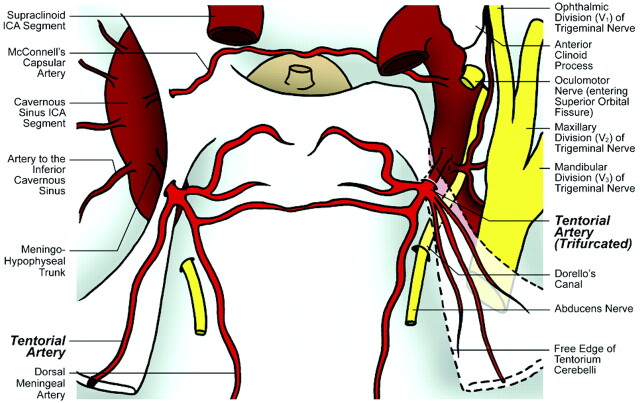
Fig 3.
Illustration of cadaver specimen 3 depicts trifurcated origin of the tentorial artery from a complete MHT on the right side and single origin of the tentorial artery from a complete MHT on the left side.
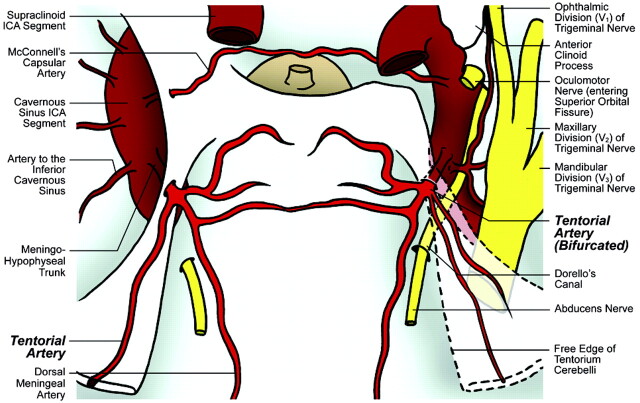
Fig 4.
Illustration of cadaver specimen 4 depicts bifurcated origin of the tentorial artery from a complete MHT on the right side and single origin of the tentorial artery from a complete MHT on the left side.
Fig 5.
Illustration of cadaver specimen 5 depicts single origin of the tentorial artery from a complete MHT on the right side and a direct origin of the tentorial artery from the cavernous sinus ICA on the left side.
Dimensions
The mean diameter of the tentorial artery was 0.7 mm (range, 0.3–0.8 mm; SD, ±0.1 mm). In the specimen with bifurcated origin, the diameter of the larger (superior) branch was 0.5 mm, and the diameter of the smaller branch was 0.2 mm. In the specimen with trifurcated origin, the diameter of the largest branch (middle) was 0.4 mm, of the inferior branch was 0.1 mm, and of the superior branch was 0.2 mm. The mean length was 15.4 mm (range, 9–23 mm; SD, ±4.4 mm). Its mean distance from the origin of the MHT was 1.7 mm (range, 1.3–2.3 mm; SD, ±0.4 mm). At the point where the tentorial artery blended with the tentorium, its mean distance from the free edge of the tentorium was 3.7 mm (range, 3–5 mm; SD, ±0.7 mm). A summary of these morphometric observations is in the Table.
Discussion
Anatomy
The tentorial artery originates usually as the terminal branch of the meningohypophyseal trunk, the most proximal intracavernous ICA branch.1 The MHT arises lateral to the dorsum sellae at or just before the apex of the medial loop of the intracavernous carotid where it turns forward after leaving the foramen lacerum.10 It divides near the roof of the cavernous sinus and typically gives rise to 3 branches: 1) the inferior hypophyseal artery that travels medially to supply the posterior pituitary capsule; 2) the dorsal meningeal artery that enters the dura of the posterior sinus wall and supplies the clival dura and sixth cranial nerve; and 3) the tentorial artery, also called the artery of Bernasconi and Cassinari. In our study, we found this complete pattern in 70% of specimens, similar to the study by Inoue et al.9 Commenting on the origin of the tentorial artery, Reisch et al6 noted a nonsingular branching pattern in 36% of specimens; we found the same in 20% of our specimens (bifurcated and trifurcated origin in 1 specimen each). In 1 of the specimens, there was a direct origin of this artery from the cavernous ICA. We also measured the average point of origin of the tentorial artery to be 9.7± 3.1 mm proximal to the entry point of the fourth cranial nerve into the cavernous sinus and 1.7 ± 0.4 mm from the origin of the MHT. Describing a similar artery in her cadaveric study, Izmailova11 measured the diameter of that artery to be in the range of 0.6–0.8 mm; we found the average diameter to be 0.7 ± 0.1 mm, with a mean length of 15.4 ± 4.4 mm.
The tentorial artery courses posterolateral to the tentorium, slightly lateral to the free edge. It sends branches to the proximal portion of the third and fourth cranial nerve as well as medial portion of the gasserian ganglion,1,12 and anastomoses with the branches of the tentorial artery from the opposite side. Similar findings were observed in our study. Lang and Schäfer13 noted that the tentorial artery runs 5 mm lateral to the free edge of the tentorium cerebelli. In our dissections, we found this distance to be 3.7 ± 0.7 mm.
In the presence of pathologic lesions supplied by the MHT and its branches, it is possible to visualize the different types of MHT based on conventional DSA, though the branching patterns could be better appreciated in 3D-DSA. As regards the tentorial artery branch of MHT, Wallace et al14 have noted that if it is visualized to be longer than 40 mm, a related pathologic lesion is considered probable. However, the tentorial artery anatomy is usually not seen distinctly in normal angiograms.3
Historical Perspective.
In summer 1956, Bernasconi and Cassinari made their observation of a “peculiar” artery supplying 5 of the 7 tentorial meningiomas. This was a seminal finding in an era devoid of microcatheter and superselective catheterization techniques.2 They proposed this finding to be pathognomonic of tentorial meningiomas and thought it to be probably arising from the external carotid artery. Similar angiographic observations were made by Wickbom and Stattin4 in 1958 and by Frugoni et al5 in 1960 (including cases of falcotentorial and parasagittal meningiomas). However, Frugoni et al, in 1 of their specimens, found that this vessel did not fill when the external carotid artery was selectively punctured; this made them doubt the external carotid origin of this artery.
In her landmark anatomic study in 1953, Izmailova11 had already demonstrated an arterial branch (the third and longest) arising from the “posterior trunk” of the intracavernous ICA, which “runs posteriorly to enter the 2 leaves of the tentorium at its free margin.” A similar description was provided by Schnurer and Stattin15; in their specimens, this branch, which they called the marginal tentorial branch, was arising from the “anterior trunk” instead. Stattin16 further angiographically described this vessel to be arising from the intracavernous carotid siphon in 80% of tentorial meningiomas, by selective internal carotid arteriography. In 1964, Frugoni et al17 finally proved the ICA origin of the tentorial artery in their seminal article.
Clinical Relevance
In addition to tentorial, posterior falcine and falcotentorial meningiomas, the tentorial artery has been implicated in myriad other pathologies, such as arteriovenous malformation and tentorial dural arteriovenous fistula,5,3,18 temporo-occipital glioblastoma,18,19 falcotentorial angioma,20 and temporo-occipital metastatic carcinoma.19
Selective catheterization and embolization of the MHT can be achieved by stabilizing the microcatheter within the MHT (and its branches) with a temporary balloon occlusion of the ICA at the site of origin of the MHT.
The embolizing material can be glue or a liquid agent (eg, ethanol). It has been considered prudent to use small aliquots of the embolizing agent along with frequent intraprocedural checking of vessel flow so as to avoid inadvertent damage to the inferior hypophyseal branch of the MHT and consequent damage to the pituitary gland.20
When a microcatheter cannot be positioned into the MHT, an indirect technique, described by Halbach et al,21 can be used. According to their method, a temporary balloon occlusion of the ICA at the site of origin of the MHT is performed so as to reduce its blood supply to the lesion. Then, if the embolizing agent is injected into a collateral artery supplying the lesion, the agent is found to be preferably perfusing into the lesion.
Because there exist a plethora of vascular and neoplastic pathologies that derive a major contribution of their blood supply from the tentorial artery, objective anatomic knowledge of this small artery and its variations is important for efficient endovascular management of these lesions.3,6
Following the seminal study by Parkinson,22 the MHT has been widely held as an invariable single trunk dividing into 3 branches. Although it was considered to be controversial by some, there remained a distinct paucity of objective evidence to the contrary. We believe that the knowledge of 5 distinct anatomic patterns (including origin and branching patterns) involving the tentorial artery and its parent vessel of origin (the MHT), as depicted in our study, will help in enhancing the precision of selective injections of these important blood vessels as well as their interpretations.
The morphometric observations and measurements of individual arteries and their variants are expected to facilitate the selection of superselective catheters of proper dimensions (including catheter diameters and tip angulations), suitable for each of these variations.
Our study also attempts to elucidate, in objective (mathematical) terms, the relationships of the MHT and its tentorial artery branch to the adjacent structures. With the advent of 3D fusion DSA,23 we hope that these spatial elaborations will further facilitate and guide precise superselective catheterizations of these salient blood vessels in various pathologies.
Inadvertent damage to this vascular setup could lead to vascular compromise of salient anatomic structures, such as the third and fourth cranial nerves as well as the medial portion of the gasserian ganglion.12 During superselective endovascular interventions involving the tentorial artery, inadvertent puncture and embolization of the inferior hypophyseal branch or the MHT itself might put the normal functioning of the pituitary-hypothalamic axis in jeopardy.24 Thus, an in-depth morphometric elucidation of the tentorial artery is of the essence in imparting greater safety to the neuroendovascular treatment of various complex lesions.
Conclusions
The artery of Bernasconi and Cassinari is an important arterial conduit for myriad vascular and tumorous pathologies in the region of the tentorium cerebelli. In this rapidly advancing era of superselective endovascular interventions, a thorough understanding of its morphometric anatomy is of importance in efficient and safe management of these lesions.
Acknowledgments
We thank Ms. Lorry Tubbs for help and contribution toward the illustrations in this article.
Abbreviations
- DSA
digital substraction angiography
- GSPN
greater superficial petrosal nerve
- ICA
internal carotid artery
- MHT
meningohypophyseal trunk
- V1
ophthalmic division of trigeminal nerve
- V2
maxillary division of trigeminal nerve
- V3
mandibular division of trigeminal nerve
References
- 1. Isolan G, De Oliveira E, Mattos JP. Microsurgical anatomy of the arterial compartment of the cavernous sinus: analysis of 24 cavernous sinus. Arq Neuropsiquiatr 2005; 63: 259–64 [DOI] [PubMed] [Google Scholar]
- 2. Bernasconi V, Cassinari V. Angiographical characteristics of meningiomas of tentorium. Radiol Med 1957; 43: 1015–26 [PubMed] [Google Scholar]
- 3. van Rooij WJ, Sluzewski M, Beute GN. Tentorial artery embolization in tentorial dural arteriovenous fistulas. Neuroradiology 2006; 48: 737–43 [DOI] [PubMed] [Google Scholar]
- 4. Wickbom I, Stattin S. Roentgen examination of intracranial meningiomas. Acta Radiol 1958; 50: 175–86 [DOI] [PubMed] [Google Scholar]
- 5. Frugoni P, Nori A, Galligioni F, et al. A particular angiographic sign in meningiomas of the tentorium: the artery of Bernasconi and Cassinari. Neurochirurgia (Stuttg) 1960; 2: 142–52 [DOI] [PubMed] [Google Scholar]
- 6. Reisch R, Vutskits L, Patonay L, et al. The meningohypophyseal trunk and its blood supply to different intracranial structures. An anatomical study. Minim Invasive Neurosurg 1996; 39: 78–81 [DOI] [PubMed] [Google Scholar]
- 7. Sanan A, Abdel Aziz KM, Janjua RM, et al. Colored silicone injection for use in neurosurgical dissections: anatomic technical note. Neurosurgery 1999; 45: 1267–71; discussion 1271–74 [DOI] [PubMed] [Google Scholar]
- 8. Jittapiromsak P, Sabuncuoglu H, Deshmukh P, et al. Greater superficial petrosal nerve dissection: back to front or front to back? Neurosurgery 2009; 64: 253–58, discussion 258–59 [DOI] [PubMed] [Google Scholar]
- 9. Inoue T, Rhoton Al, Theele D, et al. Surgical approaches to the cavernous sinus: a microsurgical study. Neurosurgery 1990; 26: 903–32 [DOI] [PubMed] [Google Scholar]
- 10. Jinkins JR. Atlas Of Neuroradiologic Embryology, Anatomy, and Variants. Philadelphia: Lippincott Williams & Wilkins; 2000: 299–329 [Google Scholar]
- 11. Izmailova IV. Arterii tverdoi oboloehki golovnogo mozga chelovecka. Arkh Anat Mozkva 1953; 30: 41–47 [PubMed] [Google Scholar]
- 12. Krisht A, Barnett DW, Barrow DL, et al. The blood supply of the intracavernous cranial nerves: an anatomic study. Neurosurgery 1994; 34: 275–79; discussion 279 [DOI] [PubMed] [Google Scholar]
- 13. Lang J, Schäfer K. The origin and ramifications of the intracavernous section of the internal carotid artery. Gegenbaurs Morphol Jahrb 1976; 122: 182–202 [PubMed] [Google Scholar]
- 14. Wallace S, Goldberg HI, Leeds NF, et al. The cavernous branches of the internal carotid artery. Am J Roentgenol Radium Ther Nucl Med 1967; 101: 34–46 [Google Scholar]
- 15. Schnurer LB, Stattin S. Vascular supply of intracranial dura from internal carotid artery with special reference to its angiographic significance. Acta Radiol (Diag) 1963; 1: 441–50 [Google Scholar]
- 16. Stattin S. Meningeal vessels of the internal carotid artery and their angiographic significance. Acta Radiol 1961; 55: 329–36 [Google Scholar]
- 17. Frugoni P, Nori A, Galligioni F, et al. Further considerations on the Bernasconi and Cassinari's artery and other meningeal rami of the internal carotid artery. Neurochirurgia (Stuttg) 1964; 108: 18–23 [DOI] [PubMed] [Google Scholar]
- 18. Cortes O, Chase Ne, Leeds N. Visualization of tentorial branches of the internal carotid artery in intracranial lesions other than meningiomas. Radiology 1964; 82: 1024–28 [DOI] [PubMed] [Google Scholar]
- 19. Krayenbühl H, Yasargil MG. Die Zerebrale Angiographie. Lehrbuch Fur Praxis. Stuttgart, Germany: Georg Thieme Verlag; 1965 [Google Scholar]
- 20. Phatouros CC, Higashida RT, Malek AM, et al. Embolization of the meningohypophyseal trunk as a cause of diabetes insipidus. AJNR Am J Neuroradiol 1999; 20: 1115–18 [PMC free article] [PubMed] [Google Scholar]
- 21. Halbach VV, Higashida RT, Hieshima GB, et al. Embolization of branches arising from the cavernous portion of the internal carotid artery. AJNR Am J Neuroradiol 1989; 10: 143–50 [PMC free article] [PubMed] [Google Scholar]
- 22. Parkinson D. Collateral circulation of the cavernous carotid artery: anatomy. Can J Surg 1964; 7: 251–68 [PubMed] [Google Scholar]
- 23. Gailloud P, Oishi S, Murphy K. Three-dimensional fusion digital subtraction angiography: new reconstruction algorithm for simultaneous three-dimensional rendering of osseous and vascular information obtained during rotational angiography. AJNR Am J Neuroradiol 2005. April; 26: 908–11. [PMC free article] [PubMed] [Google Scholar]
- 24. Bernasconi V, Cassinari V, Gori G. Diagnostic value of the tentorial arteries of the carotid siphon. (Angiographic study of a case of falcotentorial angioma). Neurochirurgia (Stuttg) 1965; 62: 67–72 [DOI] [PubMed] [Google Scholar]