Abstract
BACKGROUND AND PURPOSE:
Frontotemporal lobar degeneration is responsible for the cognitive abnormalities seen in patients with ALS. We sought to evaluate the in vivo neurochemical changes associated with this pathology indicative of neuronal loss and gliosis.
MATERIALS AND METHODS:
Twenty-four patients with ALS (2 with ALS-FTD) and 15 healthy controls were studied. High-field proton MR spectroscopy of the mesial prefrontal cortex was used to determine concentrations of NAA and mIns, markers of neuronal integrity and gliosis, respectively. Metabolite concentrations were correlated with cognitive tests (verbal fluency, ACE).
RESULTS:
NAA/mIns was decreased 17% (P =.002). Abnormalities were present to a lesser degree in the individual metabolites NAA (decreased 9%; P =.08) and mIns (increased 11%; P =.06) than the ratio of the 2 metabolites. These measures did not correlate significantly with verbal fluency or the ACE.
CONCLUSIONS:
Prefrontal lobe degeneration exists in patients with ALS as indicated by an abnormal mesial prefrontal cortex neurochemical profile. Further study is necessary to determine the potential utility of the NAA/mIns ratio as a biomarker for frontal lobe degeneration in ALS.
Progressive upper motor neuron and lower motor neuron degeneration is the hallmark of ALS. In addition, cerebral degeneration beyond the motor cortex involving frontal and temporal lobes gives rise to mild cognitive and behavioral impairment in upward of 50% of patients.1–3 These deficits may only be evident with specific neuropsychologic testing; however, in a proportion of patients (5%–15%) the severity is sufficient to meet the Neary criteria for FTD.3,4
The pathologic changes of FTLD include atrophy of the frontal and temporal lobes, neuronal loss, gliosis, and superficial spongiform degeneration.5,6 Neuronal inclusions in FTLD associated with motor neuron disease are ubiquitin-immunoreactive and τ- and α-synuclein negative. Such inclusions are present in both cognitively intact and cognitively impaired patients; however, the changes are more widespread in cognitively impaired patients,7 with the greatest abnormalities in the cingulate gyrus5 where there is also neuronal loss.8 TDP-43 is present within ubiquinated inclusions in cases of FTLD without motor neuron disease as well as in sporadic ALS with or without cognitive impairment.9 Thus, clinical, pathologic, and molecular evidence support there being common pathogenic mechanisms in the spectrum of disorders with FTLD.
An objective and sensitive biomarker is needed to detect extramotor involvement in ALS in vivo. Imaging studies have shown promise in this regard by demonstrating gray matter loss by voxel-based morphometry,10,11 decreased cerebral perfusion by single-photon emission CT12 and by PET,13 and decreased gamma-aminobutyric acid (A) receptor14 binding sites with PET. MR spectroscopy studies are few, but they have revealed impaired neuronal integrity indicated by reduced NAA.2,15,16 Whereas MRS studies have documented mIns in the motor cortex to be elevated,17–19 there are no studies quantifying this glial marker in the PFC.
We hypothesized that ALS would be associated with neurochemical abnormalities in nonmotor frontal brain regions and that these would be associated with cognitive dysfunction. MR spectroscopy was used to measure NAA and mIns in the PFC in patients with ALS. Furthermore, we assessed the NAA/mIns ratio as a biomarker of degeneration compared with the individual metabolites NAA and mIns.
Materials and Methods
Patients fulfilling the revised El Escorial criteria20 for “possible,” “probable,” “probable laboratory-supported,” or “definite” ALS and healthy controls were recruited from the University of Alberta ALS Clinic. Subjects with a prior history of cerebrovascular disease, head trauma, epilepsy, or psychiatric illness were ineligible. Participants gave informed consent and the study was approved by the Health Research Ethics Board.
The ALSFRS-R was used as an indicator of global disease status. Letter F fluency was evaluated because it is a sensitive screening measure of cognitive impairment in ALS,21 and the ACE22 was administered as a general cognitive assessment tool considered appropriate for use in patients with ALS.21
MR Imaging and Spectroscopy
Because of the technical challenges in resolving the strongly coupled resonances of mIns on typical MR imaging systems, we conducted experiments at high magnetic field (3T) and used MR images designed for its optimized detection and quantification. An 80-cm bore magnet (Magnex Scientific, UK) was used with actively shielded gradient coils and an SMIS console (Surrey Medical Imaging Systems, Surrey, United Kingdom). A standard quadrature birdcage head coil was used for both RF transmission and reception. Data were acquired by using single-voxel localized proton MRS. The voxel measured 25 × 25 × 30 mm and was centered in the mesial PFC anterior to the genu of the corpus callosum (Fig). Details of the locally developed metabolite-specific sequence used have been published previously.23 This sequence optimizes the evolution and quantification of resonances arising from the metabolites of interest, while minimizing the contribution from metabolites that in standard spectroscopy protocols complicate the spectrum. It targets the weakly coupled resonance of mIns at 4.06 ppm rather than the 3.56 ppm resonance that has traditionally been studied; quantification of the 3.56 ppm resonance is complicated by its strong coupling and overlap with a glycine singlet and the coupled resonances of threonine and choline containing compounds.24 This sequence was modified from our previously reported23 method to incorporate a dual-resonance selective RF pulse within a point-resolved spectroscopy sequence for the selective detection of NAA in addition to mIns. Spectral acquisition parameters included TR=2.4 s, TE=132 ms, number of averages=64, sweep width=2.5 kHz, and number of sampling points=2048. LCModel software25 was used to analyze the spectra, by using model spectra of mIns and NAA that were calculated with quantum mechanical simulation incorporating the shaped RF and gradient pulses.26,27 Fractions of gray matter, white matter, and CSF within the voxel were determined by using 1D projections of the water signal intensity from the selected volume.28 Double inversion recovery at echo times of 24 and 800 ms was used to discriminate between the water signals from the 3 compartments based on the differences in their T1 and T2 relaxation times. Gray matter, white matter, and CSF T1 and T2 values used were 1331, 832, and 4000 ms and 100, 75, and 1100 ms, respectively.29 Metabolites were normalized by using the water signal intensity as an internal reference incorporating T1 (NAA=1.3 seconds, mIns=1.4 seconds) and T2 (NAA=270 ms, mIns=150 ms) metabolite relaxation times reported in the literature29,30 to produce metabolite concentrations.
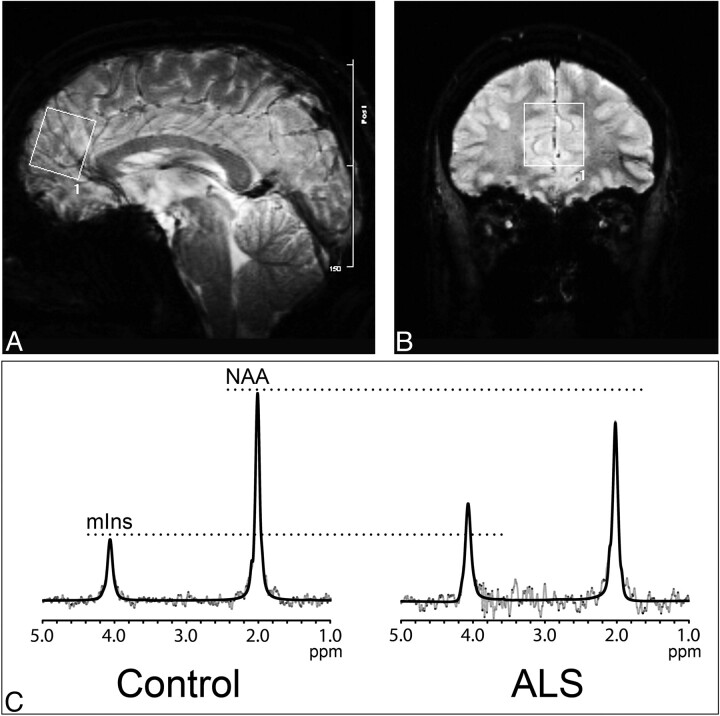
Figure.
Sagittal (A) and coronal (B) gradient-weighted MR images demonstrate voxel placement in the mesial prefrontal cortex. Representative spectra (C, gray lines) and LCModel fit (C, dark lines) with peaks identified for mIns and NAA are shown for a control subject (left) and patient with ALS (right); note the increased mIns and decreased NAA in the patient with ALS compared with the control subject.
Statistics
Group differences in metabolite concentrations were tested with a 2-tailed t test. Relationships with clinical parameters were tested by determination of Pearson (r) correlation coefficients. Statistical significance was accepted for a 2-sided P < .05.
Results
Twenty-four patients with ALS (mean age, 56 ± 14 years) and 15 healthy controls (mean age, 52 ± 16 years) were studied. The proportion of men to women was higher in patients (19:5 versus 5:10, P =.004). At the time of scanning, 4 patients had definite ALS, 15 had probable ALS, and 5 had possible ALS according to El Escorial criteria. Symptom duration at time of scanning was 23 ± 20 months (mean ± SD; median, 14 months; range, 5–75 months). Nineteen patients were limb-onset and 5 were bulbar onset. The ALSFRS-R was 40 ± 6 (median, 41; range, 26–47). Letter F fluency (words per minute) was 13 ± 3 in controls and 13 ± 4 in patients (P =.5). Some subjects deferred further cognitive testing to another day after imaging; however, 1 subject did not return due to an intercurrent illness and subsequent advancing disability, and 2 patients and 2 control subjects were lost to follow-up. Two patients had ALS-FTD, with 1 patient unable to complete the ACE due to cognitive impairment and another scoring 49/100. Excluding these patients, the ACE was not different (P =.3) between patients (89 ± 5) and controls (90 ± 5).
The most striking spectroscopic abnormality was a 17% decrease in NAA/mIns (Table 1). Abnormalities in the individual metabolites, NAA and mIns, were of lesser magnitude and did not reach statistical significance.
Table 1:
Spectroscopy results for all patients
| Metabolite | Controls |
Patients |
Difference in Patients vs Controls (%) | P Value |
|---|---|---|---|---|
| (n=15) | (n=24) | |||
| NAA/mIns | 1.95 ± 0.27 | 1.62 ± 0.31 | −17 | .002 |
| NAA (mmol/L) | 9.82 ± 1.76 | 8.92 ± 1.29 | −9 | .08 |
| mIns (mmol/L) | 5.06 ± 0.76 | 5.63 ± 0.96 | +11 | .06 |
Note:—Spectroscopy quantities are mean ± SD.
There was no significant correlation between NAA/mIns and disease duration, site of onset, ACE, or F fluency. NAA/mIns declined progressively with higher disease stages (Table 2), and spectroscopic indices were markedly abnormal in the 2 patients with ALS-FTD (NAA=8.45, 5.60 mmol/L; mIns=6.84, 5.83 mmol/L; NAA/mIns=1.23, 0.96).
Table 2:
Spectroscopy results
| Metabolite | Controls (n = 15) | ALS-All (n = 24) | El Escorial Criteria |
ALS-FTD (n = 2) | ||
|---|---|---|---|---|---|---|
| Possible (n = 5) | Probable (n = 15) | Definite (n = 4) | ||||
| NAA/mIns | 1.95 ± 0.27 | 1.62 ± 0.31 | 1.65 ± 0.23 | 1.63 ± 0.37 | 1.53 ± 0.10 | 1.10 ± 0.19 |
| NAA (mmol/L) | 9.82 ± 1.76 | 8.92 ± 1.29 | 9.22 ± 0.65 | 8.77 ± 1.36 | 9.14 ± 1.80 | 7.02 ± 2.02 |
| mIns (mmol/L) | 5.06 ± 0.76 | 5.63 ± 0.96 | 5.67 ± 0.89 | 5.06 ± 0.75 | 5.99 ± 1.14 | 6.34 ± 0.71 |
Note:—Spectroscopy quantities are mean ± SD. Patients are categorized according to disease stage by El Escorial Criteria or presence of dementia (ALS-FTD).
Discussion
High-field MRS was used to determine the status of the neuronal marker NAA and glial marker mIns in the PFC of patients with ALS. Given the known pathologic changes of neuronal loss and gliosis that would result in decreased NAA and increased mIns, it was anticipated that the ratio of the 2 (NAA/mIns) would be a more robust marker of degeneration than either NAA or mIns individually. Indeed, NAA/mIns was the most abnormal with a decrease of 17% compared with a 9% reduction in NAA and 11% increase in mIns. Notably, these metabolite differences were in a group of patients in whom only 2 had ALS-FTD. The differences in NAA and mIns did not reach statistical significance; however, the trends were in the expected direction as decreased NAA and elevated mIns are consistent with the histologic features of neuronal loss and gliosis present in this region.7,8,31 These trends are also similar to the pattern and magnitude of change reported in the motor cortex in ALS wherein NAA/mIns was reduced 22%, NAA/Cr was reduced 10%, and mIns/Cr was increased 18%.19 The more pronounced abnormalities in the motor cortex would suggest a greater burden of pathology compared with the frontal lobe, consistent with the pattern of pathologic changes including the distribution of TDP-43 pathology.7 In vivo confirmation of a spatial gradient in neurochemical abnormalities would require a spectroscopic study measuring metabolites in both regions in the same cohort of patients.
Only one other study has evaluated the mesial PFC with MRS; direct comparison of our findings is not possible because of their statistical analysis that was dichotomized according to regional onset of symptoms without a report of differences in the patients as a whole. NAA/Cr was normal in limb onset patients and reduced in the subset (n =5) of bulbar onset patients.2 We did not find a difference between bulbar onset and limb onset patients.
Although the metabolites deviated significantly from the control means in the 2 patients with ALS-FTD, a correlation was not observed with NAA/mIns and cognitive measures. This may be due to the choice of voxel location or the psychometric tests used. The mesial PFC was studied because of its prominent involvement in the ALS-FTD syndrome.5,7 Verbal fluency was specifically measured as a sensitive screening tool of cerebral degeneration beyond the motor cortex in ALS.21,32 ALS patients with impaired verbal fluency have functional and structural imaging abnormalities in the frontotemporal region, including the anterior cingulate where our voxel was placed: functional MR imaging has demonstrated impaired activation in the dorsolateral PFC and anterior cingulate gyrus during a letter fluency task,33 and white matter volume is reduced in motor and nonmotor associative tracts in frontotemporal regions and the cingulum.34 Given these imaging findings, it would be useful to additionally explore correlations of these metabolite indices from the dorsolateral PFC; although it would not be unreasonable to suspect that the degeneration in the mesial PFC is correlated to that in the dorsolateral PFC, this remains to be substantiated by pathologic investigation. At the time of this study, our MRS protocols were not optimized for study of the dorsolateral PFC. A correlation also may have been inapparent due to limited variability in cognitive impairment as evident from the similar letter F and ACE scores in patients and controls.
Mesial PFC dysfunction is associated with behavioral changes in addition to cognitive impairments, including apathy and disinhibition.35 Because such behavioral abnormalities are present in patients with ALS,36 further study is warranted to determine the association of mesial PFC neurochemistry with behavioral psychometric indices.
A disadvantage of our approach that used a sequence optimized for detection of mIns is that other metabolites of potential relevance in ALS, such as glutamate, could not be detected; separate acquisitions would be required for their measurement. However, the gain in number of metabolites accessible with other methods, namely, those incorporating short echo times, must be weighed with the inaccuracies secondary to spectral overlap and the emergence of peaks from macromolecules and lipids. This study aimed to quantify mIns as accurately as possible. Comparative studies by using other methods are required to explore the issue of spectral yield and accuracy.
Conclusions
Extramotor neurodegeneration in the PFC was demonstrated by high-field proton MRS in patients with ALS. The metabolic differences found reflecting neuronal loss and gliosis were present in a group of patients in whom a minority was demented. Further study is necessary to determine the utility of MRS to provide presymptomatic markers of future cognitive decline and in monitoring frontal lobe degeneration.
Abbreviations
- ACE
Addenbrooke's Cognitive Examination
- ALS
amyotrophic lateral sclerosis
- ALSFRS-R
ALS Functional Rating Scale-Revised
- FTD
frontotemporal dementia
- FTLD
frontotemporal lobar degeneration
- MRS
MR spectroscopy
- NAA
N-acetylaspartate
- mIns
myo-inositol
- PET
positron-emission tomography
- PFC
prefrontal cortex
- RF
radio frequency
- TDP-43
TAR DNA binding protein of 43 kDa
Footnotes
This study was funded by the ALS Association of America, ALS Society of Canada, the MSI Foundation of Alberta, the University of Alberta Hospital Foundation, and the Shelly Mrkonjic ALS Research Fund.
Disclosures: Richard Camicioli, Research Support (including provision of equipment or materials): Canadian Institute for Health Research and Michael J. Fox Foundation, Details: Two projects as coinvestigators. One examining MR imaging correlates of gait problems in Parkinson's disease (MJFF), the other looking at imaging correlates of motor response in Parkinson's disease; Wendy S. Johnston, Research Support (including provision of equipment or materials): Canadian Institute for Health Research, Details: “Dignity and Distress across End-of-Life Populations”; PI Harvey Chochinov, University of Manitoba, Role: Site PI, Amount: $587 074; Sanjay Kalra, Research Support (including provision of equipment or materials): Lundbeck, Details: Supplied trial medication for a phase II study of memantine in patients with ALS. This trial is not related to the study in question of the manuscript submitted.
References
- 1. Ringholz GM, Appel SH, Bradshaw M, et al. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology 2005; 65: 586–90 [DOI] [PubMed] [Google Scholar]
- 2. Strong MJ, Grace GM, Orange JB, et al. A prospective study of cognitive impairment in ALS. Neurology 1999; 53: 1665–70 [DOI] [PubMed] [Google Scholar]
- 3. Lomen-Hoerth C, Murphy J, Langmore S, et al. Are amyotrophic lateral sclerosis patients cognitively normal? Neurology 2003; 60: 1094–97 [DOI] [PubMed] [Google Scholar]
- 4. Barson FP, Kinsella GJ, Ong B, et al. A neuropsychological investigation of dementia in motor neurone disease (MND). J Neurol Sci 2000; 180: 107–13 [DOI] [PubMed] [Google Scholar]
- 5. Wilson CM, Grace GM, Munoz DG, et al. Cognitive impairment in sporadic ALS: a pathologic continuum underlying a multisystem disorder. Neurology 2001; 57: 651–57 [DOI] [PubMed] [Google Scholar]
- 6. Ferrer I, Roig C, Espino A, et al. Dementia of frontal lobe type and motor neuron disease. A Golgi study of the frontal cortex. J Neurol Neurosurg Psychiatry 1991; 54: 932–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Geser F, Martinez-Lage M, Robinson J, et al. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol 2009; 66: 180–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yoshida M. Amyotrophic lateral sclerosis with dementia: the clinicopathological spectrum. Neuropathology 2004; 24: 87–102 [DOI] [PubMed] [Google Scholar]
- 9. Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006; 314: 130–33 [DOI] [PubMed] [Google Scholar]
- 10. Chang JL, Lomen-Hoerth C, Murphy J, et al. A voxel-based morphometry study of patterns of brain atrophy in ALS and ALS/FTLD. Neurology 2005; 65: 75–80 [DOI] [PubMed] [Google Scholar]
- 11. Agosta F, Pagani E, Rocca MA, et al. Voxel-based morphometry study of brain volumetry and diffusivity in amyotrophic lateral sclerosis patients with mild disability. Hum Brain Mapp 2007; 28: 1430–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abe K, Fujimura H, Toyooka K, et al. Cognitive function in amyotrophic lateral sclerosis. J Neurol Sci 1997; 148: 95–100 [DOI] [PubMed] [Google Scholar]
- 13. Abrahams S, Goldstein LH, Kew JJ, et al. Frontal lobe dysfunction in amyotrophic lateral sclerosis. A PET study. Brain 1996; 119: 2105–20 [DOI] [PubMed] [Google Scholar]
- 14. Lloyd CM, Richardson MP, Brooks DJ, et al. Extramotor involvement in ALS: PET studies with the GABA(A) ligand [(11)C]flumazenil. Brain 2000; 123 (Pt 11): 2289–96 [DOI] [PubMed] [Google Scholar]
- 15. Rooney WD, Miller RG, Gelinas D, et al. Decreased N-acetylaspartate in motor cortex and corticospinal tract in ALS. Neurology 1998; 50: 1800–05 [DOI] [PubMed] [Google Scholar]
- 16. Abe K, Takanashi M, Watanabe Y, et al. Decrease in N-acetylaspartate/creatine ratio in the motor area and the frontal lobe in amyotrophic lateral sclerosis. Neuroradiology 2001; 43: 537–41 [DOI] [PubMed] [Google Scholar]
- 17. Block W, Karitzky J, Traber F, et al. Proton magnetic resonance spectroscopy of the primary motor cortex in patients with motor neuron disease: subgroup analysis and follow-up measurements. Arch Neurol 1998; 55: 931–36 [DOI] [PubMed] [Google Scholar]
- 18. Bowen BC, Pattany PM, Bradley WG, et al. MR imaging and localized proton spectroscopy of the precentral gyrus in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol 2000; 21: 647–58 [PMC free article] [PubMed] [Google Scholar]
- 19. Kalra S, Hanstock CC, Martin WR, et al. Detection of cerebral degeneration in amyotrophic lateral sclerosis using high-field magnetic resonance spectroscopy. Arch Neurol 2006; 63: 1144–48 [DOI] [PubMed] [Google Scholar]
- 20. Brooks BR, Miller RG, Swash M, et al. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000; 1: 293–99 [DOI] [PubMed] [Google Scholar]
- 21. Strong MJ, Grace GM, Freedman M, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2009; 10: 131–46 [DOI] [PubMed] [Google Scholar]
- 22. Mathuranath PS, Nestor PJ, Berrios GE, et al. A brief cognitive test battery to differentiate Alzheimer's disease and frontotemporal dementia. Neurology 2000; 55: 1613–20 [DOI] [PubMed] [Google Scholar]
- 23. Choi C, Ogilvie CJ, Malykhin N, et al. Detection of the myo-inositol 4.06-ppm resonance by selective J rewinding: application to human prefrontal cortex in vivo. Magn Reson Med 2005; 54: 1536–40 [DOI] [PubMed] [Google Scholar]
- 24. Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed 2000; 13: 129–53 [DOI] [PubMed] [Google Scholar]
- 25. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993; 30: 672–79 [DOI] [PubMed] [Google Scholar]
- 26. Thompson RB, Allen PS. Sources of variability in the response of coupled spins to the PRESS sequence and their potential impact on metabolite quantification. Magn Reson Med 1999; 41: 1162–69 [DOI] [PubMed] [Google Scholar]
- 27. Choi C, Bhardwaj PP, Seres P, et al. Measurement of glycine in human brain by triple refocusing 1H-MRS in vivo at 3.0T. Magn Reson Med 2008; 59: 59–64 [DOI] [PubMed] [Google Scholar]
- 28. Choi C, Coupland NJ, Bhardwaj PP, et al. T2 measurement and quantification of glutamate in human brain in vivo. Magn Reson Med. 2006; 56: 971–77 [DOI] [PubMed] [Google Scholar]
- 29. Wansapura JP, Holland SK, Dunn RS, et al. NMR relaxation times in the human brain at 3.0 Tesla. J Magn Reson Imaging 1999; 9: 531–38 [DOI] [PubMed] [Google Scholar]
- 30. Traber F, Block W, Lamerichs R, et al. 1H metabolite relaxation times at 3.0 Tesla: Measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. J Magn Reson Imaging 2004; 19: 537–45 [DOI] [PubMed] [Google Scholar]
- 31. Nagy D, Kato T, Kushner PD. Reactive astrocytes are widespread in the cortical gray matter of amyotrophic lateral sclerosis. J Neurosci Res 1994; 38: 336–47 [DOI] [PubMed] [Google Scholar]
- 32. Abrahams S, Leigh PN, Harvey A, et al. Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS). Neuropsychologia 2000; 38: 734–47 [DOI] [PubMed] [Google Scholar]
- 33. Abrahams S, Goldstein LH, Simmons A, et al. Word retrieval in amyotrophic lateral sclerosis: a functional magnetic resonance imaging study. Brain 2004; 127: 1507–17 [DOI] [PubMed] [Google Scholar]
- 34. Abrahams S, Goldstein LH, Suckling J, et al. Frontotemporal white matter changes in amyotrophic lateral sclerosis. J Neurol 2005; 252: 321–31 [DOI] [PubMed] [Google Scholar]
- 35. Hornak J, O'Doherty J, Bramham J, et al. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci 2004; 16: 463–78 [DOI] [PubMed] [Google Scholar]
- 36. Grossman AB, Woolley-Levine S, Bradley WG, et al. Detecting neurobehavioral changes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2007; 8: 56–61 [DOI] [PubMed] [Google Scholar]