SUMMARY:
Embolization of head and neck hypervascular tumors is a well-established therapeutic technique. Preoperative embolization reduces intraoperative blood loss, shortens the length of surgery, and decreases surgical morbility and mortality. This study assesses the safety and efficacy of preoperative embolization of meningiomas fed by the OPH by using Onyx liquid embolic agent.
Preoperative embolization of meningiomas can reduce surgical blood loss, shorten operation length, reduce the risk of damage to surrounding structures, and increase the likelihood of complete tumor resection.1–11 Embolization becomes especially important when meningiomas are located at the skull base, because of the difficulties of bleeding management in this region.2,11,12 The blood supply of meningiomas usually arises from branches of the external carotid artery, except in cases of anterior and middle skull base tumors. In these cases, hypervascularized tumors are often fed by internal carotid artery branches, such as the OPH.2–5,12–15 In this situation, the benefits of preoperative embolization must be weighed against the risk of complications, as an inadvertent interruption of blood flow to the central retinal artery may lead to retinal ischemia and visual compromise.1,8,13–16
The preoperative embolization of meningiomas supplied by the OPH requires superselective catheterization and the most distal placement of the microcatheter as possible, aiming to protect the central retinal artery of any reflux. There are several embolic materials, such as PVA particles, spheres, and glue, that were widely used in the literature.3,4,6,14–18 However, these embolic agents are usually injected by using guide-dependent microcatheters that are known to have limited distal reach.
Liquid embolic agent reflux into the OPH could lead to inadvertent occlusion of the central retinal artery.3,14–18 This article reports our experiences with the preoperative embolization of meningiomas fed by OPH branches by using Onyx (ev3, Irvine, California). This endovascular approach with Onyx was proposed as an alternative strategy for a more controlled embolization, aiming to reduce the risks of visual impairment caused by central retinal artery occlusion.
Materials and Methods
From February 2008 to January 2010, 5 patients with untreated intracranial meningiomas fed mainly by OPH branches underwent preoperative embolization with Onyx-18 at our institution. There were 2 men and 3 women, with a mean age of 56 with an SD of 11.11 years (median, 53.3 years; range, 46–74 years).
Clinical presentation included headache in 3 patients, confusion in 3, anosmia in 1 patient, decreased visual acuity in 1 patient, and epilepsy in 1 patient. No patient had appendicular deficits. All patients underwent a CT scan, MR imaging, and DSA. Hypervascular blush identified at DSA was present in all cases.
Three meningiomas were located at the olfactory groove, 1 meningioma at the left lesser sphenoid wing, and 1 meningioma in the right sphenoid ring. The mean diameter of the largest tumor was 5.14 cm (median, 5.75 cm; range, 3–7 cm). There were of 2 ± 1 (mean ± SD) arterial feeders for each tumor (median, 2; range, 1–3). The OPH, combined or isolated, was involved in all cases. The MMA was involved in 2 cases but made only a minor contribution (Table). Onyx injection was adopted as the single endovascular treatment technique for all cases. The percentage of tumor devascularization was estimated by comparing the pre- and postembolization tumor blush at DSA as follows: slight devascularization, if pre-embolization blush was reduced in <30%; moderate devascularization, if reduced in 30%–60%; extensive devascularization, if reduced in 60%–90%; and complete, if reduced in >90%.
Summary of 5 patients with meningiomas treated with Onyx injection via OPH branches
| Case No. | Age (yr), Sex | Presentation | Localization | Size (cm) | Arterial Supply | Onyx Injection | Length of Injection (min) | Devascularization | Compli-cations | Postembolization Visual Acuity |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46, M | Confusion, apathy | Olfactory groove | 6.0 | Both ACA (meningeal branches), left OPH (accessory meningeal branch) | 0.7 mL via OPH | 31 | Complete | None | Normal |
| 2 | 59, M | Chronic headache | Left lesser sphenoid wing | 4.2 | Left OPH (recurrent meningeal branch), left anterior temporal artery, left MMA | 4.0 mL via OPH | 29 | Extensive | Transitory left CN palsy, peritumoral hemorrhage | Normal |
| 3 | 74, F | Headache, confusion, anosmia, decreased left eye acuity | Olfactory groove | 5.5 | Left OPH (anterior meningeal branch) | 0.7 mL via OPH | 30 | Extensive | None | Unalterated |
| 4 | 50, F | Headache, confusion | Olfactory groove | 7.0 | Both OPH (anterior meningeal and ethmoid branches), left MMA (anterior branch) | 5.5 mL via OPH | 30 | Extensive | None | Normal |
| 5 | 51, F | Epilepsy | Right lesser sphenoid wing | 3.0 | Right OPH (recurrent meningeal branch) | 1.2 mL via OPH | 25 | Complete | None | Normal |
All patients underwent a contrast-enhanced CT scan after embolization and were followed-up clinically after the surgical treatment. Histologic samples of the tumor and intraoperative surgical findings were analyzed. Pathologic analysis confirmed the diagnosis of meningioma in all cases.
The measurement of bleeding was empiric, based on the experience of the neurosurgery team. The neurosurgeons also observed a reduction in surgical time, as well as a facilitation of the cleavage plane view of the tumor and coagulation of adjacent vessels by the presence of dark color left by Onyx agent.
Endovascular Procedure
All procedures were performed under general anesthesia. An intravenous bolus of 5,000 IU of heparin was administered at the beginning of the procedure. Vascular access was obtained through the right common femoral artery. A complete cerebral angiogram that included both internal carotid arteries, both external carotid arteries, and the ipsilateral vertebral artery, was performed before treatment to identify the arterial feeders of the tumor and possible dangerous anastomoses. A 6F guiding catheter was placed at the origin of the internal carotid artery and then a microcatheter (Marathon; ev3) was advanced over a 0.008 microguidewire (Mirage; ev3) into the OPH branch. The microcatheter was positioned distally to the origin of the central retinal artery, aiming to rest as close as possible to the tumor, or at least in position of blockade of the afferent artery. The microcatheter was washed with 0.3 mL of dimethyl-sulfoxide followed by slow injection of Onyx for 40 seconds to fill the microcatheter lumen. Short injections of Onyx were gently performed, under fluoroscopy, aiming to keep the amount of reflux as minimal as possible.
Each treatment consisted of a single Onyx injection after 1 single feeder catheterization. No provocative test was used.
Results
Immediate Postembolization Angiographic Results
After the Onyx injection, complete devascularization of the tumor was achieved in 2 patients and extensive devascularization in 3 patients, as demonstrated on the angiography performed immediately after the embolization procedure (Figs 1 and 2).
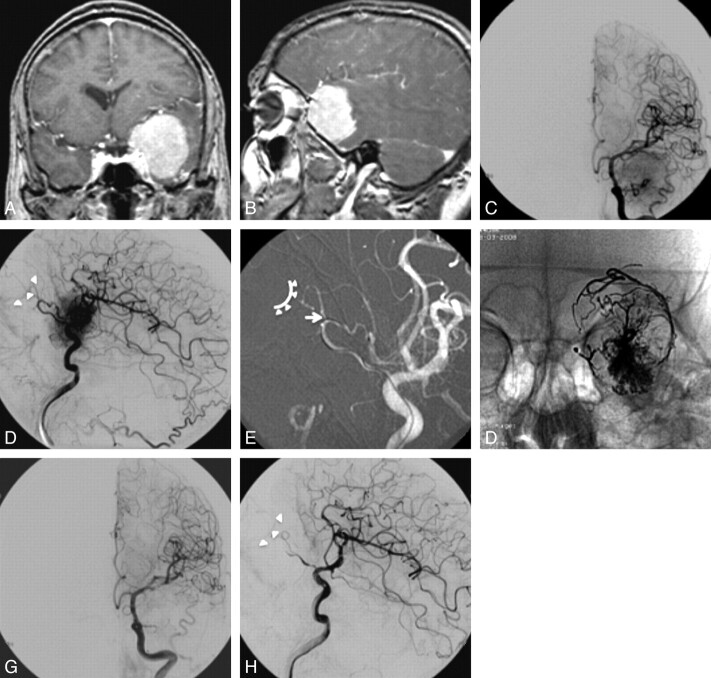
Fig 1.
Coronal (A) and sagittal (B) T1-weighted postgadolinium MR imaging showing a large meningioma, on the left great sphenoid wing, with intense and homogeneous contrast enhancement. DSA of the left ICA, in anteroposterior (C) and lateral views (D), showing intense tumoral blush, fed mainly by recurrent meningeal artery branches of the OPH. E, Roadmap of left internal carotid artery showing the microcatheter positioned in recurrent meningeal artery. Arrow shows the probable OPH segment from where the central retinal artery arises. Arrowheads and curved line delimitate the location of the choroidal crescent. F, Anteroposterior view X-ray showing the final cast of Onyx; DSA of left internal carotid artery (G) and lateral (H) views, with preservation of the choroidal crescent (arrowheads).
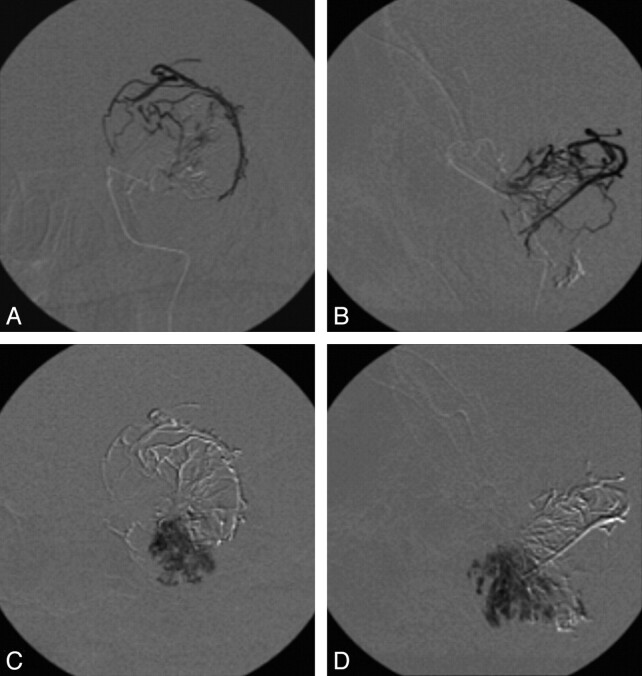
Fig 2.
Digital subtracted fluoroscopy in anteroposterior view (A) and lateral view (B) demonstrating the filling of the major afferent arteries of the meningioma. Digital subtracted fluoroscopy in anteroposterior view (C) and lateral view (D), demonstrating the deep penetration into small-to-medium tumor vessels.
All Onyx injections were performed via an ophthalmic branch, with good penetration inside the tumor. The volume of Onyx injected ranged from 0.7 to 5.5 mL per procedure (mean, 2.42 mL). The duration of the injection per pedicle ranged from 25 to 31 minutes (mean, 29 minutes).
In all cases, OPH branches to the retina were preserved. The choroidal crescent of the globe was preserved in all postembolization DSA. No hemorrhagic or thromboembolic complications occurred as a consequence of the endovascular procedure. Microcatheter withdrawal was performed without incident in all patients.
No patient experienced decreased vision function after endovascular treatment. One patient with decreased visual acuity before embolization maintained the same preprocedure status. The complication rate was 20% (1/5). One patient with a lesser sphenoid wing lesion experienced oculomotor nerve palsy after embolization, which was probably due to minor peritumoral bleeding demonstrated by CT. The palsy resolved completely after surgery.
In all cases, postembolization CT scan did not demonstrate worsening of peritumoral edema. Patients were surgically treated between the first and fourth day after embolization. All tumors were successfully removed with minimal blood loss during the operation. None of the patients needed blood transfusion. All patients had an uneventful recovery.
Discussion
Surgery of hypervascular brain tumors remains a challenge despite all the advances in microsurgical techniques,3,8 and still frequently requires blood transfusions due to intraoperative hemorrhage. Among these lesions, skull base meningiomas can pose unique challenges due to inadequate access to their feeding vessels. By comparison, convexity meningiomas often receive their vascular supply from the dura adjacent to the calvaria; thus, its vascular pedicle is among the first structures encountered in the surgical approach, making its resection significantly easier. In cranial base surgery, access to the vascular pedicle is usually obtained only after most of the lesion has been resected.9,12,13,19
For skull base meningiomas, embolization can be a valuable and effective preoperative procedure to facilitate surgery and to reduce intraoperative blood loss.3,8–10,12,13,17,19–22 However, these benefits should be analyzed considering the particularities of each case.
Serious complications of meningioma embolization are not infrequent. Previous studies by using particles had complication rates ranging from 3.6% to 12%.20 When only skull base meningiomas were selected, these rates increased to 21.6% of patients, with permanent deficits occurring in 9%.1,8,9,19,20 Ischemic or hemorrhagic events are the major cause of complications in these studies. Ischemic events may be attributed to thromboembolism or unintentional obstruction of parenchymal branches, whereas hemorrhagic events may be associated with necrosis as a result of the deep penetration of particles inside the tumor and occlusion of its draining veins.1,8,19,20 Given that anterior and middle skull base meningiomas frequently recruit a significant blood supply from OPH branches, endovascular embolization is not considered a presurgical routine for these cases, because it can lead to the risk of occlusion of the central retinal artery, causing visual compromise.9,13,14,16,22,23
Many studies reporting meningioma embolization through OPH branches have described their methods through the following steps: 1) identify the choroidal blush; 2) navigate the catheter beyond the central retinal artery; 3) use larger PVA particles; and 4) in some situations, perform an amytal/lidocaine test to evaluate the effect of the occlusion of particular vessels on vision. However, these precautions do not guarantee preservation of these vessels. False-positive and false-negative provocative tests have been reported, and PVA particles have been shown to fragment into smaller sizes during the mixing process, thus contaminating the PVA mixture with particles capable of occluding the central retinal artery.1,3,7,12,14,15 Terada et al15 reported 4 cases of presurgery embolization of meningiomas through OPH branches. In 1 case of their series, a patient who had the meningioma embolized with Gelfoam powder (Phadia, Uppsala, Sweden) presented postembolization visual deficit. Lefkowitz et al14 presented 12 cases of patients with neurosurgical lesions involving the OPH. One of the patients, who had a positive provocative test, had an orbital meningioma that was not considered suitable for embolization due to its distal location in the OPH vasculature and consequent proximity to the central retinal artery.
Thanks to the nonadhesive properties of Onyx, a more controlled intratumoral injection could be achieved. This may be partially explained by its low precipitation rate, which allows deep penetration into small-to-medium tumor vessels before its solidification. Another advantage is the possibility of retrograde filling of the dural feeders with a lower risk of inadvertent central retinal artery occlusion.2,4,17 In the present series, all patients were treated exclusively with Onyx injection through the OPH, and satisfactory tumor devascularization was obtained with no visual impairment. Understanding the anatomy of the OPH, being able to advance the microcatheter tip to a safe position, and performing very slow, intermittent Onyx injections while avoiding excessive reflux are necessary to achieve good postembolization results.
Alvarez et al24 described a “safety point” easily recognized on angiogram, which should be considered as a secure limit for embolization in OPH. Their study, based on embryologic analysis, showed that visual deficit can be avoided if embolization is restricted to the vessels anterior to this safety point, because the central retinal artery arises proximally to this point. The OPH can be divided into 3 segments: 1) a segment extending from the entrance of the OPH into the orbit, to the point where the vessel changes direction to cross over or under the optic nerve; 2) a short part of the vessel as it passes under or over the optic nerve; and 3) a segment that extends from the bend in the vessel on the medial aspect of the optic nerve, to the edge of the orbit.
The embolization can be done beyond the safety point, which is anywhere beyond segment 2 with minimal risk of embolization of the retina.
In the current study, the endovascular procedure, specifically the devascularization of the tumor, was evaluated based on a subjective analysis of the postembolization angiography.
Even with all the precautions listed, the procedure is not completely without risk as indicated by the peritumoral hemorrhage in 1 of the 5 patients (20%).
This hemorrhage may be associated with necrosis resulting from the deep and distal penetration of the Onyx agent inside the tumor and occlusion of its draining veins, similar to that described with particles.1,8,19,20
Adhering to the rules discussed in the text can decrease the possibility of complications but cannot prevent them.
Conclusions
Considering the favorable results obtained in this series, we suggest that the use of Onyx as an embolic agent for preoperative embolization of meningiomas fed by OPH branches should be regarded as a feasible option. However, even with our positive results supporting the safety and effectiveness of this procedure, comparative studies with other embolic agents, eg, particles, are necessary to draw conclusions regarding the superiority of this technique.
Abbreviations
- ACA
anterior cerebral artery
- DSA
digital subtraction angiography
- MMA
middle meningeal artery
- OPH
ophthalmic artery
- PVA
polyvinyl alcohol.
Footnotes
Disclosures: Monica Manisor, Research Support (including provision of equipment or materials): Neurosurgery, Speaker Bureau: NHC, Consultant; Prof. Mounayer, Ownership Interest: Neurovascular.
References
- 1. Carli DF, Sluzewski M, Beute GN, et al. Complications of particle embolization of meningiomas: frequency, risk factors, and outcome. AJNR Am J Neuroradiol 2010; 31: 152–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kerim AA, Bonneville F, Jean B, et al. Balloon-assisted embolization of skull base meningioma with liquid embolic agent. J Neurosurg 2010; 112: 70–72 [DOI] [PubMed] [Google Scholar]
- 3. Yoon YS, Ahn JY, Chang JH, et al. Pre-operative embolisation of internal carotid artery branches and pial vessels in hypervascular brain tumours. Acta Neurochir (Wien) 2008; 150: 447–52 [DOI] [PubMed] [Google Scholar]
- 4. Shi ZS, Feng L, Jiang XB, et al. Therapeutic embolization of meningiomas with Onyx for delayed surgical resection. Surg Neurol 2008; 70: 478–81 [DOI] [PubMed] [Google Scholar]
- 5. Kusaka N, Tamiya T, Sugiu K, et al. Combined use of TruFill DCS detachable coil system and Guglielmi detachable coil for embolization of meningioma fed by branches of the cavernous internal carotid artery. Neurol Med Chir (Tokyo) 2007; 47: 29–31 [DOI] [PubMed] [Google Scholar]
- 6. Kai Y, Hamada JI, Morioka M, et al. Clinical evaluation of cellulose porous beads for the therapeutic embolization of meningiomas. AJNR Am J Neuroradiol 2006; 27: 1146–50 [PMC free article] [PubMed] [Google Scholar]
- 7. Kunikata H, Tamai M. Cilioretinal artery occlusions following embolization of an artery to an intracranial meningioma. Graefes Arch Clin Exp Ophthalmol 2006; 244: 401–03 [DOI] [PubMed] [Google Scholar]
- 8. Bendszus M, Monoranu CM, Schütz A, et al. Neurologic complications after particle embolization of intracranial meningiomas. AJNR Am J Neuroradiol 2005; 26: 1413–19 [PMC free article] [PubMed] [Google Scholar]
- 9. Rosen CL, Ammerman JM, Sekhar LN, et al. Outcome analysis of preoperative embolization in cranial base surgery. Acta Neurochir (Wien) 2002; 144: 1157–64 [DOI] [PubMed] [Google Scholar]
- 10. Kai Y, Hamada J, Morioka M, et al. Appropriate interval between embolization and surgery in patients with meningioma. AJNR Am J Neuroradiol 2002; 23: 139–42 [PMC free article] [PubMed] [Google Scholar]
- 11. Oka H, Kurata A, Kawano N, et al. Preoperative superselective embolization of skull-base meningiomas: indications and limitations. J Neurooncol 1998; 40: 67–71 [DOI] [PubMed] [Google Scholar]
- 12. Hirohata M, Abe T, Morimitsu H, et al. Preoperative selective internal carotid artery dural branch embolization for petroclival meningiomas. Neuroradiology 2003; 45: 656–60 [DOI] [PubMed] [Google Scholar]
- 13. White DV, Sincoff EH, Abdulrauf SI. Anterior ethmoidal artery: microsurgical anatomy and technical considerations. Neurosurgery 2005; 56 (2 Suppl): 406–10, discussion 406–10 [DOI] [PubMed] [Google Scholar]
- 14. Lefkowitz M, Giannotta SL, Hieshima G, et al. Embolization of neurosurgical lesions involving the ophthalmic artery. Neurosurgery 1998; 43: 1298–303 [DOI] [PubMed] [Google Scholar]
- 15. Terada T, Kinoshita Y, Yokote H, et al. Preoperative embolization of meningiomas fed by ophthalmic branch arteries. Surg Neurol 1996; 45: 161–66 [DOI] [PubMed] [Google Scholar]
- 16. Turner T, Trobe JD, Deveikis JP. Sequential branch retinal artery occlusions following embolization of an intracranial meningioma. Arch Ophthalmol 2002; 120: 857–60 [PubMed] [Google Scholar]
- 17. Rossitti S. Preoperative embolization of lower-falx meningiomas with ethylene vinyl alcohol copolymer: technical and anatomical aspects. Acta Radiol 2007; 48: 321–26 [DOI] [PubMed] [Google Scholar]
- 18. Rodiek SO, Stölzle A, Lumenta ChB. Preoperative embolization of intracranial meningiomas with Embosphere microspheres. Minim Invasive Neurosurg 2004; 47: 299–305 [DOI] [PubMed] [Google Scholar]
- 19. Yu SC, Boet R, Wong GK, et al. Postembolization hemorrhage of a large and necrotic meningioma. AJNR Am J Neuroradiol 2004; 25: 506–08 [PMC free article] [PubMed] [Google Scholar]
- 20. Dowd CF, Halbach VV, Higashida RT. Meningiomas: the role of preoperative angiography and embolization. Neurosurg Focus 2003; 15: E10 [DOI] [PubMed] [Google Scholar]
- 21. Bendszus M, Martin-Schrader I, Schlake HP, et al. Embolisation of intracranial meningiomas without subsequent surgery. Neuroradiology 2003; 45: 451–55 [DOI] [PubMed] [Google Scholar]
- 22. Engelhard HH. Progress in the diagnosis and treatment of patients with meningiomas. Part I: Diagnostic imaging, preoperative embolization. Surg Neurol 2001; 55: 89–101 [DOI] [PubMed] [Google Scholar]
- 23. Gruber A, Killer M, Mazal P, et al. Preoperative embolization of intracranial meningiomas: a 17-years single center experience. Minim Invasive Neurosurg 2000; 43: 18–29 [DOI] [PubMed] [Google Scholar]
- 24. Alvarez H, Rodesch G, Garcia-Monaco R, et al. Embolisation of the ophthalmic artery branches distal to its visual supply. Surg Radiol Anat 1990; 12: 293–97 [DOI] [PubMed] [Google Scholar]