Abstract
BACKGROUND AND PURPOSE:
Hemodynamics have been shown to play an important role in the initiation and progress of intracranial aneurysms, and are considered well-related to vascular configuration. The purpose of this study was to quantify the vascular geometry change due to intracranial stent placement and to discuss its potential effects on hemodynamics.
MATERIALS AND METHODS:
Imaging data of patients with wide-neck AcomA aneurysms, treated with stent-assisted coiling between January 2005 and January 2010, were retrospectively analyzed. The angle between the afferent vessels (A1 segment) and the efferent vessels (ipsilateral or contralateral A2 segment) was calculated to determine the exact change in the angle after stent placement.
RESULTS:
In all 20 patients, the stent caused a distinct change in the geometry of the parent vessel. Stent-related vascular angle change ranged from 7.60 to 74.88°, with an average of 29.95°. In 10 cases, the angle changed by >30°. In the 12 patients with the distal segment of the stent placed in the ipsilateral A2 segment, the mean postoperative A1-A2 angle increased by 27.71 ± 13.17° (from 7.60° to 48.29°). In the other 8 patients with the distal segment of the stent placed in the contralateral A2 segment, the mean postoperative A1-AcomA-A2 angle increased by 33.29 ± 21.89°(from 15.49° to 74.88°).
CONCLUSIONS:
In addition to serving as a scaffold to contain coils, stent placement for AcomA aneurysms has a substantial effect on the vascular geometry, which may result in local hemodynamic changes.
With the continuing evolutions of endovascular techniques and devices, EVT for patients with intracranial aneurysms is increasingly safer and effective. Currently, the technique is widely accepted as a valid alternative to surgical clipping in the treatment of intracranial aneurysms.1 Wide-neck, large, and giant aneurysms might have posed challenges to the traditional endovascular treatment of aneurysms. Stent placement to prevent coil protruding into the parent artery has been successfully applied to intracranial aneurysm treatment. It effectively expands the indications of EVT for cerebral aneurysms.
Hemodynamics have been shown to play an important role in the initiation, growth, and rupture of intracranial aneurysms. The placement of a stent across the aneurysmal neck significantly alters hemodynamics in the aneurysmal sac.2–4 Hemodynamic parameters are well known to be especially sensitive to variations in vessel geometry, including morphology of aneurysmal sac and morphologic relationship between aneurysm and parent vessel.5–7
In this study, we retrospectively analyzed the imaging data of patients with wide-neck AcomA aneurysms treated with a stent-assisted coiling technique. The current objectives were to quantify the vascular angle changes due to intracranial stent placement and discuss the stent-associated effects on hemodynamics.
Materials and Methods
Patients
In this retrospective study, only the imaging data of wide-neck AcomA aneurysms, treated with stent-assisted coiling from January 2005 to January 2010, were analyzed. All the patients included in this series had an angiographic imaging that clearly displayed afferent and efferent vessels of aneurysms pre- and poststenting. Then, only patients with a minimal imaging follow-up period of 6 months were included.
Patients treated with stent placement in an aneurysmal sac technique were excluded. This technique refers to a procedure where the stent is placed partially into the aneurysm and into the afferent artery, and then the portion of the stent protruding into the aneurysm fundus provides neck support for the subsequent successful coiling.8 Patients with severe vasospasm (>50% luminal narrowing, which is related to subarachnoid hemorrhage) of A1 and A2 segments also were excluded.
Treatment
In all patients, endovascular embolization was performed under general anesthesia. All patients received systemic heparinization after placement of the sheath. The activated clotting time was maintained at 2–3 times the baseline throughout the procedure. For patients with unruptured or recanalized aneurysms, dual antiplatelet drugs (75 mg/day clopidogrel and 300 mg/day aspirin) were given for 3 days before the procedure. For patients with acutely ruptured aneurysms, a loading dose of clopidogrel and aspirin (300 mg each) was administered orally or rectally at 2 hours before stent placement. All the patients were maintained on aspirin and clopidogrel for 6 weeks, followed by aspirin alone, which was continued indefinitely.
A guiding catheter was placed in the distal internal carotid artery. Neuroform stents (Boston Scientific, Natick, Massachusetts) were used to treat these wide-neck AcomA aneurysms. All the aneurysms were treated with stent-assisted coiling. At the end of coiling, an image was acquired to confirm adequate coiling. After EVT, all patients were transferred to the intensive care unit, where they underwent fluid balance, neurologic status, and blood pressure close surveillances. Low-molecular-weight heparin (40 mg every 12 hours by hypodermic injection) was administered immediately after the procedure for 3 days.
Three different therapeutic strategies were used in this series: 1) stent-jacket technique in 10 aneurysms: the stent was first implanted to bridge the wide aneurysm neck, and then the coil microcatheter was positioned into the aneurysm sac through the mesh of the stent for coil embolization; 2) stent-jailing technique in 5 aneurysms: the aneurysmal sac was catheterized before stent placement. After introducing the first coil (not detached), the stent was deployed to stabilize the microcatheter. Subsequent coils were then introduced into the aneurysm sac; and 3) bailout technique in 5 other aneurysms. In these cases, we initially planned to coil alone. However, coil protrusion into the parent artery was observed after coiling. For fear of further thrombosis, an additional stent was implanted to preserve the patency of parent vessels.
Measurements
For all imaging data, 2 measurements were obtained, as follows: 1) the development situation of bilateral anterior cerebral arteries and 2) the angle between the afferent and efferent vessels before and after stent placement.
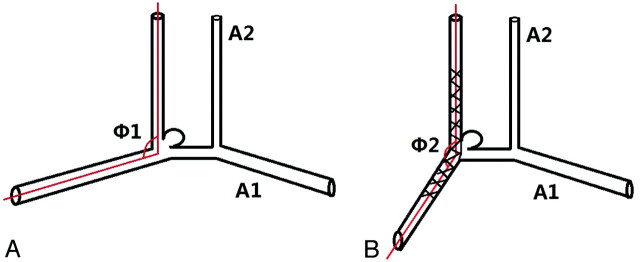
To avoid overlapping of blood vessels, we chose the branch vessels and perforating branches for reference. On this basis, we selected an angiographic view that could display the afferent and efferent vessels clearly for measurement. The diameters of the initial, middle, and terminal points of the bilateral A1 were measured in anteroposterior view of internal carotid artery angiogram with the same magnification ratio and the average value of the 3 measurement figures was calculated as the diameter of A1. Hypoplasia was defined when the difference in diameter between the left and right A1 segment was >2-fold.9 In a selected view of an angiogram image, a dot was first marked: the center of the parent vessel at the area where the aneurysmal neck was located. Then, taking the dot as the initial point, 2 lines parallel to afferent and efferent vessels were marked. The angles were then measured for 2 intersecting lines (Fig. 1). The angle change before and after stent placement was calculated.
Fig 1.
Schematic drawing showing the measurements obtained: the angles Φ1 and Φ2 formed between the A1 segment and A2 segment, before (A) and after (B) stent placement. The angle Φ was initially marked by lines. A dot was marked on the middle of aneurysmal neck. Then, 2 lines parallel to inflow tract and outflow tract were marked. The angle Φ was measured for 2 intersecting lines. The angle change between Φ1 and Φ2 was calculated.
Follow-Up
Imaging follow-up included MRA at 3, 24, and 36 months after treatment and a DSA at 6 and 12 months. Follow-up DSA and MRA were then compared with immediate postembolization angiograms. Recurrence was defined as aneurysm progression between the initial postprocedural angiographic control and the subsequent angiographic control with change in the aneurysm category. Both postembolization and follow-up angiograms were graded on a 3-point Raymond scale. (Raymond 1 = complete obliteration of aneurysm including the neck, Raymond 2 = contrast filling of the neck of the aneurysm without opacification of the aneurysm sac, and Raymond 3 = contrast filling of the sac of the aneurysm).
Statistical Analysis
All aneurysms were reviewed by 2 independent physicians. Disagreement was resolved by discussion between them. If the disagreement could not be resolved by discussion, then the opinion of a senior reviewer was solicited. The angles measured by the 2 reviewers were compared by using a t test. All statistical analyses were performed by using Statistical Program for Social Sciences version 11.0 for Windows (SPSS, Chicago, Illinois).
Results
In total, 20 patients with wide-neck AcomA aneurysms were available for analysis. The patients' mean age was 51.3 ± 10.1 years (range, 45–73 years), including 13 men and 7 women. All 20 patients were successfully treated with a stent-assisted coiling technique.
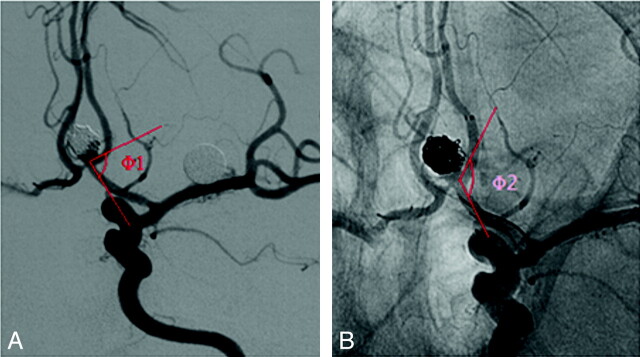
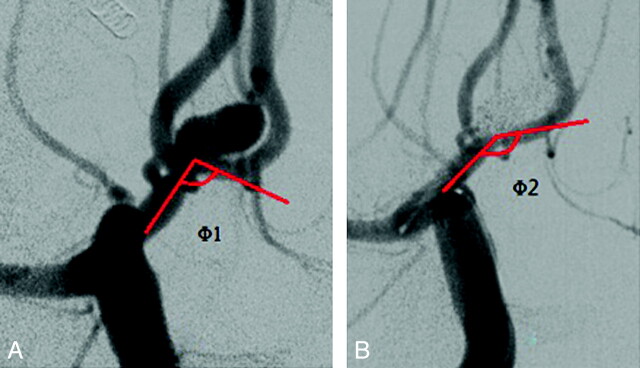
According to the distal location of the stent, it was placed in the ipsilateral A2 segment in 12 patients (Fig 2), and in the contralateral A2 segment through the AcomA in 8 other patients (Fig 3). In total, 65.0% (13/20) of the patients had AcomA aneurysms associated with hypoplasia or aplasia of the A1 segment. In these patients, 8 of the A1 segments were dominant on the right.
Fig 2.
AcomA aneurysm treated with stent-assisted coiling. A, Aneurysm recurrence (Φ1: 77.99°). B, Stent was distally placed in the ipsilateral A2 segment, and the angle of A1 segment to A2 segment increased after stent placement. (Φ2: 117.69°).
Fig 3.
AcomA aneurysm treated with stent-assisted coiling. A, Aneurysm before embolization (Φ1: 104.80°). B, Stent placed across the AcomA into the contralateral A2 segment; the A1-AcomA-A2 angle significantly increased after stent placement. (Φ2: 149.02°).
In all 20 patients, the stent caused a clear change in the geometry of the parent vessel. Stent-related vascular angle increases ranged from 7.60° to 74.88°, with an average of 29.95°. Interobserver agreement between the 2 reviewers for the measurement of the angle was substantial (P = .142, paired t test). In the 12 patients with the stent distally placed in the ipsilateral A2 segment, the mean A1-A2 angle increased by 27.71 ± 13.17° (range, 7.60° to 48.29°) after stent placement. Seven patients' A1-A2 angle increased by >30°; the angle increased between 10° and 30° in 4 patients; and the other patient's A1-A2 angle increased by <10°. In those 8 patients with the stent distally deployed in the contralateral A2 segment through the AcomA, the mean poststent A1-AcomA-A2 angle increased by 33.29 ± 21.89° (range, 15.49° to 74.88°). In 3 patients, the angle between afferent vessels and efferent vessels increased by >30°, and in the other 5, the change was from 10° to 30°.
Efficacy of aneurysm coiling was assessed by using the Raymond scale. Immediate angiography after treatment showed that 15 aneurysms achieved complete occlusion (Raymond scale, 1); 4, near-complete aneurysm occlusion (Raymond scale, 2); and 1, partial occlusion (residual aneurysm, Raymond scale, 3). Angiographic follow-ups for 6–36 months were available in all 20 patients, with a mean of 16.1 months. The partially occluded aneurysm disappeared totally in follow-up DSA, suggesting thrombosis in the aneurysm. All the other aneurysms remained stable without recurrence.
Discussion
The AcomA is the most common location for intracranial aneurysms, as reported in some large surgical or endovascular series.10 Hypoplasia of the artery is a risk factor in the process of aneurysm formation.9,11 In this study, >60% AcomA aneurysms were associated with hypoplasia of the A1 segment, including aplasia of right A1 segment in 1 case. Our findings support previous studies9 showing that hypoplasia of 1 proximal anterior cerebral artery is a risk factor for the development of AcomA aneurysms.
The long-term results of intracranial aneurysms treated with coils alone are not yet completely clear. According to the International Subarachnoid Aneurysm Trial, although there were no significant differences in rebleeding between endovascular coiling and neurosurgical clipping, a higher rate of recurrence was observed in patients who underwent endovascular treatment.12 Coil embolization alone had a higher proportion of residual aneurysm neck, especially in wide-neck aneurysms.13 In patients with residual aneurysmal neck, rates of rebleeding and recurrence were higher than those with complete occlusion.14 During the past decade, stent-assisted coiling treatment has been shown to be technically feasible. Stent placement can prevent coil from protruding into the parent artery, making it effective to treat wide-neck, fusiform, and dissecting intracranial aneurysms. In addition to a mechanical supporting role, more and more studies now focus on the effects on hemodynamics by stent placement.2–4
Hemodynamics have been shown to play an important role in the initiation, growth, and rupture of intracranial aneurysms. Both endovascular embolization and surgical clipping need to isolate the blood flow from the aneurysm. Many studies have been carried out about the role of hemodynamics in intracranial aneurysm prognosis. The role of hemodynamics in aneurysmal recurrence after endovascular embolization also has been profoundly explored.3 These studies showed that hemodynamics in the aneurysm sac are affected by many factors. These are not only affected by the size and shape of the aneurysm but also by the mode of blood flow from the artery into the aneurysm, which is related to the configuration of the parent artery.5,6
Hemodynamic parameters such as wall shear stress are especially sensitive to variations in vessel geometry. Cebral et al15 reported that specific morphology of the parent artery may predict an aneurysmal initiation and rupture. When the morphology of a parent artery is determined, blood pressure and flow velocities have little impact on hemodynamics. The apex of bifurcations is the site of maximum hemodynamic stress in a vascular network. The pressure and wall shear stress on the vessel bifurcation vary with the bifurcation angle. The arterial bifurcation also affects the wall shear stress distribution.16 Hoi et al17 analyzed the correlations between aneurysmal neck size and parent artery curvature by using an ideal model. They found that small geometric variations on the aneurysm model could significantly alter the flow-field and key hemodynamic parameters. Meng et al18 reported that aneurysm models within different curvature vessels had fundamentally different hemodynamics after stent placement. Secondary flow, easy to generate in the high-curvature parent artery, also was reported by Mantha et al.19
In all patients in this study, the deployment of the stent caused a significant change in the geometry of the vessel. Stent-related vascular angle increase ranged from 7.60° to 74.88°, with an average of 29.95°. So, vessel curvature should become small. We were not able to measure angles of curves because we could not objectively determine the beginning, middle, and ending of the curve. Taking previous analyses on aneurysm hemodynamics into consideration, we considered that stent placement directly changed the hemodynamics in aneurysm sac. In addition, stent placement indirectly affected the hemodynamics by changing the parent vascular configuration.
There was a certain recurrence rate even though aneurysms had achieved complete occlusion, which was as high as 30%, especially in wide-neck aneurysms.2 Although coil embolization could affect the hemodynamics in the aneurysm sac, it had no significant effect on hemodynamics of the parent artery. By reducing wall shear stress to the physiologic range and cutting down the impact zone,20 stent placement could promote favorable wall remodeling and potentially stabilize the aneurysm, thereby decreasing risk of rupture and recurrence. The stent could weaken the impinging force exerted on the coil mass and thereby reduce coil compaction by reducing the inflow momentum.18
Liou et al4 compared maximum blood flow velocity between straight and curved vessels before stent placement. They found that the maximum flow velocity in an aneurysmal sac with curved vessels was 15% that of the parent artery, which was approximately 15 times that of the maximum inflow velocity in a straight vessel aneurysm. This indicated that stent placement was important in the treatment of aneurysms in curved vessels by changing vascular geometry. Of the 20 cases in this study, angiographic follow-ups showed no recurrence, including 1 partially occluded aneurysm (Raymond scale, 3) that later disappeared. So, in the treatment of wide-neck AcomA aneurysms, stent-assisted coiling can probably improve the success rate of embolization and parent artery patency. In contrast, the technique, through reducing the parent arterial curvature, can decrease blood jet zone in the aneurysm neck, and this will further reduce recurrence rate. Stent placement changes the angle between afferent and efferent vessels, as well as the parent-aneurysm angle. Recently, Ford et al7 analyzed the blood flow dynamics in a basilar artery tip aneurysm by using computational fluid dynamics simulations. Their findings suggest that the phenotypes of hemodynamics may be anticipated by a relatively simple-to-measure geometric parameter: the angle of the aneurysm bulb relative to the parent artery.
This study first reported the vascular configuration and stent-related vascular angle change because of endovascular stent placement for intracranial aneurysms. In conclusion, stent placement significantly changes the angle between efferent and afferent vessels. It may play an important role in the changes of local hemodynamics, promoting the healing of aneurysms. There are some limitations in this study. First, the sample in this retrospective study was relatively small. Further studies are required with a larger number of cases and adequate follow-up, to identify whether long-term outcome of endovascular treatment is affected by vascular configuration change. Second, the measurements were all made on selected 2D angiographic images based on 3D angiography. It is very difficult to describe the morphologic changes in 3D space and determine uniform measurement standards. Moreover, to identify whether hemodynamics are affected by vascular configuration change because of endovascular stent placement, a computational fluid dynamics study is needed in patient-specific cerebral aneurysm models. Further research in the hemodynamic factors associated with specific morphologic characteristics will improve our understanding of the long-term results of stent placement.
Abbreviations
- AcomA
anterior communicating artery
- DSA
digital subtraction angiography
- EVT
endovascular treatment
- MRA
MR angiography
Footnotes
This study was supported by National Natural Science Foundation of China (grant 30901556) and the Shanghai Committee of Science and Technology (grant 08JC1406400).
References
- 1. Molyneux AJ, Kerr RS, Birks J, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol 2009;8:427–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rhee K, Han MH, Cha SH. Changes of flow characteristics by stenting in aneurysm models: influence of aneurysm geometry and stent porosity. Ann Biomed Eng 2002;30:894–904 [DOI] [PubMed] [Google Scholar]
- 3. Sforza DM, Putman CM, Cebral JR. Hemodynamics of cerebral aneurysms. Annu Rev Fluid Mech 2009;41:91–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liou TM, Li YC, Wang TC. Hemodynamics altered by placing helix stents in an aneurysm at a 45 degrees angle to the curved vessel. Phys Med Biol 2008;53:3763–76 [DOI] [PubMed] [Google Scholar]
- 5. Baharoglu MI, Schirmer CM, Hoit DA, et al. Aneurysm inflow-angle as a discriminant for rupture in sidewall cerebral aneurysms: morphometric and computational fluid dynamic analysis. Stroke 2010;41:1423–30 [DOI] [PubMed] [Google Scholar]
- 6. Sato K, Imai Y, Ishikawa T, et al. The importance of parent artery geometry in intra-aneurysmal hemodynamics. Med Eng Phys 2008;30:774–82 [DOI] [PubMed] [Google Scholar]
- 7. Ford MD, Lee SW, Lownie SP, et al. On the effect of parent-aneurysm angle on flow patterns in basilar tip aneurysms: towards a surrogate geometric marker of intra-aneurysmal hemodynamics. J Biomech 2008;41:241–48 [DOI] [PubMed] [Google Scholar]
- 8. Huang Q, Xu Y, Hong B, et al. Stent-assisted embolization of wide-neck anterior communicating artery aneurysms: review of 21 consecutive cases. AJNR Am J Neuroradiol 2009;30:1502–06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kasuya H, Shimizu T, Nakaya K, et al. Angles between A1 and A2 segments of the anterior cerebral artery visualized by three-dimensional computed tomographic angiography and association of anterior communicating artery aneurysms. Neurosurgery 1999;45:89–93, discussion 93–84 [DOI] [PubMed] [Google Scholar]
- 10. Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1267–74 [DOI] [PubMed] [Google Scholar]
- 11. Gonzalez N, Sedrak M, Martin N, et al. Impact of anatomic features in the endovascular embolization of 181 anterior communicating artery aneurysms. Stroke, 2008;39:2776–82 [DOI] [PubMed] [Google Scholar]
- 12. Mitchell P, Kerr R, Mendelow AD, et al. Could late rebleeding overturn the superiority of cranial aneurysm coil embolization over clip ligation seen in the International Subarachnoid Aneurysm Trial? J Neurosurg 200;108:437–42 [DOI] [PubMed] [Google Scholar]
- 13. Bandeira A, Raphaeli G, Baleriaux D, et al. Selective embolization of unruptured intracranial aneurysms is associated with low retreatment rate. Neuroradiology 2010;52:141–46 [DOI] [PubMed] [Google Scholar]
- 14. Hayakawa M, Murayama Y, Duckwiler GR, et al. Natural history of the neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. J Neurosurg 2000;93:561–68 [DOI] [PubMed] [Google Scholar]
- 15. Cebral JR, Castro MA, Appanaboyina S, et al. Efficient pipeline for image-based patient-specific analysis of cerebral aneurysm hemodynamics: technique and sensitivity. IEEE Trans Med Imaging 2005;24:457–67 [DOI] [PubMed] [Google Scholar]
- 16. Hademenos GJ, Massoud TF. Biophysical mechanisms of stroke. Stroke 1997;28:2067–77 [DOI] [PubMed] [Google Scholar]
- 17. Hoi Y, Woodward SH, Kim M, et al. Validation of CFD simulations of cerebral aneurysms with implication of geometric variations. J Biomech Eng 2006;128:844–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meng H, Wang Z, Kim M, et al. Saccular aneurysms on straight and curved vessels are subject to different hemodynamics: implications of intravascular stenting. AJNR Am J Neuroradiol 2006;27:1861–65 [PMC free article] [PubMed] [Google Scholar]
- 19. Mantha A, Karmonik C, Benndorf G, et al. Hemodynamics in a cerebral artery before and after the formation of an aneurysm. AJNR Am J Neuroradiol 2006;27:1113–18 [PMC free article] [PubMed] [Google Scholar]
- 20. Glagov S. Intimal hyperplasia, vascular modeling, and the restenosis problem. Circulation 1994;89:2888–91 [DOI] [PubMed] [Google Scholar]