SUMMARY:
Facial allotransplantation replaces missing facial structures with anatomically identical tissues, providing desired functional, esthetic, and psychosocial benefits far superior to those of conventional methods. On the basis of very encouraging initial results, it is likely that more procedures will be performed in the near future. Typical candidates have extremely complex vascular anatomy due to severe injury and/or multiple prior reconstructive attempts; thus, each procedure is uniquely determined by the defects and vascular anatomy of the candidate. We detail CT angiography vascular mapping, noting the clinical relevance of the imaging, the angiosome concept and noninvasive delineation of the key vessels, and current controversies related to the vascular anastomoses.
Restoring complex facial components such as eyelid and/or lip function is nearly impossible (Figs 1A and 2A) with conventional reconstructive techniques.1,2 Allotransplantation is accepted as the only option for the most complex craniofacial reconstruction (Fig 1A, -B). On the basis of the large population of patients with facial defects and encouraging initial results,3 it is likely that face transplantation will become a more common procedure. This underscores the need for radiologists to recognize the need for vascular mapping in these patients and to learn key aspects of the surgery that are important for image acquisition and reporting.
Fig 1.
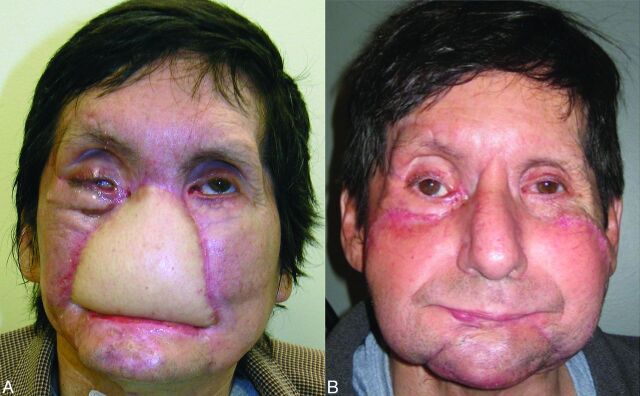
A 59-year-old man who underwent partial face transplantation. A, Severe disfigurement of the midface caused by a high-voltage burn injury is demonstrated, despite multiple conventional reconstructive attempts. B, Two-year postoperative follow-up illustrates restoration of form and function.
Fig 2.
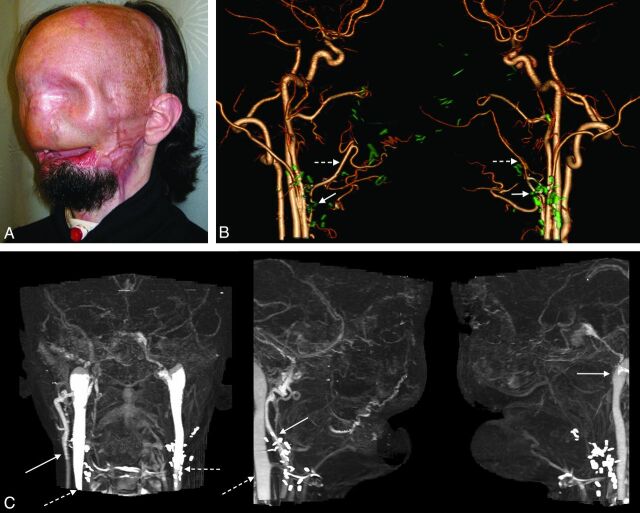
Candidate for full face transplantation. A, After catastrophic loss of facial tissues, muscle flaps and skin grafts placed during >20 surgeries rendered the patient's face featureless. B, Surgical-planning volume-rendered CT angiography depicts residual arteries after previous reconstructions using bilateral free latissimus muscle and serratus muscle flap arteries (dashed arrows), which are anastomosed end-to-end to the bilateral facial artery stumps (arrows). While not ideal, those facial and/or flap arteries are technically available and considered for microsurgical anastomoses. Prior surgical clips are rendered in green using the multiobject segmentation described in the text. C, Venous images from the same CT acquisition show occluded or absent bilateral anterior, posterior facial, and left external jugular veins. Patency of the right external jugular vein (arrow) and bilateral internal jugular veins (dashed arrows) is confirmed.
There have been approximately 20 face transplantations performed to date; 4 of these patients, including 3 from the United States,3 had “full” face transplantation,4 defined as restoration of the forehead, eyelids, nose, lips, chin, and cheeks, with or without bone. Patients with smaller defects can undergo so-called “partial” transplantation (Fig 1). Although candidate screening includes patients with malignancy and congenital etiologies, most patients to date have had severe trauma. For our patients, suitable brain dead, sex-, and skin color–matched donors are identified from our regional organ bank.
The intricacy of facial transplantation includes vascular preparation of both the recipient site and donor allograft. Long (15–22 hours) operation times5,6 and massive (up to 35 units) blood loss7 have been reported. The vascular anastomoses are the most critical part of the operation. Although 1 arterial and venous anastomosis appears adequate for perfusion of facial tissues, additional vessels are typically connected to ensure adequate facial blood flow.8,9
Open dialogue between surgeons and radiologists is critical to assess potential anastomoses and 3D relationships between vessels and other structures (eg, shrapnel, bone fragments) and to minimize the risk of critical blood loss and ischemia time.
The radiologist is charged with preoperative identification of the best target arteries. The risk of vascular anastomotic complications can be reduced if vessels with unfavorable anatomy are excluded from anastomoses. Preoperative knowledge of vascular anatomy allows the surgeons to plan ahead and prepare backup options such as vein grafts. We have observed that the elimination of vascular “unknowns” has led to more timely operations.
Face transplantation surgery is highly variable, and surgical planning for each case is unique. For the allograft, the recipient's tissue deficits determine the design, ranging from partial to full facial transplantation. The allograft includes not only the skin but also the underlying soft tissues, cartilage, and bone, depending on the defect of the recipient. This article focuses on the importance of the blood vessels. High-quality noninvasive image acquisition and postprocessing are essential because there is variation in subject anatomies, common sources for arterial and venous anastomoses are often depleted by previous reconstructive efforts or injury, and the surgical dissection can be very difficult due to scarring and fibrosis.
Literature supports CT vascular mapping for living hepatic and renal donors,10,11 considering it fundamental for safety and surgical outcomes.12,13 One distinction for facial transplantation imaging is that selection of donor vessels is affected by both allograft design and the recipient's vascular anatomy as a result of extensive defects or prior interventions. Moreover, head and neck vascular anatomy has higher patient-to-patient variability, particularly with respect to venous outflow, emphasizing the importance of preoperative mapping for reliable microvascular anastomoses and minimizing the risk of venous congestion and thrombosis in anastomotic vessels, the most common complications of microsurgery.
This article illustrates vascular CT for surgical planning. The angiosome concept is presented with respect to its influence on the donor vessel selection. Relevant controversies for vessel anastomoses are discussed, and current CT protocols are emphasized.
VASCULAR CONSIDERATIONS IN FACIAL TRANSPLANTATION
Donor Vessels and Angiosomes
The allograft design is determined by the recipient's defect (Figs 1A, 2A, and 3A). The vascular pedicles or donor vessels are chosen by using the angiosome (Fig 3D) concept,14,15 to optimize adequate perfusion. Each angiosome has a source artery that supplies 3D composite blocks of skin and underlying tissues (muscles, nerves, and bones).16 This concept suggests that multiple arteries are needed for perfusion of the facial skin and most of the facial skin is supplied by the superficial temporal, facial, and ophthalmic arteries.16 However, several anastomotic networks allow perfusion of multiple adjacent territories17–20 or across the midline.8,9 Thus, a source artery can also perfuse neighboring angiosomes when connecting potential vessels, called “choke vessels,” open, and subsequently develop into collateral vessels.15 For example, the source artery for the ophthalmic angiosome of the central forehead is typically a branch of the internal carotid artery. However, this angiosome can be supplied by reversed flow from the adjacent superficial temporal and facial artery territories.17
Fig 3.
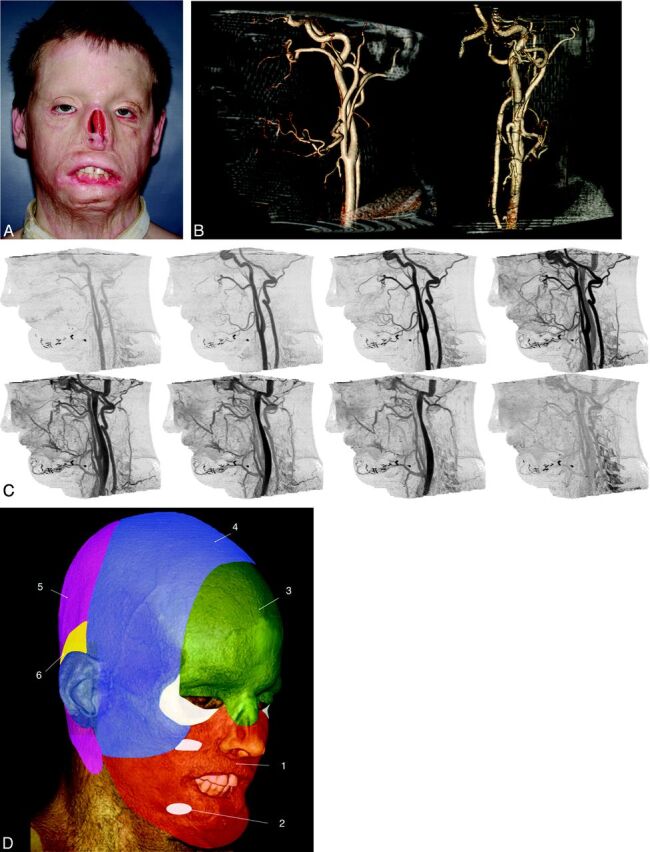
Candidate for full face transplantation. A, After a high-voltage injury, several flap and graft procedures resulted in persistent disfigurement. The patient wore a nasal prosthesis, removed for this photograph. B, Representative sagittal (left) and anteroposterior (right) projections from CT volume rendering. Volumes are viewed from an arbitrary angle to characterize branch and smaller vessels—for example, those from the external carotid artery that may be available for anastomoses. The nasal prosthesis was included in the CT acquisition. C, Sagittal cine CT images provide time resolution and enable separation of arteries and veins so that each dataset can be individually postprocessed. D, Angiosomes of the face overlaid on a volume-rendered CT image, including all soft-tissue components and numbered according to the source artery: 1) facial, 2) internal maxillary, 3) ophthalmic and internal carotid, 4) superficial temporal, 5) vertebral, and 6) posterior auricular. Lower and midface (orange) allografts can be perfused solely by facial arteries. Although the lower two-thirds of the face includes internal maxillary artery angiosomes, this territory can also be perfused by the facial artery via neighboring angiosome collateral vessels. For procedures in which the allograft includes the upper face and scalp (green and blue), the facial and superficial temporal arteries should be included, with the external carotid artery as the source vessel.
Selection of Recipient Vessels
Although the head and neck have a rich and often redundant vascular network,21 in our experience, it is common that the typical target vessels are depleted from either massive defects or multiple surgeries (Figs 2B, -C and 4). When it is available for surgery, CTA mapping of the course, caliber, contour, and 3D relationship to metal from the injury or prior surgeries is the most important preoperative step. CTA preoperatively identifies alternative recipient vessels when more suitable vessels are depleted. The usual strategy22 is to identify adjacent small vessels by CTA.23 When small neighboring vessels are not available, major neck vessels, such as the internal jugular system, can be used.22
Fig 4.
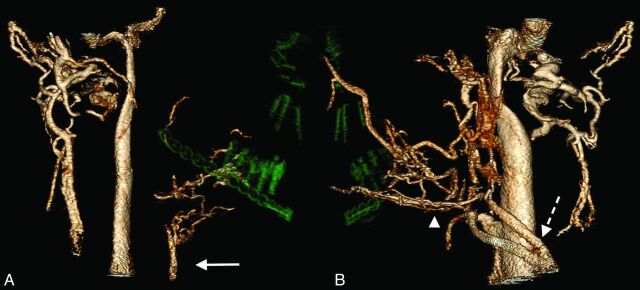
Volume-rendered venous-only reformatted images from a candidate who had a blast injury. A, Anterior and posterior facial veins and the external jugular vein are absent on the right, presumably from the injury. Imaging confirms the patency of the anterior jugular vein (arrow), a potential alternative for flap drainage. B, On the left, the external jugular vein (dashed arrow) and anterior facial vein (arrowhead) are available for flap drainage.
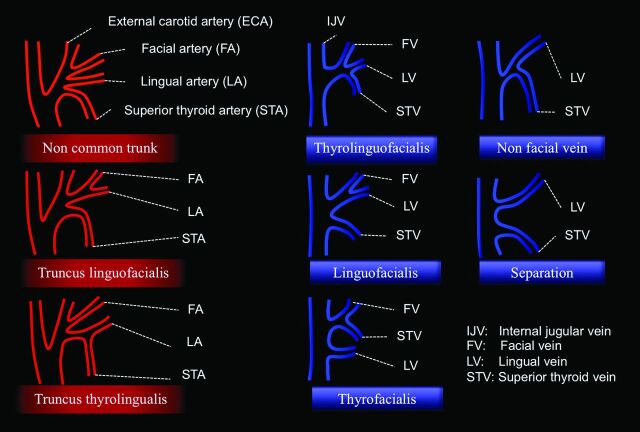
Another critical element of surgical planning is the identification of anatomic variants (Table and Fig 5).24 Both arteries and veins can have a common trunk, leading to larger caliber vessels compared with the smaller individual vessels. Larger caliber trunks are considered more favorable because the anastomosis is simpler and safer. Duplications are also common, and in general, these are considered advantageous because of increased options for anastomoses. Seventeen percent of individuals have duplicated external jugular veins, and 30% of individuals have duplicated anterior jugular veins.24
Major variations in branching patterns of the external carotid artery (3 types) and variations in the confluence of the facial, lingual, and superior thyroid veins with internal jugular vein (5 types)
| Variant Type | Description |
|---|---|
| Arterial | |
| Noncommon trunk | Facial, lingual, and superior thyroid arteries arise separately from the ECA |
| Truncus linguofacialis | Facial and lingual arteries arise from the ECA in a common trunk |
| Truncus thyrolingualis | Superior thyroid and lingual arteries arise from the ECA in a common trunk |
| Venous | |
| Thyrolinguofacialis | Facial, lingual, and superior thyroid veins form a thyrolinguofacialis vein |
| Linguofacialis | Facial and lingual veins form a venous stem |
| Thyrofacialis | Facial and superior thyroid veins join together, and separate lingua l vein joins into the IJV |
| Nonfacial vein | Superior thyroid and lingual veins join together into the IJV |
| Separation | Lingual and superior thyroid veins fuse independently with the IJV |
Note:—ECA indicates external carotid artery; IJV, internal jugular vein.
Fig 5.
Major variations in branching patterns of the external carotid artery (3 types) and variations of the confluence of the facial, lingual, and superior thyroid veins with the internal jugular vein (5 types), described by Shima et al.24 Descriptions of each variation are found in the Table.
As a general rule, 2 venous anastomoses are used to maintain sufficient outflow. Conventional thinking is that compared with the corresponding arteries, selection of drainage veins is considered less critical.25 However, it is essential to maintain sufficient drainage because venous congestion and thrombosis are the most common cause of flap failure.26,27 Additionally, there is a high incidence of venous thrombosis after neck dissection, up to 30% in the internal jugular vein.28–30
Planning Vascular Anastomoses
Once all vessels are identified, planning the vascular anastomoses considers the following: 1) optimal size match between donor and recipient vessels, 2) sufficient length between the allograft pedicle and donor vessel, and 3) surgical accessibility of the pedicle. Communication between the radiologist and surgeon by using the postprocessing methods described below is essential to portray the spatial relationships.
Surgical planning meticulously considers and matches the caliber and length of recipient and donor vessels. When these are highly compatible, it is possible to perform end-to-end anastomoses; the technique is simple and easily performed with less blood flow turbulence. However, other techniques are often required, usually from large-diameter discrepancies that increase the technical difficulty of the anastomoses and the reliability of the final conduit. Moreover, abrupt caliber changes are also undesirable because these induce turbulence and predispose to platelet aggregation.31 In related interventions, artery size discrepancy32 and/or intricate anastomoses33 have been shown to have higher complication rates.
In our experience, 3-fold diameter discrepancies between donor and recipient vessels have been encountered and are accommodated for by end-to-side anastomoses. Other options would include sleeve and so-called “fishmouth” anastomoses for the management of size discrepancies in microvascular anastomosis.31 Practically, an end-to-side anastomosis is often the only available option. Both rat microsurgery studies and human vascular surgery studies have shown no differences in patency rates between end-to-end and end-to-side anastomoses.34–36 As the number of procedures and surgical approaches evolve, selection criteria will vary among surgeons, based on personal preferences and complex patient and donor presentations.
Surgical Controversies
There are 3 main controversies in face transplantation that influence preoperative CT acquisition and interpretation.
1) Is One Arterial Anastomosis Sufficient for Perfusion of a Full Face Allograft?
Increasing evidence suggests that 1 arterial anastomosis may be sufficient for tissue perfusion.8,9,37 However, bilateral single arterial anastomoses are prudent to minimize the risk of flap ischemia due possible anastomotic complications (blood clot, stenosis, and vascular compression from head rotation). Thus, as noted in the image reformation section below, arterial maps should include all potential major branches from the external carotid system because of scar tissue and the potential for unexpected vascular findings at surgery.
2) Can the Facial Artery Alone Perfuse the Maxilla?
Cadaveric studies have suggested that the facial artery cannot adequately perfuse the maxilla.9,17 However, there is accumulating evidence that the facial artery can adequately perfuse both the entire maxilla and the mandible anteriorly from the insertion of the masseter muscle.37,38 A successful, defined by improved esthetic and functional outcome after surgery, facial transplantation suggests that the facial artery alone can perfuse a midfacial allograft that includes the maxilla.8
3) Should Bilateral External Carotid Artery Anastomoses Be Performed?
We are cautious regarding bilateral end-to-end anastomoses because there is a presumed increased risk of ischemia in certain external carotid territories such as the hypopharynx.8 On the other hand, Meningaud et al9 have reported bilateral end-to-end external carotid artery anastomoses,7 suggesting that this is often the best option because of sufficient arterial length for cervical connections and the large diameter that is safer for anastomosis. Of note, these reports7,9 did not specify the exact location of the anastomoses, and the relationship to the lingual artery (ie, proximal or distal) was not clear. Although experience to date with bilateral external carotid artery anastomoses is largely anecdotal, there is a recognized risk of catastrophic ocular ischemia from anastomosis between the external carotid and the ophthalmic arteries.39 Alizai et al40 reported 2 cases of ocular ischemic syndrome due to bilateral external carotid artery occlusion.
IMAGING FOR PREOPERATIVE SURGICAL PLANNING
Rationale
We hypothesize that meticulous vascular mapping can reduce the procedure time, similar to results in breast reconstruction, by using abdominal perforator flaps41 and anterolateral thigh flaps.42 We also postulate that catastrophic iatrogenic vascular injury can be avoided with CTA and careful surgical planning. Of note, Liu et al42 demonstrated that preoperative CTA for patients undergoing an anterolateral thigh flap was associated with a significant reduction in major surgical complications, as well as the length of surgery and the need for a secondary debulking procedure. A shortened operation time enhances patient safety. Finally, although details regarding imaging and surgery related to bone allografts or prostheses are beyond the scope of this review, 3D and 2D reformatted CT is the best technique43 to provide accurate assessment of severe bone defects,37,38 facilitating 3D understanding of skeletal stability, rotation, and displacement of bony fragments. Volumetric CT also enables 3D cephalometric measurements and creation of physical models of bones, which can be used for designing or improving bone allografts or prostheses.
CT Image Acquisition
Presurgical vascular mapping is challenging because the vessels are small, the FOV must be large enough to include the full extent of the external carotid artery and those branches that could be used for anastomoses, and there may be substantial metal artifacts (implants and shrapnel). In addition, patients are generally young, and thus cumulative radiation doses are a concern, particularly because both arterial and venous maps are needed.
At our institution, all surgical-planning CT images23 are acquired axially with 320 × 0.5 mm detector row CT (Aquilion One; Toshiba Medical Systems, Tochigi-ken, Japan), which covers the entire FOV,44 to enable multiple phases by using intermittent dynamic volumes with a 0.75-second gantry rotation. The protocol (Fig 6), initially developed45 for brain perfusion, includes pure arterial and venous volumes for input to postprocessing software. For patients with at least partial vision, the gantry is angulated to limit radiation to the orbits; and regarding the inferior aspect of the FOV, care is taken to limit radiation to the thyroid,46 usually by limiting the craniocaudal coverage to 14 cm (280 × 0.5 mm detector row acquisition).
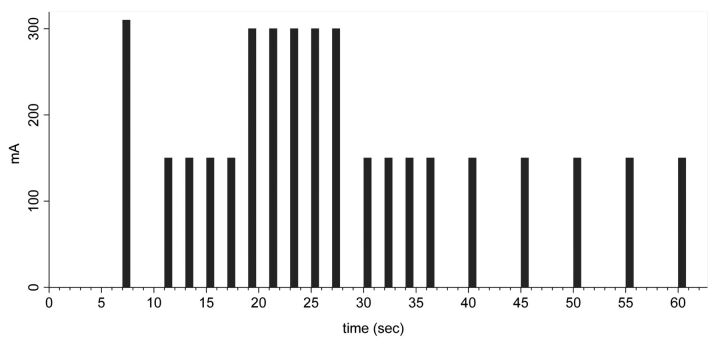
Fig 6.
Timing diagram for a 320–detector row CT acquisition for face transplantation candidates. Each bar refers to 1 phase of the multiphasic axial acquisition; each volume includes the entire anatomy required for surgical planning.
The contrast injection is timed by using a 20-mL test bolus to time the dual (EmpowerCTA; ACIST Medical, New York, New York) main injection (60-mL iopamidol, 370 mg I/mL, Isovue-370; Bracco Diagnostics, Princeton, New Jersey), followed by 40-mL normal saline.
CT Image Postprocessing
Volume rendering illustrates the spatial relationships between skin, soft tissues, vessels, and bones (Fig 7). Vascular overlays show the presurgical orientation of different facial structures (Fig 8) that are important for planning the dissection.
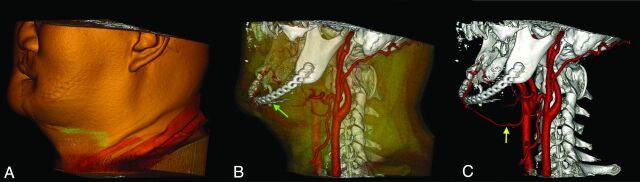
Fig 7.
Candidate for full face transplantation. A, Volume-rendered image including full soft tissues. B, Skin and superficial soft tissues have been shadowed. This view depicts the relationship of the bones, postsurgical hardware (green arrow), and segmented arteries. C, Volume rendering that exclusively shows the bones, postsurgical hardware, and arteries. The superior thyroid artery (yellow arrow) is used for the anastomosis of free flap, and its surgically altered course is demonstrated.
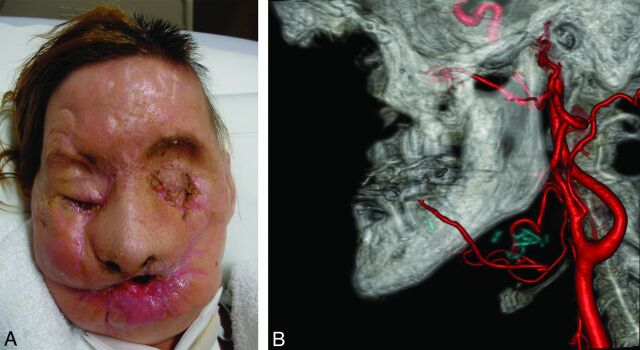
Fig 8.
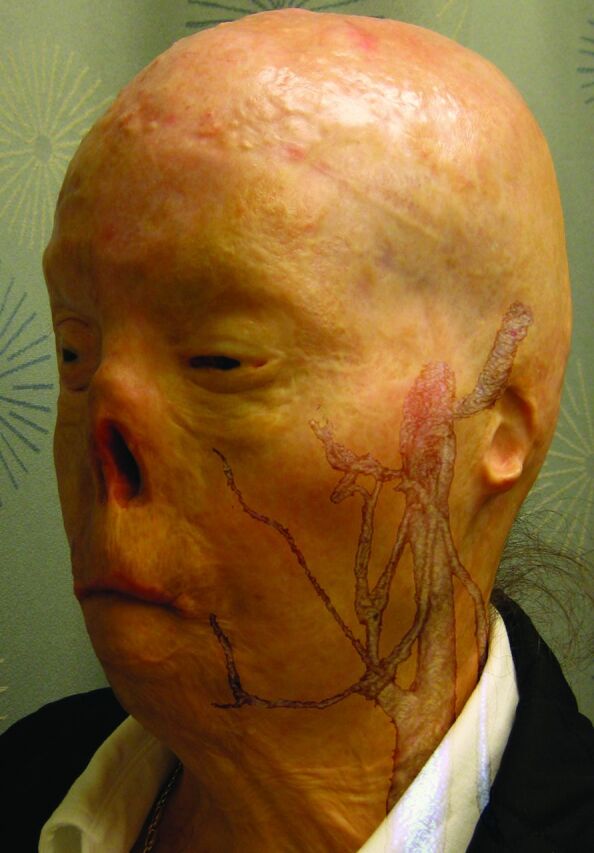
Face transplantation candidate shown with fusion of the preoperative photograph and CT venography images. Meticulous CT segmentation and feature mapping are used to depict preoperative structures critical for rapid, precise, surgical dissection.
There are several considerations related to the anastomoses. The course and branches of the external carotid artery should be documented. In our experience, it is important to map and annotate the facial, superior thyroid, lingual, and superficial temporal arteries (Fig 3B) because these are common targets for recipient vessels. Although there is often a close correlation between arteries and veins, venous drainage does not always parallel arterial territories.16 While the use of the external jugular and facial veins has been reported, there is a rich venous network, and preoperative imaging should include venous enhancement to identify all veins, including the anterior jugular vein, internal jugular vein, and vein grafts from prior procedures. Imaging characterization includes patency, diameter, and variation. Vascular diameters are essential for surgical planning, and manual correction of vessel segmentation is required. Specifically, caliber changes and specific branching patterns must be meticulously illustrated to minimize the risk of complications and provide secondary and tertiary surgical options.
Regarding shrapnel, metal implants from prior reconstruction, and bone fragments from initial trauma, postprocessing considerations include the 3D spatial relationships of blood vessels and neighboring structures and the presence of artifacts. Multiplanar reformation, maximum intensity projections, and volume rendering define the 3D spatial relationships of blood vessels and neighboring structures. We routinely use multiobject segmentation (Vitrea fX 6.0; Vital Images, A Toshiba Medical Systems Group Company, Minnetonka, Minnesota) to portray the spatial relationships (Fig 9B). Metal artifacts can limit the assessment of blood vessels, though these artifacts are generally less severe than those in corresponding MR images.47
Fig 9.
Candidate for full face transplantation who was attacked by a chimpanzee. A, Photograph of the victim after multiple conventional reconstructive surgeries for catastrophic facial injury, demonstrating the limitation of conventional surgical options. B, Volume-rendered reformations of CT images by using multiobject segmentation to rapidly communicate information to the surgical team. In red, the arteries, including the course of right facial artery, are clearly demonstrated. The facial artery was used for the anterolateral thigh flap immediately after the injury. The anastomosis can be identified via surgical clips rendered in green.
4D volumes (3 spatial planes plus time) enable cine (Fig 3C) assessment of small-caliber vessels similar to that described in the lower extremity.48 The drawback of multiphase CT is increased radiation exposure in comparison with fewer phases that could, in theory, give the same static information. However, preoperative acquisition of multiple phases largely ensures that essential pure arterial and venous images are available for postprocessing and interpretation. As in other high-flow body parts,48 the timing for separation of small arteries versus veins can be challenging, particularly if there are unexpected findings such as a postinjury or postoperative fistula or asymmetric blood flow from injury or previous reconstructive surgeries. Thus, we advocate multiphase imaging, keeping in mind that the risks associated with life-long immunosuppression and the operation itself are far greater than those from surgical planning vascular CTA.
Future Directions
For most vascular mapping applications before transplantation,49,50 CT angiography has replaced invasive conventional angiography. The challenges for CT, namely small-caliber vessels, rapid transit time, and metal artifacts, also pose challenges for advanced MR imaging acquisitions. For face transplantation candidates, initial comparisons between time-resolved imaging51,52 suggested the superiority of CT when compared to MR.47 Moreover, axial wide-area-detector CT protocols provide datasets amenable for future studies of tissue perfusion after surgery and in the clinical setting of tissue rejection.
MR angiography is an attractive alternative for follow-up studies, particularly in patients with few metallic implants and thus relatively low susceptibility artifacts. For suspected complications, specialized imaging such as diffusion-weighted sequences for allograft function53 and high-resolution vessel wall sequences54,55 for rejection can be performed. These can be complementary to 3D high-spatial-resolution MR angiography and time-resolved sequences for arterial and venous separation.
CONCLUSIONS
Face transplantation is now accepted as the only option for the most complex craniofacial reconstruction. With initial technical successes, the number of patients will continue to increase, emphasizing the need for radiologists to understand the surgical relevance, preoperative arterial and venous mapping, and current controversies regarding vascular anastomoses. Face transplantation candidates require complex CT protocols to depict arterial and venous imaging of the external carotid arteries and veins, their branches, and changes from severe facial injuries and prior attempts at reconstruction. The complexity of this process is reflected in the technical challenges of image acquisition and interpretation in patients with unexpected anatomy from severe deformity and prior surgical reconstructions. Meticulous communication between radiologists and surgeons related to vascular anastomoses will facilitate preoperative planning and optimization of the surgical technique.
Footnotes
Disclosures: Ericka Bueno—RELATED: Department of Defense,* Comments: U.S. Department of Defense research contract with my institution (Contract W911QY-09-C-021). David Enterline—UNRELATED: Consultancy: Bayer Healthcare, DFine Inc, Grants/Grants Pending: Siemens Healthcare,* Guerbet Group,* Payment for Lectures (including service on Speakers Bureaus): Bayer Healthcare, Bracco Diagnostics, Stock/Stock Options: Pioneer Surgical Technology, Medical Simulation Corp. Frank Rybicki—RELATED: Grant: United States Department of Defense,* Contract W911QY-09-C-021; grant support to the institution from Toshiba Medical Systems Corp. and Bracco Diagnostics. *Money paid to the institution.
Paper previously presented in part and awarded a Radiological Society of North America 2010 Certificate of Merit, a Radiological Society of North America 2011 Excellence in Design Award, and an Excellent Educational Poster Award at: 71st Annual Meeting of the Japan Radiological Society, April 12–15, 2012; Yokohama, Japan.
REFERENCES
- 1. Spinelli HM, Jelks GW. Periocular reconstruction: a systematic approach. Plast Reconstr Surg 1993;91:1017–24, discussion 1025–26 [PubMed] [Google Scholar]
- 2. Langstein HN, Robb GL. Lip and perioral reconstruction. Clin Plast Surg 2005;32:431–45, viii [DOI] [PubMed] [Google Scholar]
- 3. Pomahac B, Pribaz J, Eriksson E, et al. Three patients with full facial transplantation. N Engl J Med 2012;366:715–22 [DOI] [PubMed] [Google Scholar]
- 4. Lengelé BG. Current concepts and future challenges in facial transplantation. Clin Plast Surg 2009;36:507–21 [DOI] [PubMed] [Google Scholar]
- 5. Meningaud JP, Paraskevas A, Ingallina F, et al. Face transplant graft procurement: a preclinical and clinical study. Plast Reconstr Surg 2008;122:1383–89 [DOI] [PubMed] [Google Scholar]
- 6. Siemionow MZ, Papay F, Djohan R, et al. First US near-total human face transplantation: a paradigm shift for massive complex injuries. Plast Reconstr Surg 2010;125:111–22 [DOI] [PubMed] [Google Scholar]
- 7. Lantieri L, Meningaud J, Grimbert P, et al. Repair of the lower and middle parts of the face by composite tissue allotransplantation in a patient with massive plexiform neurofibroma: a 1-year follow-up study. Lancet 2008;372:639–45 [DOI] [PubMed] [Google Scholar]
- 8. Pomahac B, Lengele B, Ridgway EB, et al. Vascular considerations in composite midfacial allotransplantation. Plast Reconstr Surg 2010;125:517–22 [DOI] [PubMed] [Google Scholar]
- 9. Meningaud JP, Benjoar MD, Hivelin M, et al. Procurement of total human face graft for allotransplantation: a preclinical study and the first clinical case. Plast Reconstr Surg 2010;126:1181–90 [DOI] [PubMed] [Google Scholar]
- 10. Rankin SC, Jan W, Koffman CG. Noninvasive imaging of living related kidney donors: evaluation with CT angiography and gadolinium-enhanced MR angiography. AJR Am J Roentgenol 2001;177:349–55 [DOI] [PubMed] [Google Scholar]
- 11. Kamel IR, Kruskal JB, Pomfret EA, et al. Impact of multidetector CT on donor selection and surgical planning before living adult right lobe liver transplantation. AJR Am J Roentgenol 2001;176:193–200 [DOI] [PubMed] [Google Scholar]
- 12. Caruso S. Imaging in liver transplantation. World J Gastroenterol 2009;15:675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singh A, Sahani D. Imaging of the renal donor and transplant recipient. Radiol Clin North Am 2008;46:79–93, vi [DOI] [PubMed] [Google Scholar]
- 14. Taylor GI, Palmer JH. “Angiosome theory.” Br J Plast Surg 1992;45:327–28 [DOI] [PubMed] [Google Scholar]
- 15. Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg 1987;40:113–41 [DOI] [PubMed] [Google Scholar]
- 16. Houseman ND, Taylor GI, Pan WR. The angiosomes of the head and neck: anatomic study and clinical applications. Plast Reconstr Surg 2000;105:2287–313 [DOI] [PubMed] [Google Scholar]
- 17. Banks ND, Hui-Chou HG, Tripathi S, et al. An anatomical study of external carotid artery vascular territories in face and midface flaps for transplantation. Plast Reconstr Surg 2009;123:1677–87 [DOI] [PubMed] [Google Scholar]
- 18. Jeng SF, Wei FC, Noordhoff MS. Replantation of amputated facial tissues with microvascular anastomosis. Microsurgery 1994;15:327–33 [DOI] [PubMed] [Google Scholar]
- 19. Hammond DC, Bouwense CL, Hankins WT, et al. Microsurgical replantation of the amputated nose. Plast Reconstr Surg 2000;105:2133–36, quiz 2137, discussion 2138 [DOI] [PubMed] [Google Scholar]
- 20. Blanco-Dávila F, Arrendondo G, De La Garza O, et al. Anatomical study of the blood supply to the skin in rhytidectomy. Aesthetic Plast Surg 1995;19:175–81 [DOI] [PubMed] [Google Scholar]
- 21. Yazar S. Selection of recipient vessels in microsurgical free tissue reconstruction of head and neck defects. Microsurgery 2007;27:588–94 [DOI] [PubMed] [Google Scholar]
- 22. Takamatsu A, Harashina T, Inoue T. Selection of appropriate recipient vessels in difficult, microsurgical head and neck reconstruction. J Reconstr Microsurg 1996;12:499–507; discussion 508–13 [DOI] [PubMed] [Google Scholar]
- 23. Soga S, Ersoy H, Mitsouras D, et al. Surgical planning for composite tissue allotransplantation of the face using 320-detector row computed tomography. J Comput Assist Tomogr 2010;34:766–69 [DOI] [PubMed] [Google Scholar]
- 24. Shima H, von Luedinghausen M, Ohno K, et al. Anatomy of microvascular anastomosis in the neck. Plast Reconstr Surg 1998;101:33–41 [DOI] [PubMed] [Google Scholar]
- 25. Taylor GI, Caddy CM, Watterson PA, et al. The venous territories (venosomes) of the human body: experimental study and clinical implications. Plast Reconstr Surg 1990;86:185–213 [DOI] [PubMed] [Google Scholar]
- 26. Nahabedian MY, Momen B, Manson PN. Factors associated with anastomotic failure after microvascular reconstruction of the breast. Plast Reconstr Surg 2004;114:74–82 [DOI] [PubMed] [Google Scholar]
- 27. Kubo T, Yano K, Hosokawa K. Management of flaps with compromised venous outflow in head and neck microsurgical reconstruction. Microsurgery 2002;22:391–95 [DOI] [PubMed] [Google Scholar]
- 28. Fisher CB, Mattox DE, Zinreich JS. Patency of the internal jugular vein after functional neck dissection. Laryngoscope 1988;98:923–27 [DOI] [PubMed] [Google Scholar]
- 29. Leontsinis TG, Currie AR, Mannell A. Internal jugular vein thrombosis following functional neck dissection. Laryngoscope 1995;105:169–74 [DOI] [PubMed] [Google Scholar]
- 30. Brown DH, Mulholland S, Yoo JH, et al. Internal jugular vein thrombosis following modified neck dissection: implications for head and neck flap reconstruction. Head Neck 1998;20:169–74 [DOI] [PubMed] [Google Scholar]
- 31. López-Monjardin H, de la Pena-Salcedo JA. Techniques for management of size discrepancies in microvascular anastomosis. Microsurgery 2000;20:162–66 [DOI] [PubMed] [Google Scholar]
- 32. Chuang DC, Jeng SF, Chen HT, et al. Experience of 73 free groin flaps. Br J Plast Surg 1992;45:81–85 [DOI] [PubMed] [Google Scholar]
- 33. Ploteau S, Rogez JM, Donnez J, et al. Which are the ideal donor and recipient vessels for a whole ovarian transplantation? Fertil Steril 2011;95:751–55 [DOI] [PubMed] [Google Scholar]
- 34. Miyamoto S, Takushima A, Okazaki M, et al. Comparative study of different combinations of microvascular anastomosis types in a rat vasospasm model: versatility of end-to-side venous anastomosis in free tissue transfer for extremity reconstruction. J Trauma 2009;66:831–34 [DOI] [PubMed] [Google Scholar]
- 35. Dotson RJ, Bishop AT, Wood MB, et al. End-to-end versus end-to-side arterial anastomosis patency in microvascular surgery. Microsurgery 1998;18:125–28 [DOI] [PubMed] [Google Scholar]
- 36. Schouten O, Hoedt MT, Wittens CH, et al. End-to-end versus end-to-side distal anastomosis in femoropopliteal bypasses; results of a randomized multicenter trial. Eur J Vasc Endovasc Surg 2005;29:457–62 [DOI] [PubMed] [Google Scholar]
- 37. Guo S, Han Y, Zhang X, et al. Human facial allotransplantation: a 2-year follow-up study. Lancet 2008;372:631–38 [DOI] [PubMed] [Google Scholar]
- 38. Siemionow M, Papay F, Alam D, et al. Near-total human face transplantation for a severely disfigured patient in the USA. Lancet 2009;374:203–09 [DOI] [PubMed] [Google Scholar]
- 39. Geibprasert S, Pongpech S, Armstrong D, et al. Dangerous extracranial-intracranial anastomoses and supply to the cranial nerves: vessels the neurointerventionalist needs to know. AJNR Am J Neuroradiol 2009;30:1459–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alizai AM, Trobe JD, Thompson BG, et al. Ocular ischemic syndrome after occlusion of both external carotid arteries. J Neuroophthalmol 2005;25:268–72 [DOI] [PubMed] [Google Scholar]
- 41. Masia J, Larranaga J, Clavero JA, et al. The value of the multidetector row computed tomography for the preoperative planning of deep inferior epigastric artery perforator flap: our experience in 162 cases. Ann Plast Surg 2008;60:29–36 [DOI] [PubMed] [Google Scholar]
- 42. Liu SC, Chiu WK, Chen SY, et al. Comparison of surgical result of anterolateral thigh flap in reconstruction of through-and-through cheek defect with/without CT angiography guidance. J Craniomaxillofac Surg 2011;39:633–38 [DOI] [PubMed] [Google Scholar]
- 43. Avery LL, Susarla SM, Novelline RA. Multidetector and three-dimensional CT evaluation of the patient with maxillofacial injury. Radiol Clin N Am 2011;49:183–203 [DOI] [PubMed] [Google Scholar]
- 44. Rybicki FJ, Otero HJ, Steigner ML, et al. Initial evaluation of coronary images from 320-detector row computed tomography. Int J Cardiovasc Imaging 2008;24:535–46 [DOI] [PubMed] [Google Scholar]
- 45. Yahyavi-Firouz-Abadi N, Wynn BL, Rybicki FJ, et al. Steroid-responsive large vessel vasculitis: application of whole-brain 320-detector row dynamic volume CT angiography and perfusion. AJNR Am J Neuroradiol 2009;30:1409–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shu KM, MacKenzie JD, Smith JB, et al. Lowering the thyroid dose in screening examinations of the cervical spine. Emerg Radiol 2006;12:133–36 [DOI] [PubMed] [Google Scholar]
- 47. Soga S, Pomahac B, Mitsouras D, et al. Preoperative vascular mapping for facial allotransplantation: four-dimensional computed tomographic angiography versus magnetic resonance angiography. Plast Reconstr Surg 2011;128:883–91 [DOI] [PubMed] [Google Scholar]
- 48. Sommer WH, Helck A, Bamberg F, et al. Diagnostic value of time-resolved CT angiography for the lower leg. Eur Radiol 2010;20:2876–81 [DOI] [PubMed] [Google Scholar]
- 49. Bueschen AJ, Lockhart ME. Evolution of urological imaging. Int J Urol 2011;18:102–12 [DOI] [PubMed] [Google Scholar]
- 50. Chen YS, Cheng YF, De Villa VH, et al. Evaluation of living liver donors. Transplantation 2003;75:S16–19 [DOI] [PubMed] [Google Scholar]
- 51. Korosec FR, Frayne R, Grist TM, et al. Time-resolved contrast-enhanced 3D MR angiography. Magn Reson Med 1996;36:345–51 [DOI] [PubMed] [Google Scholar]
- 52. Ersoy H, Goldhaber SZ, Cai T, et al. Time-resolved MR angiography: a primary screening examination of patients with suspected pulmonary embolism and contraindications to administration of iodinated contrast material. AJR Am J Roentgenol 2007;188:1246–54 [DOI] [PubMed] [Google Scholar]
- 53. Eisenberger U, Thoeny HC, Binser T, et al. Evaluation of renal allograft function early after transplantation with diffusion-weighted MR imaging. Eur Radiol 2010;20:1374–83 [DOI] [PubMed] [Google Scholar]
- 54. Mitsouras D, Owens CD, Conte MS, et al. In vivo differentiation of two vessel wall layers in lower extremity peripheral vein bypass grafts: application of high-resolution inner-volume black blood 3D FSE. Magn Reson Med 2009;62:607–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rybicki FJ, Mitsouras D, Owens CD, et al. Multi-contrast high spatial resolution black blood inner volume three-dimensional fast spin echo MR imaging in peripheral vein bypass grafts. Int J Cardiovasc Imaging 2010;26:683–91 [DOI] [PMC free article] [PubMed] [Google Scholar]