Hemodyalisis may not prevent brain damage resulting from accumulation of urea and other metabolites as previously believed. These investigators used voxelwise DTI to assess the white matter of 28 patients with end-stage renal disease. All DTI parameters were abnormal, especially in the callosum, sagittal stratum, and pons.
Abstract
BACKGROUND AND PURPOSE:
ESRD results in excessive accumulation of urea and toxic metabolites. Hemodialysis is usually performed to maintain health in patients with ESRD; however, it may cause silent white matter alterations in the earlier stages. Hence, this study aimed to perform voxelwise diffusion tensor analysis for global detection of subtle white matter alterations in patients with ESRD.
MATERIALS AND METHODS:
Twenty-eight patients with ESRD and 25 age-matched control subjects were enrolled in this study. Each subject underwent CASI assessment and DTI. After spatial normalization of DTI images, voxelwise statistical analyses were performed to compare DTI parameters between the 2 groups.
RESULTS:
In patients with ESRD, AD, RD, and MD values were significantly increased, whereas the FA value was significantly decreased, mostly in the corpus callosum, bilateral sagittal stratum, and pons. Multiple regression analysis further revealed that both RD and MD were positively correlated with the duration of hemodialysis in the pons; however, no significant correlation was observed with FA. Negative correlations of RD and MD and a positive correlation of FA with the CASI score were observed in the corona radiata.
CONCLUSIONS:
We concluded that voxelwise DTI analysis is helpful in the detection of white matter alterations caused by hemodialysis.
ESRD has become an increasing problem, with a growing number of patients undergoing hemodialysis in the United States in recent years.1 ESRD has been characterized as the failure of renal functions, which permanently decrease to <10% of the normal status, and is accompanied by multiple organ dysfunction.1 To maintain their health, patients with ESRD usually undergo regular hemodialysis 3 times per week to remove excess urea and other toxic metabolites from the body. However, uremic neuropathy has been reported in patients with ESRD undergoing hemodialysis. These neurologic complications may be related to ESRD itself or to dialysis. These neurologic complications include acute reactions such as dialysis disequilibrium syndrome and osmotic myelinolysis and chronic changes such as encephalopathy, dementia, and stroke.2,3 These acute complications are presumably related to brain swelling or tissue edema caused by the osmotic gradient between plasma and brain tissue during hemodialysis.2,4 Excessive edema may gradually lead to damaged brain tissue.
For direct measurement of water diffusion, in a previous animal MR imaging study, DWI was performed in nephrectomized rats.5 The results showed that ADC was significantly increased in the rat brain immediately after hemodialysis. Similar results were also observed in a human brain study in which ADC measurement was performed in multiple regions of the brain of patients with ESRD by using region-of-interest analysis.6 That study found that before hemodialysis, ADC values were significantly higher for both the gray and white matter in patients with ESRD than in control subjects. After the initial hemodialysis, ADC values were further increased in the frontal white matter; this change implied that immediate hemodialysis led to increased water diffusion in brain tissue.
Because hemodialysis may cause white matter alterations, another previous study used the DTI technique to investigate the effects of long-term hemodialysis on white matter integrity by comparing FA values between patients with ESRD and healthy subjects by using manual region-of-interest analysis.7 The results showed that FA values were significantly reduced in many white matter regions and that negative correlations were evident between reduction in FA values and the duration of hemodialysis. Recently, a DTI tractography study further showed an association between abnormalities of fiber tracts and cognitive function.8
However, manual region-of-interest analysis has been used in the studies in which diffusion measurements of the brain tissue of patients with ESRD were performed. This method is known to be effort-intensive, and the results are dependent on the locus and size of the region of interest drawn by the operators.9 In contrast, voxel-based analysis is an automatic method that normalizes whole-brain DTI to a standard coordinate system in which 2 datasets are compared on a voxel-by-voxel basis.10 This approach has been widely used to detect disease-, drug-, or age-related white matter alterations in the human brain11–13; however, no study has used this technique in patients with ESRD, to our knowledge. Recently, better registration accuracy was demonstrated with diffeomorphic image registration.14,15 Combining voxel-based analysis with diffeomorphic registration may provide more accurate results than conventional methods.16 Moreover, in DTI, AD and RD represent the diffusivity in directions parallel and perpendicular to fiber orientations, respectively. Both can provide in-depth insight into underlying biophysical changes in the axon and myelin. Thus, white matter alterations may be better characterized in patients with ESRD by using AD, RD, and MD values together with FA values.
This study aimed to perform voxelwise DTI analysis with diffeomorphic registration for accurate characterization of white matter alterations in patients with ESRD. AD, RD, and MD values were combined with FA values in this study, and the relationships of these values with the duration of hemodialysis and cognitive function in subjects with ESRD were explored.
Materials and Methods
Subjects
This study was approved by the institutional review board of Hsiao-Kang Municipal Hospital. Twenty-eight patients with ESRD (male/female ratio = 14:14; 39 ± 8 years of age) and 25 age-matched control subjects (male/female ratio = 11:14; 39 ± 7 years of age) were enrolled in this study. Patients who had undergone hemodialysis for >1 year were regarded as long-term dialysis subjects. The mean of their duration of dialysis was 7 ± 5 years, and their last hemodialysis was conducted 2 days before MR imaging. Table 1 shows the demographic characteristics of the enrolled subjects. All participants completed an informed consent form. Subjects who had a history of diabetes, alcoholism, drug abuse, psychiatric disorders, and major neurologic disorders or who had claustrophobia were excluded from this study. All participants completed the overall evaluation of cognitive functions in approximately 30 minutes, by using the CASI assessment.17 The CASI covers a broad range of cognitive domains and is usually used in evaluating cognitive changes clinically.
Table 1:
Demographic characteristics of enrolled subjectsa
| Patients with ESRD | Control Subjects | |
|---|---|---|
| Age (yr) | 39 ± 8 | 39 ± 7 |
| Sex (M/F) | 14/14 | 11/14 |
| Dialysis duration (yr) | 7 ± 5 | N/A |
| CASI score | 93.5 ± 7.2b | 96.8 ± 3.7b |
Note:—N/A indicates not applicable.
Data are means.
Statistically significant difference (P < .05).
Data Acquisition
All brain MR imaging data were acquired from a 1.5T MR imaging scanner (Signa Excite; GE Healthcare, Milwaukee, Wisconsin). After triplanar scans and acquisition of calibration data for array spatial sensitivity encoding technique parallel imaging, 20 axial T1WI, T2WI, and T2-FLAIR images were sequentially acquired from each subject. Those anatomic images were used to diagnose pre-existing lesions in patients, and those who had lesions diagnosed were excluded from this study.
DTI acquisitions were performed by using single-shot twice-refocused spin-echo echo-planar diffusion-weighted sequences with an 8-channel phased array neurovascular coil. Thirty axial sections were placed to cover the whole-brain region with orientation parallel to the anterior/posterior commissure line. Other imaging parameters were as follows: TR/TE = 8000/82.8 ms, matrix size = 128 × 128, b=1000 s/mm2, number of noncollinear diffusion directions = 33, B0 = 1, FOV = 240 × 240 mm, NEX = 1, array spatial sensitivity encoding technique factor = 2.0, section thickness = 4.4 mm, and no gap between sections. The scanning time for the DTI acquisition was 4 minutes 48 seconds.
DTI Analysis
All data were transferred to a stand-alone workstation and were processed by using the fMRI of the Brain Software Library (http://www.fmrib.ox.ac.uk/) to obtain diffusion tensor maps. First, the eddy-current distortions were corrected by using affine registration to minimize the diffusion gradient–induced eddy-current distortions in 33 DWIs with b=0 as the reference image. Subsequently, a diffusion tensor was fitted with least-squares estimation on a voxel-by-voxel basis to obtain 3 eigenvalues, from which AD, RD, MD, and FA values were calculated.
Voxel-Based DTI Analysis
In voxel-based analysis, whole-brain FA maps were spatially normalized to an International Consortium for Brain Mapping FA template18 by using affine registration to minimize global differences, followed by nonparametric diffeomorphic demon registration14 to further minimize local differences between individual and template images. The displacement maps generated from the affine and demon registrations were used to spatially normalize the corresponding AD, RD, and MD maps, respectively. Subsequently, the voxel-based analysis was conducted by using SPM5 (Wellcome Department of Imaging Neuroscience, London, UK) on a Matlab platform (MathWorks, Natick, Massachusetts). The statistical comparisons of DTI indices between patients with ESRD and healthy subjects were performed by using a 2-sample t test. In addition, multiple regression analysis was used to reveal the associations of DTI indices with the duration of hemodialysis and the CASI score, respectively, whose net effects were extracted by nulling the age and sex influences. In this study, the areas with statistically significant differences (uncorrected P < .01 and cluster of >50 voxels) were displayed as red-yellow colors superimposed on the averaged AD, RD, MD, and FA maps, respectively.
Results
Age, Sex, CASI Score, and Duration of Hemodialysis
In this study, the sex distributions of patients with ESRD and healthy subjects were not well-matched but did not have a significant difference. The results of 2-sample t tests revealed significantly lower CASI scores in patients with ESRD than in healthy subjects (P < .05). Correlation testing found no significant correlations between CASI scores and age, sex, or duration of hemodialysis in patients with ESRD or healthy subjects.
Axial Diffusivity
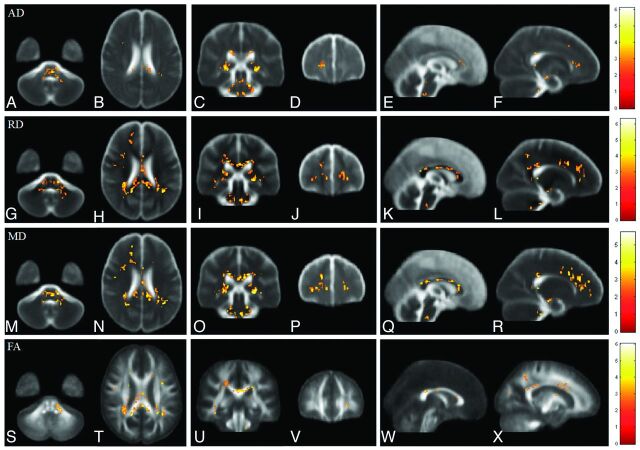
In patients with ESRD, AD values were significantly higher than those in control subjects in the bilateral stria terminalis extending laterally to the retrolenticular part of the internal capsule, left anterior forceps minor, and pons, as shown in Fig 1A–F. Montreal Neurological Institute coordinates of areas with significant AD differences are shown in Table 2. No significant correlation was observed among AD values, the duration of dialysis, and CASI scores in patients with ESRD.
Fig 1.
Voxelwise comparisons of AD, RD, MD, and FA between patients with ESRD and healthy subjects. Red-yellow colors indicate significant increases of AD (A–F), RD (G–L), and MD (M–R) and significant decreases of FA (S–X) in patients with ESRD. Color bars show the ranges of t-scores.
Table 2:
MNI coordinates of major areas with significant AD differences along with means of AD values
| Brain Regions | MNI Coordinate (mm) |
AD (mean) × 10−3 mm2/s |
|||
|---|---|---|---|---|---|
| X | Y | Z | ESRD | Healthy | |
| Rt RLIC | 25 | −16 | −12 | 1.43 ± 0.17 | 1.39 ± 0.14 |
| Lt RLIC | −27 | −14 | −14 | 1.66 ± 0.21 | 1.57 ± 0.13 |
| Lt Fminor | −21 | 52 | −12 | 1.43 ± 0.07 | 1.33 ± 0.07 |
| Pons | 1 | −14 | −56 | 1.85 ± 0.18 | 1.73 ± 0.11 |
Note:—Fminor indicates forceps minor; RLIC, retrolenticular part of the internal capsule; MNI, Montreal Neurological Institute; Rt, right; Lt, left.
Radial Diffusivity
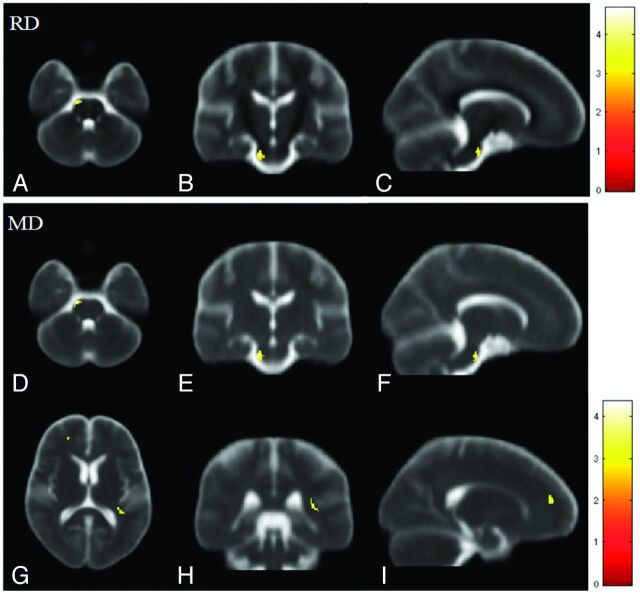
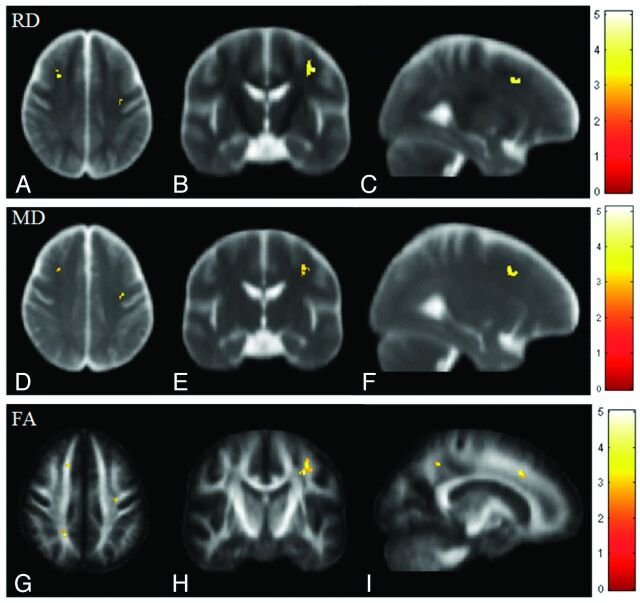
Compared with control subjects, significant increases in RD values were observed in multiple white matter areas in patients with ESRD, including the genu, splenium, and body of the corpus callosum; the bilateral retrolenticular part of the internal capsule; the bilateral forceps major; the bilateral anterior corona radiata; the bilateral posterior corona radiata; the pontine region; and the bilateral cerebellum, as shown in Fig 1G–L. Montreal Neurological Institute coordinates of areas with significant RD differences are shown in Table 3. Multiple regression analysis revealed a positive correlation between RD and the duration of hemodialysis in the pontine region and a negative correlation between RD and the CASI score in the left anterior corona radiata and right superior corona radiata in patients with ESRD, as shown in Figs 2A–C and 3 A–C, respectively.
Table 3:
MNI coordinates of major areas with significant RD differences along with means of RD values
| Brain Regions | MNI Coordinate (mm) |
RD (mean) × 10−3 mm2/s |
|||
|---|---|---|---|---|---|
| X | Y | Z | ESRD | Healthy | |
| GCC | 1 | 39 | −1 | 0.94 ± 0.2 | 0.86 ± 0.14 |
| SCC | 1 | −14 | 5 | 0.65 ± 0.15 | 0.56 ± 0.08 |
| Rt. Fmajor | 24 | −54 | −8 | 0.66 ± 0.11 | 0.62 ± 0.09 |
| Lt. Fmajor | −28 | −42 | −8 | 0.67 ± 0.09 | 0.61 ± 0.11 |
| Rt. PCR | 25 | −23 | 7 | 0.64 ± 0.09 | 0.63 ± 0.04 |
| Lt. PCR | −28 | −20 | 7 | 0.66 ± 0.09 | 0.65 ± 0.04 |
| Rt. ACR | 17 | 51 | −6 | 0.64 ± 0.06 | 0.62 ± 0.05 |
| Lt. ACR | −20 | 56 | −8 | 0.63 ± 0.06 | 0.59 ± 0.05 |
| Pons | 3 | −13 | −54 | 0.82 ± 0.19 | 0.71 ± 0.12 |
Note:—ACR indicates anterior corona radiata; Fmajor, forceps major; PCR, posterior corona radiata; SCC, splenium of the corpus callosum; MNI, Montreal Neurological Institute; GCC, genu of the corpus callosum; Rt, right; Lt, left.
Fig 2.
Voxelwise multiple regression analysis of DTI indices and the duration of hemodialysis in patients with ESRD. Red-yellow colors indicate the significant positive correlation in RD (A–C) and MD (D–I). Color bars show the ranges of t-scores.
Fig 3.
Voxelwise multiple regression analysis of DTI indices and CASI scores in patients with ESRD. Red-yellow colors indicate the significant negative correlations in RD (A–C) and MD (D–F) and a positive correlation in FA (G–I). Color bars show the ranges of t-scores.
Mean Diffusivity
In patients with ESRD, widespread significant increases in MD were observed in the white matter regions, including the genu, splenium, and body of the corpus callosum; the bilateral posterior corona radiata; the bilateral anterior corona radiata; the bilateral forceps major; and the bilateral retrolenticular part of the internal capsule. These increases extended to the bilateral sagittal stratum, pontine crossing tracts, and middle cerebellar peduncle, as shown in Fig 1M–R. Montreal Neurological Institute coordinates of areas with significant MD differences are shown in Table 4. Multiple regression analysis revealed a positive correlation between MD and the duration of hemodialysis in the pons, the right retrolenticular part of the internal capsule, and the left anterior frontal white matter, as shown in Fig 2D–I. A significant negative correlation between MD and the CASI score was also observed in the left anterior corona radiata and right superior corona radiata, as shown in Fig 3D–F.
Table 4:
MNI coordinates of major areas with significant MD differences along with means of MD values
| Brain Regions | MNI Coordinate (mm) |
MD (mean) × 10−3 mm2/s |
|||
|---|---|---|---|---|---|
| X | Y | Z | ESRD | Healthy | |
| GCC | 1 | 39 | −8 | 1.16 ± 0.07 | 1.08 ± 0.05 |
| SCC | 1 | −13 | 5 | 1.12 ± 0.13 | 1.02 ± 0.06 |
| Rt. SS | 31 | −31 | −9 | 1.27 ± 0.22 | 1.21 ± 0.15 |
| Lt. SS | −39 | −24 | −15 | 1.07 ± 0.12 | 1.04 ± 0.08 |
| Rt. Fmajor | 25 | −42 | −3 | 0.96 ± 0.11 | 0.89 ± 0.04 |
| Lt. Fmajor | −30 | −34 | −3 | 1.15 ± 0.11 | 0.99 ± 0.09 |
| Rt. PCR | 27 | −25 | 4 | 1.07 ± 0.12 | 0.92 ± 0.06 |
| Lt. PCR | −29 | −25 | 1 | 1.21 ± 0.18 | 1.11 ± 0.15 |
| Pons | 2 | −14 | −56 | 1.28 ± 0.13 | 1.13 ± 0.09 |
Note:—Fmajor indicates forceps major; PCR, posterior corona radiata; SCC, splenium of the corpus callosum; SS, sagittal stratum; MNI, Montreal Neurological Institute; GCC, genu of the corpus callosum; Rt, right; Lt, left.
Fractional Anisotropy
Similarly, FA values were significantly decreased in multiple areas of the brain in patients with ESRD. Major differences between patients with ESRD and healthy subjects were found in clusters in the genu and splenium of the corpus callosum and the bilateral retrolenticular part of the internal capsule extending to the bilateral sagittal stratum. Some scattered clusters were found in the middle cerebellar peduncle, as shown in Fig 1S–X. Montreal Neurological Institute coordinates of areas with significant FA differences are listed in Table 5. Multiple regression analysis revealed a positive correlation between FA values and the CASI score in the left anterior corona radiata, left posterior corona radiata, and right superior corona radiata in patients with ESRD, as shown in Fig 3G–I. However, no significant correlations were observed between FA and the duration of hemodialysis. Montreal Neurological Institute coordinates of areas with significant correlations of RD, MD, and FA values to the duration of hemodialysis and CASI score are shown in Table 6.
Table 5:
MNI coordinates of major areas with significant FA differences along with means of FA values
| Brain Regions | MNI Coordinate (mm) |
FA (mean) |
|||
|---|---|---|---|---|---|
| X | Y | Z | ESRD | Healthy | |
| GCC | 3 | 40 | −8 | 0.6 ± 0.06 | 0.67 ± 0.04 |
| SCC | 3 | −14 | 5 | 0.65 ± 0.05 | 0.72 ± 0.05 |
| Rt. SS | 31 | −28 | −6 | 0.50 ± 0.04 | 0.56 ± 0.04 |
| Lt. SS | −27 | −39 | −6 | 0.46 ± 0.05 | 0.56 ± 0.07 |
| Rt. MCP | 13 | −18 | −58 | 0.45 ± 0.03 | 0.53 ± 0.04 |
Note:—MCP indicates middle cerebellar peduncle; SCC, splenium of the corpus callosum; SS, sagittal stratum; MNI, Montreal Neurological Institute; GCC, genu of the corpus callosum; Rt, right; Lt, left.
Table 6:
MNI coordinates of areas with significant correlations along with their correlation coefficients
| Brain Regions | MNI Coordinate (mm) |
Correlation Coefficient | ||
|---|---|---|---|---|
| X | Y | Z | ||
| RD and duration | ||||
| Lt. Pons | −16 | −3 | −46 | 0.7093 |
| RD and CASI | ||||
| Lt. ACR | −28 | 33 | 19 | −0.7442 |
| Rt. SCR | 31 | 7 | 21 | −0.6154 |
| MD and duration | ||||
| Rt. RLIC | 33 | −20 | −6 | 0.6009 |
| Lt. FWM | −21 | 63 | −5 | 0.6287 |
| Lt. Pons | −12 | −3 | −46 | 0.6675 |
| MD and CASI | ||||
| Lt. ACR | −28 | 33 | 19 | −0.7657 |
| Rt. SCR | 31 | 7 | 21 | −0.5463 |
| FA and CASI | ||||
| Lt. ACR | −17 | 40 | 18 | 0.7067 |
| Lt. PCR | −20 | −29 | 24 | 0.6117 |
| Rt. SCR | 33 | 6 | 26 | 0.6815 |
Note:—ACR indicates anterior corona radiata; PCR, posterior corona radiata; RLIC, retrolenticular part of the internal capsule; SCR, superior corona radiata; MNI, Montreal Neurological Institute; FWM, frontal white matter; Rt, right; Lt, left.
Discussion
In this study, voxelwise analysis of DTI was performed in patients with ESRD on long-term hemodialysis to reveal global white matter alterations not only in the cerebrum but also in the infratentorial structures. In general, AD, RD, and MD values increased significantly, whereas FA decreased significantly in multiple white matter areas in patients with ESRD. Significant increases in MD were observed in many white matter regions in patients with ESRD that are similar to those of a previous DWI study6; however, in this study, increases in AD, RD, and MD and a decrease in FA that were not mentioned previously were also observed in the pons and cerebellum. Although these changes were observed in patients with ESRD who underwent an MR imaging 2 days after the last weekly hemodialysis, part of these results may very likely be attributed to the immediate effects of hemodialysis, which were shown to impact cognitive functions longer than 2 days.19
Unlike cognitive function, white matter alterations in the infratentorial regions after hemodialysis were only discussed in a few studies using conventional MR imaging.20–22 Dialysis disequilibrium syndrome, first described in 1962 by Kennedy et al,3 is commonly described as an acute neurologic disorder that occurs in patients undergoing hemodialysis. In addition, osmotic demyelination syndrome, which has been observed in patients with ESRD, is characterized by transient edema and demyelination in the pons and extrapontine regions after hemodialysis.21 Although both syndromes occur less often in patients on long-term dialysis, the results of this study suggest that dialysis-associated changes may occur not only in cerebral white matter but also in cerebellar and brain stem regions. These changes may have been associated with both syndromes.
In analyses of diffusivity, AD and RD were associated with white matter changes in patients with ESRD. In most regions with increased MD, significant increases in RD but not AD were found. Similar findings have been reported in previous animal and human studies in which defective myelin was shown to increase RD more than AD.23,24 Although the mechanisms of uremic neuropathy are complex, the results of this study suggest that demyelination of white matter is the major neuropathy in patients with ESRD with long-term hemodialysis. Furthermore, a positive correlation of MD and RD with the duration of hemodialysis was found. These findings may reflect progressive changes of demyelination, which is the subsequent change in uremic neuropathy. Because a previous study demonstrated that neurologic complications, whether due to the uremic state or its treatment, play an important role in the morbidity and mortality of patients with ESRD,25 monitoring the damage to brain microstructures is important in these patients. The results of this study suggest that MD and RD may be good indices for monitoring changes in demyelination in patients with ESRD.
Similar to our previous study, this study identified significant decreases in FA in multiple areas of the brains of patients with ESRD.7 However, these decreases were observed not only in the cerebral white matter but also in the middle cerebellar peduncle. Similar to that in previous studies, a decrease in diffusion anisotropy is recommended as a good index for neural degeneration.23,26 In patients with ESRD, decreases in FA and increases in MD were caused by reduced microstructural integrity with macroscopic tissue loss or interstitial edema, whereas decreases in FA and normal MD were attributed to microstructural changes without gross tissue loss or with the occurrence of gliosis. However, decreases in FA and increases in RD occurred because of demyelination of white matter tissue. In multiple regression analysis, though no significant correlation was observed between FA and the duration of hemodialysis, a trend of negative correlations was observed when the statistical criteria were lowered (P < .05), suggesting that long-term hemodialysis slightly and gradually compromised the integrity of white matter tissue in these patients.
Two-sample t test analysis revealed significantly lower CASI scores in patients with ESRD than in healthy subjects. Multiple regression analysis further revealed significant correlations of RD, MD, and FA values with CASI scores in the frontal and parietal white matter that are likely responsible for cognitive function of the brain. The increases in RD and MD values and decreases in FA values may highlight microstructural nerve damage, including axon injury and demyelination, which generally parallel the degree of clinical and pathologic impairment. Previous studies demonstrated that DTI can detect early neurodegenerative changes and subtle changes in clinical function.27,28 The results of this study suggested that DTI can detect uremic neuropathy, which is associated with cognitive function.
The small number of participants with ESRD is a limitation of this study. The inclusion criteria that patients had to be young and undergoing hemodialysis may have been responsible for the small sample size. An extended study with a larger sample size and inclusion of those undergoing peritoneal dialysis, which led to higher mortality rates than hemodialysis in a previous study,29 may provide more information about the effects of different factors. A further follow-up study would be helpful for understanding the long-term effects of dialysis methods. In terms of data acquisition, this study acquired DTI data with a section thickness of 4.4 mm, which is larger than the in-plane resolution and has more partial volume averaging in the through-plane direction. Hence, the results of this study may have been affected by the partial volume effects of imaging in the through-plane direction. In cognitive assessment, this study did not record the subject's education level. Because the CASI score is associated with education,30 the results of this study may also have been affected by the educational difference between the 2 groups.
Conclusions
The voxel-based analysis method was used in this study for global detection of white matter alterations in patients with ESRD on long-term hemodialysis. The results showed that AD, RD, and MD were significantly increased, whereas FA was significantly decreased in many white matter regions of the brain of patients with ESRD. The interpretation of these white matter alterations by using all DTI indices led to the conclusion that long-term hemodialysis caused increased interstitial edema in both the supra- and infratentorial regions and gradually led to axonal demyelination in the pons. Finally, voxel-based DTI analysis was helpful in characterizing white matter alterations in patients with ESRD on long-term hemodialysis.
ABBREVIATIONS:
- AD
axial diffusivity
- CASI
Cognitive Abilities Screening Instrument
- ESRD
end-stage renal disease
- FA
fractional anisotropy
- MD
mean diffusivity
- RD
radial diffusivity
Footnotes
This work was supported in part by grant NSC-99–2314-B-037–070-MY2 from the National Science Council of Taiwan.
REFERENCES
- 1. Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States renal data system. J Am Soc Nephrol 2007;18:2644–48 [DOI] [PubMed] [Google Scholar]
- 2. Arieff AI. Dialysis disequilibrium syndrome: current concepts on pathogenesis and prevention. Kidney Int 1994;45:629–35 [DOI] [PubMed] [Google Scholar]
- 3. Kennedy AC, Eaton JC, Linton AL. Urea levels in cerebrospinal fluid after haemodialysis. Lancet 1962;1:410–11 [DOI] [PubMed] [Google Scholar]
- 4. Pappius HM, Oh JH, Dossetor JB. The effects of rapid hemodialysis on brain tissues and cerebrospinal fluid of dogs. Can J Physiol Pharmacol 1967;45:129–47 [DOI] [PubMed] [Google Scholar]
- 5. Galons JP, Trouard T, Gmitro AF, et al. Hemodialysis increases apparent diffusion coefficient of brain water in nephrectomized rats measured by isotropic diffusion-weighted magnetic resonance imaging. J Clin Invest 1996;98:750–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen CL, Lai PH. A preliminary report of brain edema in patients with uremia at first hemodialysis: evaluation by diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2007;28:68–71 [PMC free article] [PubMed] [Google Scholar]
- 7. Hsieh TJ, Chang JM, Chuang HY, et al. End-stage renal disease: in vivo diffusion-tensor imaging of silent white matter damage. Radiology 2009;252:518–25 [DOI] [PubMed] [Google Scholar]
- 8. Kim H, Park J, Bai D, et al. Diffusion tensor imaging findings in neurologically asymptomatic patients with end stage renal disease. NeuroRehabilation 2011;29:111–16 [DOI] [PubMed] [Google Scholar]
- 9. Snook L, Plewes C, Beaulieu C. Voxel based versus region of interest analysis in diffusion tensor imaging of neurodevelopment. Neuroimage 2007;34:243–52 [DOI] [PubMed] [Google Scholar]
- 10. Ashburner J, Friston KJ. Voxel-based morphometry: the methods. Neuroimage 2000;11:805–21 [DOI] [PubMed] [Google Scholar]
- 11. Della Nave R, Foresti S, Pratesi A, et al. Whole-brain histogram and voxel-based analyses of diffusion tensor imaging in patients with leukoaraiosis: correlation with motor and cognitive impairment. AJNR Am J Neuroradiol 2007;28:1313–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu HS, Chou MC, Chung HW, et al. Potential long-term effects of MDMA on the basal ganglia-thalamocortical circuit: a proton MR spectroscopy and diffusion-tensor imaging study. Radiology 2011;260:531–40 [DOI] [PubMed] [Google Scholar]
- 13. Grieve SM, Williams LM, Paul RH, et al. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. AJNR Am J Neuroradiol 2007;28:226–35 [PMC free article] [PubMed] [Google Scholar]
- 14. Vercauteren T, Pennec X, Perchant A, et al. Diffeomorphic demons: Efficient non-parametric image registration. Neuroimage 2009;45:S61–72 [DOI] [PubMed] [Google Scholar]
- 15. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007;38:95–113 [DOI] [PubMed] [Google Scholar]
- 16. Takahashi R, Ishii K, Miyamoto N, et al. Measurement of gray and white matter atrophy in dementia with Lewy bodies using diffeomorphic anatomic registration through exponentiated lie algebra: a comparison with conventional voxel-based morphometry. AJNR Am J Neuroradiol 2010;31:1873–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teng EL, Hasegawa K, A. H., et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr 1994;6:45–58 [DOI] [PubMed] [Google Scholar]
- 18. Mori S, Oishi K, Jiang HY, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 2008;40:570–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williams MA, Sklar AH, Burright RG, et al. Temporal effects of dialysis on cognitive functioning in patients with ESRD. Am J Kidney Dis 2004;43:705–11 [DOI] [PubMed] [Google Scholar]
- 20. Lakadamyali H, Ergun T. MRI for acute neurologic complications in end-stage renal disease patients on hemodialysis. Diagn Interv Radiol 2011;17:112–17 [DOI] [PubMed] [Google Scholar]
- 21. Tarhan NC, Agildere AM, Benli US, et al. Osmotic demyelination syndrome in end-stage renal disease after recent hemodialysis: MRI of the brain. AJR Am J Roentgenol 2004;182:809–16 [DOI] [PubMed] [Google Scholar]
- 22. Ağildere AM, Benli S, Erten Y, et al. Osmotic demyelination syndrome with a disequilibrium syndrome: reversible MRI findings. Neuroradiology 1998;40:228–32 [DOI] [PubMed] [Google Scholar]
- 23. Song SK, Sun SW, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002;17:1429–36 [DOI] [PubMed] [Google Scholar]
- 24. Metwalli NS, Benatar M, Nair G, et al. Utility of axial and radial diffusivity from diffusion tensor MRI as markers of neurodegeneration in amyotrophic lateral sclerosis. Brain Res 2010;1348:156–64 [DOI] [PubMed] [Google Scholar]
- 25. Brouns R, De Deyn PP. Neurological complications in renal failure: a review. Clin Neurol Neurosurg 2004;107:1–16 [DOI] [PubMed] [Google Scholar]
- 26. Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology 2001;56:304–11 [DOI] [PubMed] [Google Scholar]
- 27. Huang J, Friedland RP, Auchus AP. Diffusion tensor imaging of normal-appearing white matter in mild cognitive impairment and early Alzheimer disease: preliminary evidence of axonal degeneration in the temporal lobe. AJNR Am J Neuroradiol 2007;28:1943–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kantarci K, Senjem ML, Avula R, et al. Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology 2011;77:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bloembergen WE, Port FK, Mauger EA, et al. A comparison of mortality between patients treated with hemodialysis and peritoneal-dialysis. J Am Soc Nephrol 1995;6:177–83 [DOI] [PubMed] [Google Scholar]
- 30. McCurry SM, Edland SD, Teri L, et al. The Cognitive Abilities Screening Instrument (CASI): data from a cohort of 2524 cognitively intact elderly. Int J Geriatr Psychiatry 1999;14:882–88 [PubMed] [Google Scholar]