Abstract
BACKGROUND AND PURPOSE:
Atherosclerotic disease of the carotid artery is an important cause of ischemic stroke. We evaluated carotid plaque morphologic features by using CTA in addition to stenosis in the setting of symptomatic hemispheric TIA/stroke to identify factors that may predict plaque activity.
MATERIALS AND METHODS:
Six hundred seventy-three patients (408 men; ages, 18–91 years; mean, 65.8 ± 15.2 years) presenting with hemispheric ischemic symptoms and having a CTA that included imaging of both carotid arteries within 24 hours were studied. Scans were interpreted for morphologic features, such as plaque length and width, attenuation, shape, surface, presence and degree of calcification, and ILT in addition to stenosis.
RESULTS:
Univariable analysis showed that carotid occlusions (P = .01, OR = 5.27), high-grade stenosis (70%–99%) (P = .06, OR = 1.8), and the presence of ILT (P = .01, OR = 4.33) were highly predictive of the symptomatic side. Smooth plaque (P = .01, OR = 0.73) and extensive calcification (P = .03, OR = 0.72) were more commonly associated with the asymptomatic side. There was no correlation between plaque hypoattenuation (P = .7, OR = 1.06) or ulcerated plaque (P = .74, OR = 0.955) in predicting the symptomatic side. In a multivariable logistic regression model, the presence of ILT was still found to be significantly associated with the symptomatic side (P = .048, OR = 3.1) and the presence of extensive calcification, with the asymptomatic carotid artery (P = .047, OR = 0.69).
CONCLUSIONS:
In addition to higher stenosis grades, the presence of ILT is highly predictive of the symptomatic side in carotid disease. Smooth plaque and extensive calcification seem to afford a protective effect. This information may be useful in radiologic risk stratification in carotid disease in addition to the current evidence available based on stenosis criteria alone.
Carotid artery atherosclerotic disease is an important cause of ischemic stroke, and thromboembolism is the predominant relevant stroke mechanism. Current clinical guidelines for revascularization strategies in carotid disease are based on stenosis criteria alone. These guidelines are derived from strong clinical trial data that examined primarily the long-term risk of stroke.1–3 Although degree of stenosis is a good surrogate marker for atherosclerotic vascular disease, based on the plausible assumption that vessel narrowing is caused by plaque accumulating in the lumen of the artery, there may be other factors that could potentially predict clinical behavior of atherosclerotic plaques. Plaque characteristics such as lipid-rich core, fibrous cap thickness, and intraplaque hemorrhage have been demonstrated on noninvasive imaging modalities such as sonography4–6 and MR imaging,7–14 and it is suggested that these ancillary features, taken in conjunction with the degree of stenosis, may be important predictors of immediate and long-term stroke risk. More recently, especially with advances in multidetector technology, CT and CTA have also been found useful in the evaluation of patients with symptomatic carotid disease.15–17
In clinical practice, patients are seen who have clinical events that are not explained by the stenosis severity and who have negative findings on a work-up for other potential proximal embolic sources. With the increasing availability of noninvasive vascular imaging modalities such as Doppler sonography, MR imaging, and CTA, patients are also encountered with no clear clinical event but who have relatively high stenosis grades. It is also common to see severe stenotic disease on the side contralateral to the presenting hemispheric event.
We evaluated CTA features of carotid plaque that would potentially predict symptomatic carotid disease in addition to the degree of stenosis. At our dedicated stroke center, we perform CTA in patients with acute ischemic events as part of our clinical protocol for vascular and parenchymal imaging. We hypothesized that certain morphologic characteristics of atherosclerotic plaque could serve as an adjunct to stenosis grade in potentially helping to decide revascularization options among patients who may not have been offered these same options based on stenosis criteria alone, especially if recurrent strokes occur with maximal medical therapy.
Materials and Methods
We retrospectively evaluated CTA data, obtained as part of standard clinical care for patients with stroke presenting to our institution, with institutional review board approval. At our center, patients with suspected acute ischemic stroke undergo evaluation with a plain CT followed by CTA of the cervical and intracranial vasculature as per institutional protocol. We identified consecutive patients from June 2004 to August 2007 who presented to the stroke neurology service, usually through the emergency department, with hemispheric ischemic symptoms and/or TIA, including amaurosis fugax, and who also had a CTA that included imaging of both carotid arteries within 24 hours of admission. All patients were reviewed clinically by a stroke neurologist and had a baseline NIHSS score documented on admission. Patients with technically inadequate CTA studies were excluded. The affected hemispheric side was determined, and the ipsilateral carotid artery was designated as the “case” carotid. The contralateral carotid artery served as the “control.” The use of contralateral carotid arteries has advantages because it automatically accounts for age, sex, and cardiovascular risk factors.
CTA Technique
CTA was performed by using multidetector scanners. Both a 4-section CT scanner (LightSpeed Plus; GE Healthcare, Milwaukee, Wisconsin) and a 64-section scanner (SOMATOM Sensation 64; Siemens, Erlangen, Germany) were used. The distribution of patients between both types of scanners was roughly equal. Both scanners used an automated trigger technique for injection of contrast at 5 mL/s for a total of 90–120 mL Scanning was performed from the aortic arch to the vertex. The 4-section scanner used a collimation of 4 × 1.25 mm, a pitch of 0.9, and a rotation time of 0.5 seconds to acquire the raw data, which was reconstructed at 2.5-mm-thick overlapping sections for axial images. Thinner sections (1.25 mm at 50% overlap) were used to reconstruct axial images of the circle of Willis. Images of the cervical carotid arteries were reconstructed in the sagittal plane at a 4-mm thickness. With the 64-section scanner, a collimation of 64 × 0.6 mm and pitch of 0.9 for a rotation time of 0.5 seconds was used to acquire the raw data, which were reconstructed at 1-mm overlapping sections for the axial images. Reconstructions for axial, coronal, and sagittal images of the circle of Willis as well as the carotid arteries on each side were performed at a 3-mm thickness at 1-mm intervals.
Evaluation of Findings
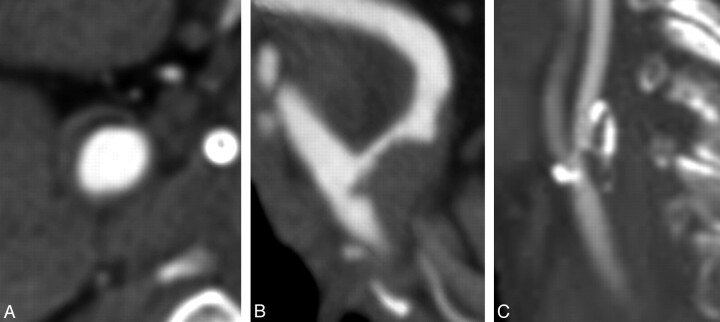
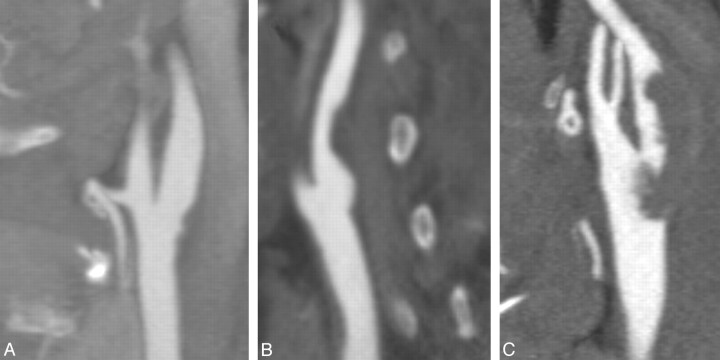
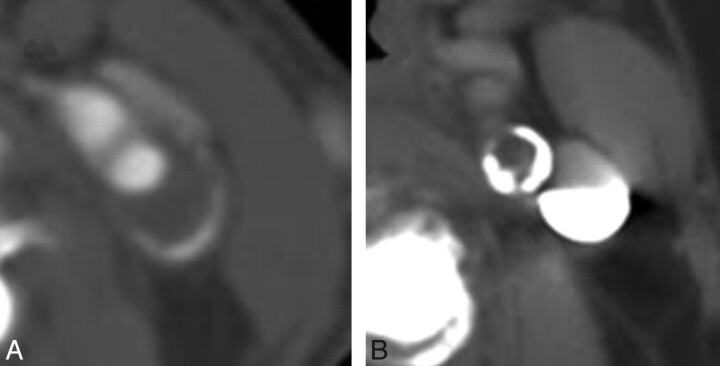
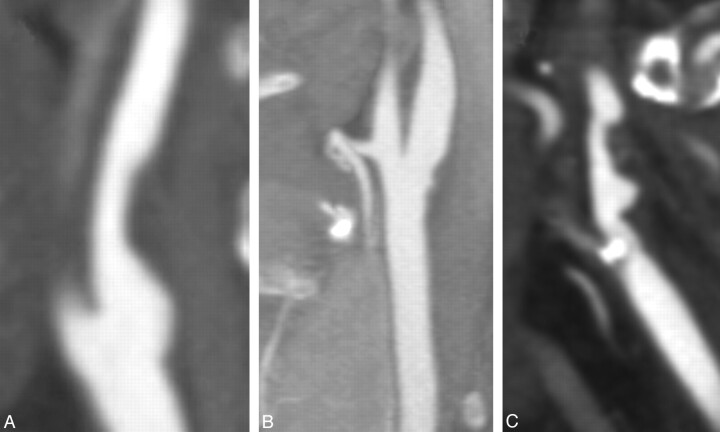
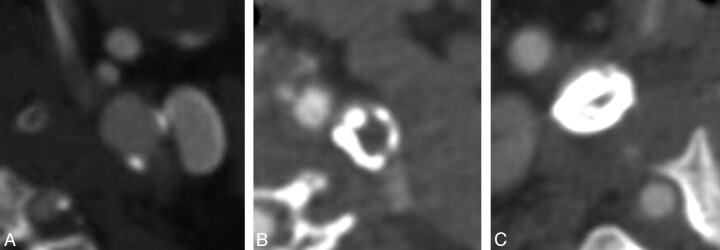
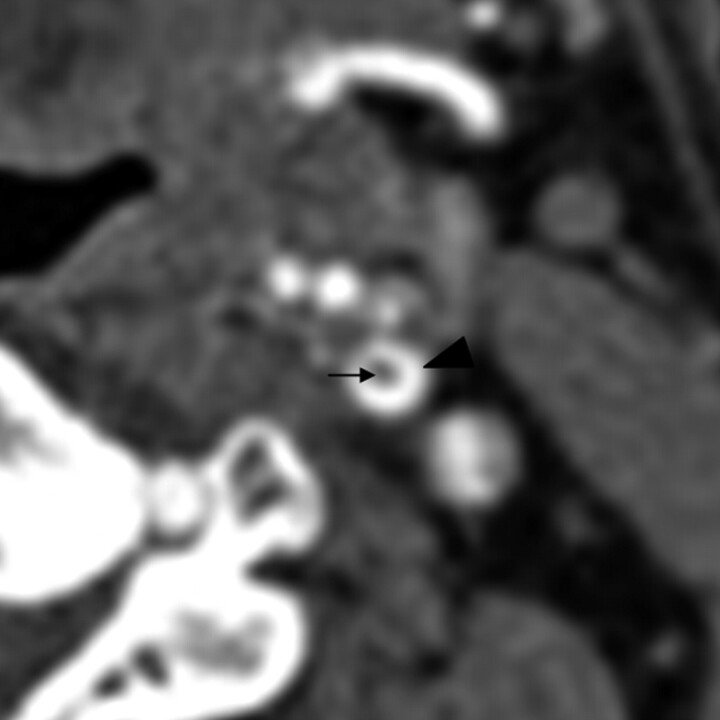
The CTA images were evaluated by 4 readers, who analyzed the images in groups of 2, 1 neurologist and 1 neuroradiologist who were experienced in the evaluation of CT and CTA images in patients with acute stroke. The readers were blinded to the symptom side as well as other clinical information. Stenosis was calculated from sagittal images reconstructed along the long axis of the internal carotid artery, by using the NASCET approach.1 The morphologic features including plaque attenuation (Fig 1), plaque shape (Figs 2 and 3), plaque surface (Fig 4), and degree of calcification (Fig 5) were determined from a review of all images. The various plaque shapes were classified as linear, crescent, sessile, pedunculated, or circumferential. The plaque surface was classified as smooth, irregular without ulceration, and ulcerated. The length and width of the plaque were also documented as was the presence of an intraluminal thrombus (Fig 6). An “intraluminal thrombus” was defined as an eccentric pedunculated filling defect projecting into the lumen of the vessel.
Fig 1.
CTA images in separate patients showing hypoattenuated (A), isoattenuated (B), and heterogeneous attenuation (C) plaques.
Fig 2.
Sagittal reformatted CTA images of the carotid bifurcation in separate patients showing linear (A), sessile (B), and pedunculated (C) plaque shapes.
Fig 3.
Axial CTA images of the carotid artery in separate patients showing crescentic (A) and circumferential (B) plaque shapes.
Fig 4.
Sagittal reformatted images from the CTAs of separate patients showing smooth (A), irregular (B), and ulcerated (C) plaque surfaces.
Fig 5.
Axial images from CTAs of the carotid arteries in separate patients showing mild (A), moderate (B), and extensive (C) calcification.
Fig 6.
Axial CTA image showing an intraluminal thrombus (black arrow) as a central filling defect surrounded by hyperattenuating contrast agent (arrowhead).
Discrepant findings were resolved by consensus.
Statistical Methods
Statistical analysis was performed with the STATA statistical software, Version 10 (StataCorp, College Station, Texas). For evaluation, the degree of stenosis was categorized as follows: <30%, 30%–49%, 50%–69%, 70%–99%, and occlusion. Data were reported by using standard descriptive summary statistics. Univariable analysis was used to assess the relationship between each individual plaque feature in addition to the category of stenosis and symptomatic side status. A multivariable logistic regression model was developed by using backward stepwise elimination of imaging factors to provide an overall assessment of the role of these factors in predicting the symptomatic side. A parsimonious model was sought so that those factors not significant at P < .05 were dropped. Age and sex were forced variables in this model. We accounted for the dependence of variables within patients by clustering the analysis within patients.
Results
There were 988 patients who had evaluations of the carotid arteries between June 2004 and August 2007. Of these patients, 972 patients had scans that were adequate for interpretation. Of these patients, 673 (408 male patients and 265 female patients; ages, 18–91 years; mean, 65.8 ± 15.2 years) were identified who had a clear hemispheric event, and these patients constituted the study population. Three hundred forty-nine patients had right-hemispheric symptoms, and 324 patients had left-hemispheric symptoms. The ipsilateral carotid arteries served as cases (n = 673) and the contralateral side, as controls (n = 673). The demographic information for the study population is given in Table 1.
Table 1:
Patient demographics
| Demographic | Total (N = 673), Instances (%) |
|---|---|
| Male | 408 |
| Female | 265 |
| Median age (range) | 69 (18–91) |
| Median NIHSS score at baseline (range) | 5 (0–25) |
| Vascular risk factors | |
| Hypertension | 401 (59.6) |
| Diabetes | 114 (16.9) |
| Atrial fibrillation | 95 (14.1) |
| Valvular heart disease | 25 (3.7) |
| History of TIA/stroke | 129 (19.2) |
| Recent MI | 10 (1.5) |
| Family history of stroke | 65 (9.7) |
| Current smoker | 165 (24.5) |
| Former smoker | 74 (11.0) |
| Hypercholesterolemia | 195 (29.0) |
Univariable analysis (Table 2) showed that carotid occlusions (P = .01, OR = 5.27), high-grade stenosis (70%–99%) (P = .06; OR = 1.9), and the presence of intraluminal thrombus (P = .01, OR = 4.33) were highly predictive of the symptomatic side. In addition, some features were more commonly associated with the asymptomatic side, such as smooth plaque surface (P = .01, OR = 0.73) and extensive calcification (P = .03, OR = 0.72). There was no correlation between plaque hypoattenuation (P = .7; OR = 1.06) or ulcerated plaque (P = .74, OR = 0.955) in predicting the symptomatic side.
Table 2:
Results of univariable and multivariable logistic regression
| Variable | Oddsa Ratio | P Value |
|---|---|---|
| Univariable logistic regression | ||
| 30%–49% Stenosis | 0.90 | .64 |
| 50%–69% Stenosis | 1.27 | .28 |
| 70%–99% Stenosis | 1.90 | .06 |
| Occlusions | 5.27 | .01 |
| Plaque length | 0.99 | .51 |
| Plaque width | 0.98 | .43 |
| Smooth surface | 0.73 | .01 |
| Irregular surface | 0.86 | .20 |
| Ulcerated surface | 0.96 | .74 |
| No calcification | 0.76 | .08 |
| Mild calcification | 0.83 | .16 |
| Moderate calcification | 0.87 | .28 |
| Extensive calcification | 0.72 | .03 |
| Hypodense plaque | 1.06 | .70 |
| Isodense plaque | 0.83 | .29 |
| Heterogeneous plaque | 0.81 | .05 |
| Intraluminal thrombus | 4.33 | .01 |
| Multivariable logistic regression of degree of stenosis accounting for age and sex | ||
| 30%–49% | 1.00 | .99 |
| 50%–69% | 1.38 | .25 |
| 70%–99% | 1.90 | .07 |
| Occlusions | 4.99 | .00 |
| Multivariable logistic regression of ILT and calcification accounting for age, sex, and degree of stenosis | ||
| Intraluminal thrombus | 3.10 | .05 |
| Extensive calcification | 0.69 | .05 |
OR of a finding being associated with the symptomatic side and P value.
In a multivariable logistic regression model accounting for age, sex, and degree of stenosis, the presence of intraluminal thrombus was still found to be significantly associated with the symptomatic side (P = .048, OR = 3.1) and the presence of extensive calcification, with the asymptomatic carotid (P = .047; OR = 0.69). This also demonstrated a graded increase in the magnitude of effect as the degree of stenosis increased toward occlusion (Table 2). The frequency of significant findings in cases versus controls is shown in Table 3.
Table 3:
Percentage of significant findings in cases versus controls
| Cases (%) | Controls (%) | |
|---|---|---|
| Occlusions | 44 (6.54) | 9 (1.34) |
| ILT | 17 (2.53) | 4 (0.59) |
| Hypodense plaque | 64 (9.51) | 56 (8.32) |
| Plaque ulceration | 85 (12.63) | 82 (12.18) |
| Smooth plaque | 103 (15.30) | 130 (19.32) |
| Extensive calcification | 53 (7.88) | 68 (10.10) |
Discussion
Existing measures for the effectiveness of endarterectomy among patients with symptomatic carotid disease have been based on the degree of stenosis from strong clinical trial data.1–3,18 The degree of stenosis is also the basis for surgical intervention in asymptomatic patients19 and in endovascular treatment for carotid disease.20 The premise that various pathologic stages in the evolution of carotid atheroma are predictive of clinical events remains to be convincingly proved in patients with stroke. Our understanding of this process in carotid atheromas has been extrapolated from the histologic classification of coronary plaques developed by the AHA.21,22 Noninvasive modalities can image atherosclerotic carotid vascular disease and depict these various pathologic stages. Plaque characteristics such as lipid-rich core, fibrous cap thickness, and intraplaque hemorrhage have been demonstrated on sonography4–6 and MR imaging,7–14,23–25 and it is suggested that these ancillary features, taken in conjunction with the degree of stenosis, may be important predictors of future stroke risk. CT angiography is being increasingly used to noninvasively image carotid stenosis26,27 and has also been shown to be useful in the evaluation of carotid plaque morphology.15,17,28–31
The findings from our study indicate that apart from increasing degree of stenosis, the presence of an intraluminal thrombus is highly predictive of the symptomatic side in patients with carotid disease. In addition, we observed that extensive calcification and smooth plaques were more likely to be associated with the asymptomatic side. Whether this in fact correlates with protective mechanisms requires large-scale prospective trials looking at natural history data. Other studies have shown that patients with extensive calcification of plaques on endarterectomy specimens are less likely to have symptomatic disease.32
The presence of an intraluminal thrombus has implications in the management of patients with complicated carotid plaques33,34 and was seen in ≤1.8% of patients in the NASCET trial.1 In our series, this was defined as an eccentric protruding filling defect within the lumen, which, on cross-section, appears to lie centrally within the lumen, surrounded by contrast (Fig 6). CT angiography may be more sensitive to the detection of intraluminal thrombus because it is less prone to the volume averaging effects from surrounding contrast as in a conventional catheter angiogram. Some of the patients with intraluminal thrombus have shown resolution of the thrombotic filling defect on follow-up imaging after aggressive antithrombotic medical treatment. These observations suggest that the presence of this finding might indicate plaque activity based on an interaction of factors such as platelet activation and aggregation and continued exposure to thrombogenic plaque contents.
We also observed that the presence of a smooth-appearing plaque and extensive calcification seems to afford a protective effect. As a corollary to the discussion on intraluminal thrombus, it is plausible that unless it is causing a hemodynamic effect, a smooth appearance of a plaque may indicate relative plaque stability and a lower grade plaque without complication (AHA I-V).21,22 Calcification may be protective by preventing adhesion, and activation and subsequent aggregation forming platelet-rich thrombi and corresponding to AHA type VII.22
Wintermark et al,17 in a smaller cross-sectional retrospective study, by using a custom automated computer algorithm to evaluate various wall descriptors, showed that wall volume, fibrous cap thickness, the number and location of lipid clusters, and the number of calcium clusters were significantly associated with acute carotid stroke. Of 136 patients, they identified 40 patients with “acute carotid stroke” and 50 patients with “noncarotid strokes.” They analyzed features between the 2 groups and also between the symptomatic and asymptomatic sides for the 40 patients with acute carotid stroke. Their study was robust in that their use of a computerized algorithm to quantitatively assess a battery of carotid features yielded objective parameters for studying carotid atherosclerotic disease. Also using a noncarotid stroke population as a control allows a truer determination of factors contributing to atherosclerotic carotid disease. In our study, we included many of those wall descriptors but in a manner that allows their identification by visual inspection from scans obtained within existing clinical protocols, and we used only contralateral carotid arteries as controls.
In our series, we did not find evidence to suggest that hypoattenuated plaque or the presence of ulceration is significantly associated with the symptomatic side. It could be possible that in the absence of thrombi formation, the mere presence of a lipid-rich necrotic core may not be a causative factor for ischemic symptoms in the acute setting or that CT may not be accurately detecting attenuation changes within plaque to the level required to achieve statistical significance. Most interesting, in our patients, the univariable logistic regression did not show that the presence of an ulcer was significantly associated with the symptomatic side. In their study of 406 patients by using CTA, de Weert et al30 showed that complicated plaques, which included both irregular and ulcerated plaques, were more often present in the symptomatic carotid artery than in the contralateral asymptomatic carotid artery (25% versus 18%, P < .01). A multivariable analysis, however, suggested that this could be attributed to the significantly higher stenosis grade present in symptomatic arteries.
MR imaging has been, in general, the more common technique for detecting complicated plaque. Murphy et al10 demonstrated the utility of direct thrombus imaging in patients with carotid disease by using MR imaging to suggest that high signal intensity on T1-weighted images may represent the intraplaque hemorrhage component of complicated plaques. In a progress review, Wasserman et al11 suggested that high-resolution black-blood MR imaging may predict vulnerable lesions even in low-grade carotid stenosis. In a recent study, Bitar et al13 showed that T1 hyperintense intraplaque signal intensity on 3D high spatial resolution on in vivo imaging represents areas of intraplaque hemorrhage on histologic samples. The utility of CT to detect intraplaque hemorrhage as a component of vulnerable plaque is limited by inadequate contrast resolution. Wasserman et al12 also demonstrated that increased contrast enhancement within the fibrous cap and outer wall of a carotid atheroma after administration of intravenous gadolinium might reflect sites of active inflammation. This may be indicative of the neovascularity seen with plaque instability. We think that it is unlikely that CT-based techniques would be able to depict such inflammatory change.
Our study should be interpreted in the context of the following limitations: We acknowledge that the study was performed in a retrospective manner. We also note that there are multiple factors involved in the pathophysiologic mechanisms underlying cerebrovascular ischemia, and in our patients, we believe that symptoms related to hemispheric ischemia originated in the carotid arteries. Using contralateral carotid arteries as controls has advantages in that various factors such as age and cardiovascular risk factors are accounted for and an adequate sample size of controls is obtained. The alternative would have been to use age-matched controls with noncarotid disease (such as patients with a proximal embolic source). We also acknowledge that certain findings may be related to the inherent nature of CT technology, especially when compared with MR imaging−based studies. We think that CT may not be adequate to demonstrate some features of complicated plaque such as intraplaque hemorrhage, neovascularity, and small surface ulcerations. The inability to detect a significant difference with hypoattenuated plaques in our study may relate to the fact that attenuation values were not objectively measured. Also, it is unclear whether the differences in scanner technology used (4-detector scanner versus 64-detector) had an effect on the data.
In summary, our findings suggest that certain morphologic characteristics of carotid plaque on CTA are seen more commonly with symptomatic carotid disease at the time of presentation and may predict clinical behavior in addition to the degree of stenosis alone. This may help in the decision-making process with regard to the need and timing of carotid revascularization in the appropriate clinical setting.
Acknowledgments
We acknowledge the help of Jayanta Roy, Imanuel Dzialowski, Volker Puetz, Christine O'Reilly, and Sherif Idris.
Abbreviations
- AHA
American Heart Association
- CTA
CT angiography
- ILT
intraluminal thrombus
- MI
myocardial infarction
- NASCET
North America Symptomatic Carotid Endarterectomy Trial
- NIHSS
National Institutes of Health Stroke Scale
- OR
odds ratio
- TIA
transient ischemic attack
Footnotes
Paper previously presented as an abstract at: Annual Meeting of the American Society of Neuroradiology, May 18–21, 2009; Vancouver, British Columbia, Canada.
References
- 1. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis: North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1991;325:445–53 [DOI] [PubMed] [Google Scholar]
- 2. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70%–99%) or with mild (0%–29%) carotid stenosis—European Carotid Surgery Trialists' Collaborative Group. Lancet 1991;337:1235–43 [PubMed] [Google Scholar]
- 3. Mayberg MR, Wilson SE, Yatsu F, et al. for the Veterans Affairs Cooperative Studies Program 309 Trialist Group . Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. JAMA 1991;266:3289–94 [PubMed] [Google Scholar]
- 4. Gray-Weale AC, Graham JC, Burnett JR, et al. Carotid artery atheroma: comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J Cardiovasc Surg (Torino) 1988;29:676–81 [PubMed] [Google Scholar]
- 5. Grønholdt ML, Nordestgaard BG, Schroeder TV, et al. Ultrasonic echolucent carotid plaques predict future strokes. Circulation 2001;104:68–73 [DOI] [PubMed] [Google Scholar]
- 6. Grønholdt ML. Ultrasound and lipoproteins as predictors of lipid-rich, rupture-prone plaques in the carotid artery. Arterioscler Thromb Vasc Biol 1999;19:2–13 [DOI] [PubMed] [Google Scholar]
- 7. Hatsukami TS, Ross R, Polissar NL, et al. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation 2000;102:959–64 [DOI] [PubMed] [Google Scholar]
- 8. Takaya N, Yuan C, Chu B, et al. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation 2005;111:2768–75 [DOI] [PubMed] [Google Scholar]
- 9. Yuan C, Zhang SX, Polissar NL, et al. Identification of fibrous cap rupture with magnetic resonance imaging is highly associated with recent transient ischemic attack or stroke. Circulation 2002;105:181–85 [DOI] [PubMed] [Google Scholar]
- 10. Murphy RE, Moody AR, Morgan PS, et al. Prevalence of complicated carotid atheroma as detected by magnetic resonance direct thrombus imaging in patients with suspected carotid artery stenosis and previous acute cerebral ischemia. Circulation 2003;107:3053–58 [DOI] [PubMed] [Google Scholar]
- 11. Wasserman BA, Wityk RJ, Trout HH, et al. Low-grade carotid stenosis: looking beyond the lumen with MRI. Stroke 2005;36:2504–13 [DOI] [PubMed] [Google Scholar]
- 12. Wasserman BA, Smith WI, Trout HH, 3rd, et al. Carotid artery atherosclerosis: in vivo morphologic characterization with gadolinium-enhanced double-oblique MR imaging initial results. Radiology 2002;223:566–73 [DOI] [PubMed] [Google Scholar]
- 13. Bitar R, Moody AR, Leung G, et al. In vivo 3D high-spatial-resolution MR imaging of intraplaque hemorrhage. Radiology 2008;249:259–67 [DOI] [PubMed] [Google Scholar]
- 14. Yamada N, Higashi M, Otsubo R, et al. Association between signal hyperintensity on T1-weighted MR imaging of carotid plaques and ipsilateral ischemic events. AJNR Am J Neuroradiol 2007;28:287–92 [PMC free article] [PubMed] [Google Scholar]
- 15. Oliver TB, Lammie GA, Wright AR, et al. Atherosclerotic plaque at the carotid bifurcation: CT angiographic appearance with histopathologic correlation. AJNR Am J Neuroradiol 1999;20:897–901 [PMC free article] [PubMed] [Google Scholar]
- 16. Josephson SA, Bryant SO, Mak HK, et al. Evaluation of carotid stenosis using CT angiography in the initial evaluation of stroke and TIA. Neurology 2004;63:457–60 [DOI] [PubMed] [Google Scholar]
- 17. Wintermark M, Arora S, Tong E, et al. Carotid plaque computed tomography imaging in stroke and nonstroke patients. Ann Neurol 2008;64:149–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med 1998;339:1415–25 [DOI] [PubMed] [Google Scholar]
- 19. Endarterectomy for asymptomatic carotid artery stenosis: Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995;273:1421–28 [PubMed] [Google Scholar]
- 20. Yadav JS, Wholey MH, Kuntz RE, et al. for the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators . Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004;351:1493–501 [DOI] [PubMed] [Google Scholar]
- 21. Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol 1995;15:1512–31 [DOI] [PubMed] [Google Scholar]
- 22. Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol 2000;20:1177–78 [DOI] [PubMed] [Google Scholar]
- 23. O'Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults: Cardiovascular Health Study Collaborative Research Group. N Engl J Med 1999;340:14–22 [DOI] [PubMed] [Google Scholar]
- 24. Coombs BD, Rapp JH, Ursell PC, et al. Structure of plaque at carotid bifurcation: high-resolution MRI with histological correlation. Stroke 2001;32:2516–21 [DOI] [PubMed] [Google Scholar]
- 25. Yuan C, Mitsumori LM, Ferguson MS, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation 2001;104:2051–56 [DOI] [PubMed] [Google Scholar]
- 26. Bartlett ES, Walters TD, Symons SP, et al. Quantification of carotid stenosis on CT angiography. AJNR Am J Neuroradiol 2006;27:13–19 [PMC free article] [PubMed] [Google Scholar]
- 27. Bartlett ES, Walters TD, Symons SP, et al. Carotid stenosis index revisited with direct CT angiography measurement of carotid arteries to quantify carotid stenosis. Stroke 2007;38:286–91 [DOI] [PubMed] [Google Scholar]
- 28. Wintermark M, Jawadi SS, Rapp JH, et al. High-resolution CT imaging of carotid artery atherosclerotic plaques. AJNR Am J Neuroradiol 2008;29:875–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walker LJ, Ismail A, McMeekin W, et al. Computed tomography angiography for the evaluation of carotid atherosclerotic plaque: correlation with histopathology of endarterectomy specimens. Stroke 2002;33:977–81 [DOI] [PubMed] [Google Scholar]
- 30. de Weert TT, Cretier S, Groen HC, et al. Atherosclerotic plaque surface morphology in the carotid bifurcation assessed with multidetector computed tomography angiography. Stroke 2009;40:1334–40 [DOI] [PubMed] [Google Scholar]
- 31. Saba L, Sanfilippo R, Pascalis L, et al. Carotid artery wall thickness and ischemic symptoms: evaluation using multi-detector-row CT angiography. Eur Radiol 2008;18:1962–71 [DOI] [PubMed] [Google Scholar]
- 32. Hunt JL, Fairman R, Mitchell ME, et al. Bone formation in carotid plaques: a clinicopathological study. Stroke 2002;33:1214–19 [DOI] [PubMed] [Google Scholar]
- 33. Buchan A, Gates P, Pelz D, et al. Intraluminal thrombus in the cerebral circulation: implications for surgical management. Stroke 1988;19:681–87 [DOI] [PubMed] [Google Scholar]
- 34. Pelz DM, Buchan A, Fox AJ, et al. Intraluminal thrombus of the internal carotid arteries: angiographic demonstration of resolution with anticoagulant therapy alone. Radiology 1986;160:369–73 [DOI] [PubMed] [Google Scholar]