Abstract
BACKGROUND AND PURPOSE:
Stents have been reported as an option for improvement of the recanalization rate in AIS. The authors have also used intracranial stents in failed cases of IAT with pharmacologic and mechanical methods since 2004. We retrospectively reviewed our cases of intracranial stent use for IAT of AIS for recanalization and as a rescue procedure for iatrogenic intracranial vascular dissection during IAT.
MATERIALS AND METHODS:
Thirty-two patients, who were diagnosed with AIS, were treated with intracranial stents (28 balloon-mounted and 7 self-expandable stents) at our neurovascular center between April 2004 and December 2008. The stent use for all 32 patients was the final attempt to recanalize occluded vessels after various trials of pharmacologic or mechanical thrombolysis or to treat iatrogenic vascular dissection.
RESULTS:
Among the 32 patients, immediate poststenting angiographic recanalization was achieved in 100% with TIMI/TICI 2 (15 of 32 lesions, 46.9%) or TIMI/TICI 3 (17 of 32 lesions, 53.1%). However, complication rates were also high. Major symptomatic intracerebral hemorrhage (1 case of procedural symptomatic hemorrhage and 3 cases of delayed symptomatic hemorrhage) occurred in 4 (12.5%); intracranial vascular dissection, in 4 (12.5%); extracranial vascular dissection, in 3 (9.4%); immediate IST, in 4 (12.5%); subacute (within 1 week) IST, in 2; late (>1 week) IST, in 1, and 1 case of in-stent restenosis occurred twice (at 5 and 17 months).
CONCLUSIONS:
Intracranial stent placement for AIS management has an excellent recanalization rate. However, it is associated with high complication risks as our series showed. We believe that the decision to treat AIS with intracranial stent placement should be made after careful consideration of potential benefits and risks.
Various treatment modalities for AIS have gradually evolved during the past decade.1–12 Among them, stents have been reported as an option for improvement of the recanalization rate and have shown excellent results with an acceptably low complication rate.6–12 The authors have also used intracranial stents in failed cases of IAT with pharmacologic and mechanical methods since 2004. We also experienced good results in terms of recanalization. But at the same time, we felt that stent should be selected as an option with care. Its use has often been accompanied by complications that could be directly and indirectly related to the stent. In this article, the authors summarize the radiologic and clinical outcomes of 32 patients with AIS treated with intracranial stents (28 balloon-mounted stents and 7 self-expandable stents).
Materials and Methods
We retrospectively reviewed patients with AIS treated by intracranial stent placement between April 2004 and December 2008 at our neurovascular center. During that period, a total of 212 IAT procedures were performed. Of these, intracranial stents were placed in 32 (15.1%) patients. The stent use for all 32 patients was the final attempt either to recanalize occluded vessels after various attempts with pharmacologic or mechanical methods or to solve iatrogenically developed cerebrovascular dissection.
Clinical and angiographic data were reviewed for the following characteristics: demographics, medical comorbidities, time of symptom onset, occlusion location, time to angiography, angiographic characteristics including collaterals, pharmacologic agents for IAT, mechanical devices (wire, balloon) for IAT, periprocedural complications, NIHSS scores (at admission, postprocedural, and discharge), and mRS scores (at discharge and 6 months after the procedure). Immediate postprocedural angiographic results were evaluated by the TIMI/TICI grading scale.13,14
MR Imaging Protocol for AIS
MR imaging for acute stroke was performed with an Intera 1.5T (Philips Healthcare, Best, the Netherlands) or an Achieva 3T (Philips Healthcare) MR imaging unit. The acute stroke MR imaging protocol consisted of T2*-weighted GRE imaging, DWI, perfusion-weighted imaging, T2 FLAIR, contrast-enhanced T1-weighted imaging, and 3D time-of-flight MR angiography. Follow-up MR imaging was performed within 1 week. It included T2*-weighted GRE, DWI, T2 FLAIR, and contrast-enhanced MR angiography covering the aortic arch, neck, and intracranial arteries. MR imaging according to the acute stroke protocol was performed on all 32 patients treated with intracranial stent placement.
Intracranial Stent Placement Procedures
All procedures were performed with local anesthetics. A standard transfemoral approach was used, and a 6F guide sheath (Shuttle sheath; Cook, Bloomington, Indiana) or a larger guide catheter was placed in the lesion side of the common carotid artery and a 6F conventional guide catheter was placed coaxially into the ICA.
IAT was performed with UK and/or GP IIb-IIIa RBs (abciximab [ReoPro] tirofiban). Along with UK, intra-arterial infusion of GP IIb-IIIa RBs (0.25 mg/kg ReoPro, maximal dose 10 mg; tirofiban, maximal dose, 10 μg/kg) was almost routinely used for >85% of our IAT patients to facilitate thrombolysis and to reduce recurrent thrombosis. In general, the lowest dose possible was infused at the proximal part of the thrombus or within the thrombus. IV ReoPro or tirofiban infusion was not routinely used during and after the procedure.
In case of stent placement, dual oral antiplatelet agents (300 mg clopidogrel and 300 mg aspirin) were given orally or through a nasogastric tube after confirming no intracranial hemorrhage on postprocedural brain CT. Daily clopidogrel (75 mg) and aspirin (100 mg) were administered thereafter.
IV heparin was not administered during and after IAT. However, in case of cardiac embolic occlusion, low molecular heparin was added 24 hours after IAT, which was changed to oral warfarin several days later. Activated clotting time was not checked during IAT.
Mechanical IAT was performed with microwires, microcatheters, and balloons (either coronary or compliant balloons). Merci (Concentric Medical, Mountain View, California) or Penumbra (Alameda, California) retrievers were not available in our country.
Intracranial stent placement was considered in 2 situations: the failure of pharmacologic and/or mechanical IAT and newly developed iatrogenic vascular dissection during mechanical thrombolysis.
As balloon-mounted stents, Driver (Medtronic, Santa Rosa, California) and FlexMaster (Abbott Laboratories, Abbott Park, Illinois) with various diameters and lengths were used. Neuroform 3 (Boston Scientific/Target, Fremont, California) and Enterprise (Cordis, Miami Lakes, Florida) were used as self-expandable stents. The choice of stents was made according to the characteristics of the occluded vessels, for example, the presence of arterial stenosis at the occlusion site, degree of atherosclerotic change, and arterial tortuosity. We mainly selected balloon-mounted stents if arterial stenosis at the occluded site was anticipated or really present. Self-expandable stents were not commonly used because difficulty on further IAT was expected after deploying open-cell-type stents. The closed-cell-type self-expandable stent has only been available recently in our country.
Results
In 33 patients with AIS, intracranial stent placement was attempted, and there was 1 failure in advancing a stent intracranially due to vascular tortuosity. The mean age of the 32 patients was 64.7 years (range, 35–90 years), and the male/female ratio was 19:13. The mean NIHSS score at admission was 15 ± 5.9 (range, 4–25), and the follow-up period was 14.8 ± 9.2 months (range, 6–38 months).
The time interval from symptom onset to initiating IAT, excluding 3 patients whose time intervals were extremely long (2160, 2520, 2880 minutes), was 120.9 ± 102.5 minutes (range, 30–360 minutes). The 3 patients with the long time intervals had vertebrobasilar occlusions with delayed detection and were given life-saving treatment. IV-tPA was administered in 24 patients (75%) before IAT (On-line Table 1).
Twenty-eight balloon-mounted stents were used in 25 patients (Driver in 10, FlexMaster in 18) and 7 self-expandable stents (Neuroform 3 in 3, Enterprise in 4) were used in 7 patients. Multiple stents were placed in 2 patients because of incomplete lesional coverage or newly developed iatrogenic intracranial vascular dissection.
The occlusion sites were located in the anterior circulation (n = 21) and in the posterior circulation (n = 11) (On-line Table 1). The pharmacologic agents that were used for IAT were UK and GP IIb-IIIa RBs (ReoPro, tirofiban). UK was administered intra-arterially in 29 of 32 patients, and intra-arterial infusion of GP IIb-IIIa RBs was used in 28 of 32 patients (ReoPro in 16, tirofiban in 12).
Mechanical thrombolysis with wires and balloons was performed in all 32 patients. For 28 patients (87.5%), stents were the final attempt to recanalize occluded vessels after various unsuccessful trials of pharmacologic or mechanical thrombolysis, and in the 4 remaining patients (12.5%), stent placement was performed due to iatrogenic intracranial vascular dissection during mechanical IAT: 1 case of intracranial vascular dissection that occurred during wire manipulation; 2 cases, after balloon inflation; and 1 case, after placement of a balloon-mounted stent.
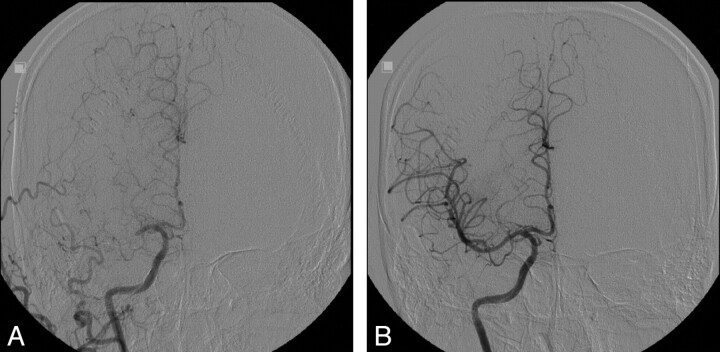
Immediate poststenting angiographic recanalization was achieved in 100% with TIMI/TICI 2 (15 of 32 lesions, 46.9%) or TIMI/TICI 3 (17 of 32 lesions, 53.1%) (Fig 1).
Fig 1.
A 39-year-old man with left hemiparesis and dysarthria was transferred to our institution for IAT. The initial NIHSS score was 15. IV-tPA was not given because of delayed arrival (270 minutes). A, Initial right carotid angiogram, demonstrates proximal M1 occlusion. B, Recanalization (TIMI/TICI 3) is achieved after deploying a balloon-mounted stent (2.25 × 12 mm, Micro-Driver, Medtronic). The postoperative NIHSS score was 4.
However, the complication rate associated with the procedure was high. Major symptomatic ICH occurred in 4 (12.5%); intracranial vascular dissection, in 4 (12.5%); extracranial vascular dissection, in 3 (9.4%); immediate IST, in 4 (12.5%); subacute (within 1 week) IST, in 2; late (>1 week) IST, in 1; and 1 case of in-stent restenosis occurred twice (at 5 and 17 months).
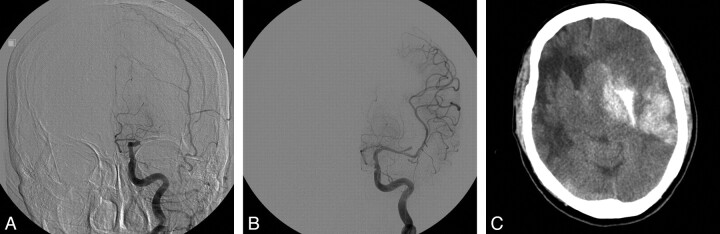
Specifically, 2 cases of procedural hemorrhage occurred, which were detected by immediate postoperative CT. One was a small amount of basal ganglia ICH with unknown cause without symptoms. The other was a symptomatic ICH caused by a lenticulostriate artery tear during wire manipulation. Delayed major cerebral hemorrhage developed in 3 patients. All were symptomatic, and 2 of them died with massive cerebral swelling (Fig 2). Overall, radiologic hemorrhagic transformations including asymptomatic lesions occurred in 15 patients (46.9%).
Fig 2.
A 77-year-old woman with acute left M1 occlusion. The initial NIHSS score was 17. IV-tPA was given. A, Initial left carotid angiogram demonstrates proximal M1 occlusion. B, A 2.75 × 12 mm FlexMaster (Abbott Laboratories) stent is deployed across the focal area of occlusion, and partial recanalization (TIMI/TICI 2A) is achieved. C, CT 7 hours after IAT demonstrates a large basal ganglia hematoma. The patient died with massive cerebral swelling.
Regarding thrombotic complications, immediate IST occurred in 4 patients (12.5%). All were successfully treated with intra-arterial UK and GP IIb-IIIa RBs. Subacute IST (within 1 week) developed in 2 patients (6.25%), 1 day after stent placement for 1 patient and on the fifth day in the other. These 2 patients finally developed large infarctions. One became vegetative and the other died. Late IST (>1 week) developed in 1 patient (3.13%) 1 month after stent placement and was treated successfully by balloon angioplasty with good results. In-stent restenosis (≥50%) occurred in 1 patient (3.13%) during follow-up, in whom it occurred twice, at 5 and 17 months after stent placement. Each time, balloon angioplasty was performed successfully.
Extracranial vascular dissection (2 cases of cervical ICA, 1 case of cervical vertebral artery origin) developed in 3 patients during navigation of intracranial stents, all with balloon-mounted stents, and was managed well with other stent placement, with carotid stents in 2 patients, and a coronary stent in 1 patient with vertebral artery dissection. All 4 cases of vascular dissection directly related to the stent placement procedure itself (1 intracranial and 3 extracranial) were coincidently caused by balloon-mounted stents, not by the self-expandable stents in our study.
On-line Table 1 summarizes the periprocedural NIHSS scores and clinical outcome at discharge and 6-month follow-up. At discharge, the clinical outcome on the basis of the mRS was as follows: 13 patients (40.6%) with mRS 0–2, 9 (28.1%) with mRS 3, three (9.4%) with mRS 4, five (15.7%) with mRS 5, and 2 (6.2%) with mRS 6. Two patients with mRS 6 died with massive cerebral swelling.
Discussion
Angiographic recanalization has been reported to be strongly associated with good outcome after AIS.13 Pharmacologic IAT only in AIS has a low recanalization rate and a high ICH rate, leading to poor outcome.1,14 Therefore, several mechanical thrombolytic devices have been introduced and successfully used.2–5 Several authors have reported that intracranial stents could be used for AIS management with high recanalization and low complication rates.6–10 Levy et al7 reported 19 cases of intracranial stent placement for recanalizing vessels that were resistant to standard thrombolytic techniques, demonstrating a 79% recanalization rate of TIMI/TICI 2 or 3 with balloon-mounted stents. One year later, Levy et al8 also reported 18 cases of self-expandable stents for recanalization of acute cerebrovascular occlusion, demonstrating a 79% recanalization rate of TIMI/TICI 2 or 3 with Neuroform 3 and Wingspan stents (Boston Scientific/Target). Self-expandable stents have been known to be easier to navigate through intracranial vasculature and safer to deploy in atherosclerotic vessels than coronary balloon-mounted stents.8,15 Zaidat et al10 also reported an 89% recanalization rate of TIMI/TICI 2 or 3 in 9 patients with AIS with Neuroform and Wingspan stents. Brekenfeld et al9 achieved better results with a 92% recanalization rate of TIMI/TICI 2 or 3 with 12 cases of Wingspan stents only. Likewise, our study, which included 32 patients with intracranial stent placement, the largest one reported from a single center to our knowledge, shows a 100% of recanalization rate. Intracranial stent placement has excellent results in terms of recanalization in AIS.
However, as our results demonstrate, intracranial stent placement for AIS is not a safe procedure. Our patients experienced many complications, such as vascular dissection (4 intracranial and 3 extracranial, overall 22%), ICH (overall 47%, major symptomatic 12.5%), and IST (4 immediate, 2 subacute, 1 late, overall 22%) during and after the procedures. To our knowledge, IST was not reported in previous studies though it was a well-known event that could be associated with stent use. We experienced it during immediate, subacute, and later periods. Nevertheless, it prompted us to perform IAT again through the stent. Sometimes, it resulted in complete occlusion or distal emboli, which led to a large infarction. The overall mortality rate of our series was 18.8% (6 of 32 patients) in 6 months. We believe that more prudent and careful decision-making would be necessary to treat AIS with intracranial stent placement.
In our series, the incidence of intracranial (12.5%) and extracranial (9.4%) vascular dissection was high compared with that in previous reports.3–10,15 We assume that the high rate of dissection might be caused by relatively old age, vessel tortuosity, the previously existing vascular stenosis, and the emergent procedures with narrow time windows. In addition, all dissections were related to manipulation of balloon-mounted stents, not to self-expandable stents. Therefore, vascular dissection seems to be related to the stiffness of balloon-mounted stents rather than self-expandable stents.
The major symptomatic ICH incidence of 12.5% (4 of 32 patients) in this study also seems to be higher than the previously reported rate of an AIS stent-placement series (5.3%∼11.1%).7,8,10 Brekenfeld et al9 reported a 0% rate of ICH. However, except for our 1 case of iatrogenic tear of an M1 with a wire, we do not believe that there was a definite cause in our IAT, including timing of IAT, doses of thrombolytic agents, intra-arterial injection methods of thrombolytic agents, and techniques of mechanical thrombolysis by using a wire and balloons. The difference was that there was difficulty in recanalization, so intracranial stents were introduced, navigated, and deployed. In addition, the antiplatelet agent use after stent placement might facilitate a high incidence of hemorrhagic transformation and delayed hemorrhage.
Conclusions
Our study demonstrates that intracranial stent placement has an excellent efficacy in recanalization of AIS. However, it also shows that the complication rate was high. We believe that the decision to treat AIS with intracranial stent placement should be made after careful consideration of potential benefits and risks.
Abbreviations
- ACA
anterior cerebral artery
- AIS
acute ischemic stroke
- BA
basilar artery
- DWI
diffusion-weighted imaging
- FLAIR
fluid-attenuated inversion recovery
- Gp IIb-IIIa RB
glycoprotein IIb-IIIa receptor blocker
- GRE
gradient recalled-echo
- IAT
intra-arterial thrombolysis
- ICA
internal carotid artery
- ICH
intracerebral hemorrhage
- IST
in-stent thrombosis
- IV
intravenous
- IV-tPA
intravenous tissue plasminogen activator
- MCA
middle cerebral artery
- mRS
modified Rankin Scale
- NIHSS
National Institutes of Health Stroke Scale
- Sx Hemo
symptomatic hemorrhage
- T
T occlusion
- TIMI/TICI
Thrombolysis in Myocardial Ischemia/Thrombolysis in Cerebral Ischemia
- UK
urokinase
- VA
vertebral artery
- VBA
vertebrobasilar artery
Footnotes
Indicates article with supplemental on-line table.
References
- 1. Tissue plasminogen activator for acute ischemic stroke: The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995;333:1581–87 [DOI] [PubMed] [Google Scholar]
- 2. Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke: The PROACT II study—a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 1999;282:2003–11 [DOI] [PubMed] [Google Scholar]
- 3. Gobin YP, Starkman S, Duckwiler GR, et al. MERCI 1: a phase 1 study of Mechanical Embolus Removal in Cerebral Ischemia. Stroke 2004;35:2848–54. Epub 2004 Oct 28 [DOI] [PubMed] [Google Scholar]
- 4. Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005;36:1432–38 [DOI] [PubMed] [Google Scholar]
- 5. Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke 2008;39:1205–12 [DOI] [PubMed] [Google Scholar]
- 6. Gupta R, Vora NA, Horowitz MB, et al. Multimodal reperfusion therapy for acute ischemic stroke: factors predicting vessel recanalization. Stroke 2006;37:986–90 [DOI] [PubMed] [Google Scholar]
- 7. Levy EI, Ecker RD, Horowitz MB, et al. Stent-assisted intracranial recanalization for acute stroke: early results. Neurosurgery 2006;58:458–63 [DOI] [PubMed] [Google Scholar]
- 8. Levy EI, Mehta R, Gupta R, et al. Self-expanding stents for recanalization of acute cerebrovascular occlusions. AJNR Am J Neuroradiol 2007;28:816–22 [PMC free article] [PubMed] [Google Scholar]
- 9. Brekenfeld C, Schroth G, Mattle HP, et al. Stent placement in acute cerebral artery occlusion: use of a self-expandable intracranial stent for acute stroke treatment. Stroke 2009;40:847–52 [DOI] [PubMed] [Google Scholar]
- 10. Zaidat OO, Wolfe T, Hussain SI, et al. Interventional acute ischemic stroke therapy with intracranial self-expanding stent. Stroke 2008;39:2392–95 [DOI] [PubMed] [Google Scholar]
- 11. The Thrombolysis in Myocardial Infarction (TIMI) trial: phase I findings—TIMI Study Group. N Engl J Med 1985;312:932–36 [DOI] [PubMed] [Google Scholar]
- 12. Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003;34:e109–37 [DOI] [PubMed] [Google Scholar]
- 13. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 2007;38:967–73 [DOI] [PubMed] [Google Scholar]
- 14. Brekenfeld C, Remonda L, Nedeltchev K, et al. Symptomatic intracranial haemorrhage after intra-arterial thrombolysis in acute ischaemic stroke: assessment of 294 patients treated with urokinase. J Neurol Neurosurg Psychiatry 2007;78:280–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henkes H, Miloslavski E, Lowens S, et al. Treatment of intracranial atherosclerotic stenoses with balloon dilatation and self-expanding stent deployment (WingSpan). Neuroradiology 2005;47:222–28 [DOI] [PubMed] [Google Scholar]