Abstract
BACKGROUND AND PURPOSE:
Surgical treatment of VA-PICA dissecting aneurysms is difficult and complication of LCNP is common. These lesions can be approached easily with endovascular technique, but complete obliteration can only be achieved in a small number of cases. Our aim was to report the clinical outcomes of VA-PICA dissecting aneurysms treated by endovascular embolization.
MATERIALS AND METHODS:
Between 2001 and 2007, the authors treated 22 consecutive patients (15 men and 7 women; ranging in age from 12 to 59 years; mean age, 43 years) with VA-PICA dissecting aneurysms. Diagnosis of VA-PICA dissecting aneurysm was based on clinical, MR imaging, and cerebral angiography studies.
RESULTS:
Of the 22 patients, 6 had unruptured aneurysms. One patient presented with headaches, whereas the remaining 5 patients showed brain stem ischemia. Four were treated with stent-only or stent-coil embolization, and 2 were treated with unilateral VA occlusion. Among 16 patients presenting with SAH, 10 were treated with stent-only or stent-coil embolization. The other 6 patients with SAH were treated by using unilateral endovascular VA occlusion. One patient could not return to his previous daily activities.
CONCLUSIONS:
VA-PICA aneurysms are rare lesions associated with significant morbidity, and endovascular treatment strategies for these lesions were stent deployment with or without coil embolization and VA occlusion. Favorable clinical outcomes can be achieved with endovascular techniques.
Few articles have been published describing patient outcome after treatment of a VA-PICA aneurysm.1 VA-PICA aneurysms present difficult treatment challenges.1–4 Proximal or parent artery occlusions, trapping procedures, and clip reconstructions are surgical techniques used to treat aneurysms that are not amenable to direct clip application.5–7 Endovascular options include parent vessel coil occlusion, stent placement, or combinations of coil and stent therapies.6,8 However, to our knowledge, the results of long-term endovascular series for treatment of patients with VA-PICA aneurysms are not yet available. In this study, we report our clinical outcomes of ruptured and unruptured VA-PICA aneurysms.
Materials and Methods
Patients and Techniques
Between 2001 and 2007, we treated 63 patients with arterial aneurysms confined to the VA, whereas 22 (35%) patients had aneurysms involving the VA-PICA, and these cases are considered in this article. The 22 patients included 15 men and 7 women who ranged from 12 to 59 years of age (mean age, 43 years). No patient had a history of head trauma. Diagnosis was based on clinical presentations and findings of radiologic examinations, including CT scanning, MR imaging, and cerebral angiography. Intramural thrombus on T1-weighted images and intimal flaps on T2-weighted images were major findings indicating a dissecting aneurysm (Fig 1). All patients underwent cerebral angiography, which demonstrated such typical results as double lumen, tapered narrowing or occlusion with or without aneurysm dilation, and retention of contrast medium. A sequential change in luminal configuration also supported a diagnosis of dissection. In 16 patients with ruptured VA-PICA dissecting aneurysms, 6 were treated with VA occlusion (detachable balloons in 2 patients and detachable coils 4 patients), and 10 were treated with stent deployment with or without additional coil embolization. In 6 patients with unruptured dissecting aneurysms, 2 were treated with VA occlusion by using detachable balloons and 4 were treated with stent deployment with or without additional coil embolization.
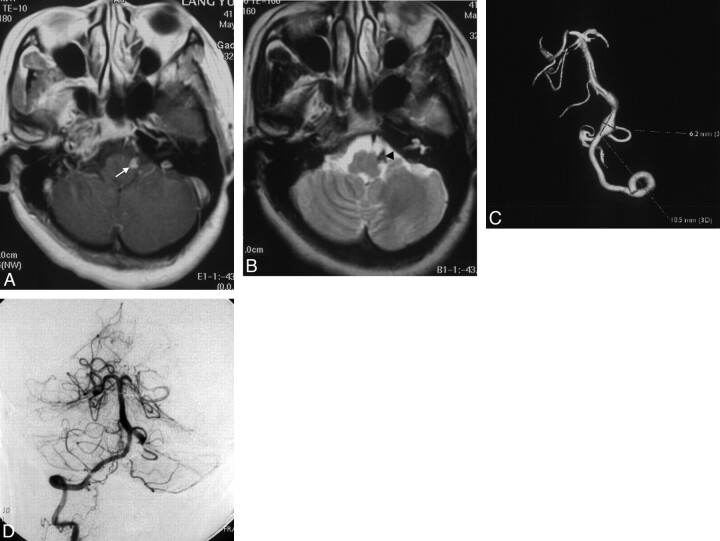
Fig 1.
A, T1-weighted MR image. B, T2-weighted MR image. Note the intramural thrombus (arrow) and intimal flap (arrowhead). C, Left VA injection (3D reconstruction) shows a fusiform aneurysm of the left VA-PICA. D, After endovascular treatment, a control angiogram of the right VA injection demonstrates that the left PICA is well filled and the aneurysm is completely occluded.
All patients were reviewed with respect to clinical data, serial radiologic imaging, treatment, and functional outcome. Postoperative angiography was classified as follows: complete, subtotal, or incomplete. Results of follow-up angiography were also classified in the same way, and clinical outcomes were evaluated according to GOS scores. The patients with SAH were evaluated according to the HH scale.
All procedures were performed or supervised by the senior author (Z.W.). They were performed in the neurointerventional suite via the transfemoral arterial route with the patient under general anesthesia with full systemic heparinization to maintain the activated clotting time around twice the base value. Stents were used for unruptured lesions or lesions in which the contralateral VA was hypoplastic; parent-vessel occlusions were used for other lesions. In this series, 16 stents (4 Bx stents, Cordis, Miami Lakes, Florida [before 2003]; 8 Neuroform and 2 Wingspan stents, Boston Scientific, Natick, Massachusetts; and 2 LEO stents, Balt, Montmorency, France) were deployed for 14 aneurysms with additional coils (Guglielmi detachable coil, Boston Scientific; Orbit, Cordis; and Microplex, MicroVention, Aliso Viejo, California) used to fill 7 aneurysms. Eight patients underwent endovascular parent vessel occlusion without a bypass procedure. If a stent was placed, patients were administered 325 mg clopidogrel and 300 mg aspirin orally 2 hours before the endovascular procedure for ruptured lesions and 75 mg clopidogrel and 300 mg aspirin orally at least 3 days before the endovascular procedure for unruptured lesions. Patients were each maintained on the same dosage daily for at least 4 weeks, and aspirin was continued indefinitely.
Results
All VA-PICA aneurysms were dissecting. Clinical and radiographic results are summarized in the Table. Sixteen patients had ruptured VA-PICA aneurysms, whereas 6 had unruptured lesions. On MR imaging, intramural hematoma was a major finding in 3 patients, and intimal flap, in 12. Cerebral angiography demonstrated a double lumen in 2 patients, tapered narrowing or occlusion with or without aneurysm dilation in 15, and retention of contrast medium in 5.
Summary of characteristics of 22 patients with VA-PICA dissecting aneurysms
| Patient No. | Age (yr)/Sex | Presentation | Aneurysm Size (mm) | Treatment | Angiographic Results | Follow-Up (Mo) | Clinical Outcome (GOS) |
|---|---|---|---|---|---|---|---|
| 1 | 53/F | CN VI palsy, headaches | 10 × 8 | VA occlusion | In | 36 | 5 |
| 2 | 59/M | Brain stem, ischemia | 6 × 4 | VA occlusion | In | 72 | 5 |
| 3 | 12/M | Headaches | 15 × 6 | Stent | In | 72 | 5 |
| 4 | 40/M | Brain stem, ischemia | 12 × 7 | Stent | In | 84 | 5 |
| 5 | 42/M | Brain stem, ischemia | 7 × 6 | Stent coil | In | 48 | 5 |
| 6 | 41/M | Brain stem, ischemia | 13 × 6 | Stent coil | Co | 12 | 5 |
| 7 | 39/F | SAH | 30 × 30 | VA occlusion | In | 84 | 5 |
| 8 | 45/M | SAH | 12 × 6 | VA occlusion | In | 36 | 5 |
| 9 | 40/M | SAH | 8 × 8 | VA occlusion | In | 12 | 5 |
| 10 | 34/F | SAH | 10 × 7 | VA occlusion | In | 60 | 5 |
| 11 | 42/F | SAH | 11 × 6 | VA occlusion | Su | 5 | 5 |
| 12 | 59/F | SAH | 12 × 7 | VA occlusion | In | 12 | 5 |
| 13 | 47/M | SAH | 5 × 4 | Stent | In | 72 | 5 |
| 14 | 30/M | SAH | 12 × 10 | Stent | In | 36 | 5 |
| 15 | 51/M | SAH | 14 × 7 | Stent | In | 10 | 5 |
| 16 | 41/M | SAH | 15 × 6 | Stent | In | 60 | 5 |
| 17 | 46/M | SAH | 10 × 10 | Stent coil | Co | 11 | 5 |
| 18 | 44/F | SAH | 12 × 8 | Stent coil | Co | 12 | 5 |
| 19 | 44/F | SAH | 12 × 8 | Stent coil | In | 6 | 5 |
| 20 | 37/M | SAH | 7 × 7 | Stent | Co | 9 | 5 |
| 21 | 45/M | SAH | 9 × 11 | Stent coil | Co | 6 | 5 |
| 22 | 53/M | SAH | 23 × 10 | Stent coil | Co | 36 | 5 |
Ruptured VA-PICA Aneurysms
Sixteen patients presented with SAH. The HH scale grades were the following: 1 in 4, 2 in 5, and 3 in 7. No patient required insertion of a ventriculoperitoneal shunt for normal-pressure hydrocephalus post-SAH. The lesion was successfully treated through endovascular occlusion of the VA in 6 patients. After treatment, the PICAs filled well via the contralateral VA as demonstrated on follow-up angiography at 4 months. Three lesions were stented only, and 7 lesions were embolized with coils after stent placement.
Unruptured VA-PICA Aneurysms
Six patients had unruptured VA-PICA aneurysms, 2 patients were managed with stent-only, 2 were managed with stent-coil embolization, and 2 were treated with unilateral VA occlusion. Among these patients, 4 had symptoms of brain stem ischemia. One patient initially experienced transient loss of consciousness but fully recovered without any neurologic deficits.
There were no treatment-related complications or neurologic deterioration during or after treatment. All patients were clinically followed up at a mean of 37 months (range, 6–84 months). All patients who were treated by the endovascular method had a favorable outcome. Ten patients (45%) underwent follow-up conventional angiography at a mean of 9 months (range, 3–18 months). In 8 of these 10 patients, follow-up angiograms revealed complete resolution of the dilation with reconstruction of the VA. In 1 patient who was treated by double stents with coiling for a ruptured VAPICA aneurysm, a 9-month follow-up angiogram revealed minimal contrast media filling outside the stent. In the other patient who was treated by double-stent placement, a 4-month follow-up angiogram revealed in-stent occlusion of the VA.
Illustrative Cases
Case 11.
A 42-year-old woman was referred with SAH. MR imaging showed intramural thrombus on T1-weighted images and intimal flap on T2-weighted images. Immediate cerebral angiography demonstrated a left VA-PICA aneurysm. The left PICA was well filled through the right VA. Endovascular coil occlusion of the left VA was performed, and the post-treatment angiograms showed nearly complete obliteration of the aneurysmal lumen.
Case 12.
A 59-year-old woman presented with sudden severe headache and vomiting, followed by loss of consciousness. CT performed on admission exhibited massive SAH (Fig 2). Cerebral angiographic studies performed on the same day demonstrated fusiform dilation of the right VA associated with the PICA origin. The right PICA was well filled through the left VA. Endovascular coil occlusion of the right VA was performed on day 2 postadmission. The patient gradually recovered.
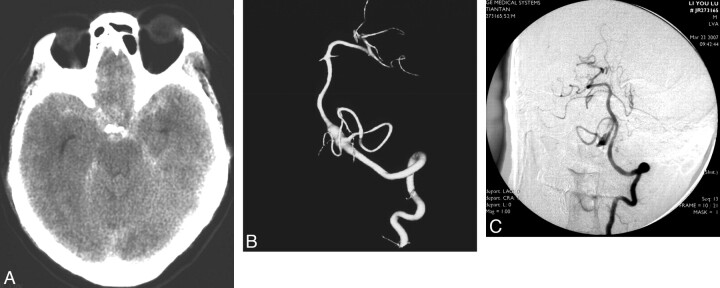
Fig 2.
Images obtained in a 59-year-old man who presented with SAH due to a ruptured right VA-PICA aneurysm. A, CT scan. B, 3D reconstruction of the right VA injection. The patient had undergone right VA coil occlusion, after which he recovered. C, Left VA angiogram after right VA occlusion reveals that the right PICA is filled via the left VA and the aneurysm is decreased in size.
Discussion
During recent decades, considerable improvement has occurred in endovascular techniques for posterior circulation aneurysms.4–6,9 Aneurysms of the VA-PICA represent only 0.5%–3% of all aneurysms and 20% of those in the posterior fossa.5 In our series, there was a male predominance (68.2%) and all aneurysms were fusiform, but the incidence of dissecting VA-PICA aneurysm is not well known. However, the entity is being recognized with increased frequency and is particularly common in adult patients. Several series studying surgically treated patients have been published, but they have focused on major morbidity and mortality.1–3,7,10,11
Surgery for VA-PICA aneurysms has unique difficulties in clipping the neck because of the deep location of aneurysm and its close relationship with the lower brain stem and lower cranial nerves.1,10,11 Dysphagia, hoarseness, diplopia, lateral medullary syndrome, and aspiration pneumonia have been reported as postoperative complications of VA-PICA aneurysm surgery.1,3 The main obstacles are the jugular tubercle, occipital condyle, and lower cranial nerves.10,11 LCNP is especially significant after surgery for aneurysms located at the PICA origin (ie, VA-PICA aneurysms). The incidence of lower cranial nerve dysfunction reported in the literature after VA-PICA aneurysm surgery varies from 10% to 45%.1,3 With the advent of alternative options to treat patients with aneurysms (eg, endovascular therapy), accurate assessment of the frequency, severity, and outcome of lower cranial nerve dysfunction is crucial.4
VA-PICA aneurysms have been reported much less frequently, however.4,5 Apart from a few cases mentioned in a series on VA-PICA aneurysms, the natural history, treatment, and outcome of VA-PICA aneurysms has rarely been analyzed. A review of VA-PICA aneurysms in patients presenting with SAH included 52 patients treated by direct surgery with a death rate of 50% and a good recovery in only 25% of patients.1 Bragg and Duckworth3 reported 6 patients with dissection of the BA, 1 of whom died of catastrophic recurrent hemorrhage. The other 5 patients were treated conservatively, with 3 eventually making a good recovery. In these literature reviews, the neurosurgery for VA-PICA aneurysms thus carried significant morbidity and death, whether the patients presented with ischemia or SAH.
VA aneurysms often present with SAH, with rebleeding and consequent death being frequent. Early treatment seems essential for improving prognosis in these patients.12 However, surgery for aneurysms of the VA-PICA traditionally has been performed in a delayed fashion, several days to weeks after SAH.1 Treatment of an arterial dissection by endovascular stent-supported coil embolization has been described.9 Ongoing advances in endovascular techniques ultimately may make stent placement with or without coil embolization one of the best management strategies for these lesions. Both the clinical course and the therapeutic approach for an unruptured VA-PICA aneurysm are complex. Ischemic symptoms from VA-PICA aneurysms initially were characterized by a grave neurologic condition. In patients with VA-PICA, lateral medullary syndrome is a common clinical presentation of brain stem ischemia.8 In our series, 3 of 5 patients with ischemia had severely impaired consciousness on admission. Although 21 patients recovered quite well from initial symptoms, the remaining patient without SAH could not return to previous activities. All VA-PICA aneurysms were packed suboptimally to maintain patency of the parent vessel.
Most important, lesions caused by dissection often resolved spontaneously. Byrne et al4 achieved stable angiographic aneurysm occlusion in 86% of small and large aneurysms; recurrent filling occurred in 14.7% of 259 aneurysms. Aneurysm recurrence was associated with bleeding risk, ranging from 0.6% to 2.4% during the first 3 years after aneurysm embolization. Clinical follow-up in 22 patients with neurologic deficits was reported to reveal spontaneous recovery in 21 and improvement in 1. Although the exact mechanisms of progression in this subcategory remain unclear, several researchers have indicated that these dissections form a spectrum of vascular abnormalities ranging from small fusiform aneurysms to symptomatic giant so-called dolichoectatic aneurysms.8 When these lesions involve the BA, they may cause serious progressive brain stem compression or ischemia.
Conclusions
VA-PICA aneurysms are rare lesions associated with significant morbidity, and endovascular treatment strategies for these lesions were stent deployment with or without coil embolization and VA occlusion. Favorable clinical outcomes can be achieved with endovascular techniques.
Abbreviations
- BA
basilar artery
- CN VI
sixth cranial nerve
- Co
complete
- GOS
Glasgow Outcome Scale
- HH
Hunt and Hess
- In
incomplete
- LCNP
lower cranial nerve palsy
- SAH
subarachnoid hemorrhage
- Su
subtotal
- VA
vertebral artery
- VA-PICA
VA-posterior inferior cerebellar artery complex
References
- 1. Al-khayat H, Al-Khayat H, Beshay J, et al. Vertebral artery-posteroinferior cerebellar artery aneurysms: clinical and lower cranial nerve outcomes in 52 patients. Neurosurgery 2005;56:2–11 [PubMed] [Google Scholar]
- 2. Benes L, Kappus C, Sure U, et al. Treatment of a partially thrombosed giant aneurysm of the vertebral artery by aneurysm trapping and direct vertebral artery-posterior inferior cerebellar artery end-to-end anastomosis: technical case report. Neurosurgery 2006;59(1 suppl 1):ONSE166–67 [DOI] [PubMed] [Google Scholar]
- 3. Bragg TM, Duckworth EA. Contralateral far-lateral approach for clipping of a ruptured vertebral artery-posterior inferior cerebellar artery aneurysm. Neurosurg Focus 2008;25:E9. [DOI] [PubMed] [Google Scholar]
- 4. Byrne JV, Sohn MJ, Molyneux AJ, et al. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg 1999;90:656–63 [DOI] [PubMed] [Google Scholar]
- 5. Coert BA, Chang SD, Do HM, et al. Surgical and endovascular management of symptomatic posterior circulation fusiform aneurysms. J Neurosurg 2007;106:855–65 [DOI] [PubMed] [Google Scholar]
- 6. Ecker RD, Hanel RA, Levy EI, et al. Contralateral vertebral approach for stenting and coil embolization of a large, thrombosed vertebral-posterior inferior cerebellar artery aneurysm: case report. J Neurosurg 2007;107:1214–16 [DOI] [PubMed] [Google Scholar]
- 7. Hamada J, Todaka T, Yano S, et al. Vertebral artery-posterior inferior cerebellar artery bypass with a superficial temporal artery graft to treat aneurysms involving the posterior inferior cerebellar artery. J Neurosurg 2002;96:867–71 [DOI] [PubMed] [Google Scholar]
- 8. Iihara K, Murao K, Yamada N, et al. Growth potential and response to multimodality treatment of partially thrombosed large or giant aneurysms in the posterior circulation. Neurosurgery 2008;63:832–44 [DOI] [PubMed] [Google Scholar]
- 9. Wu Z, Lv X, Yang X, et al. Ruptured vertebro-inferoposterior cerebellar artery dissecting aneurysm treated with the Neuroform stent deployment and vertebral artery occlusion. Eur J Radiol Extra 2009;70:e100–03 [Google Scholar]
- 10. Matsushima T, Matsukado K, Natori Y, et al. Surgery on a saccular vertebral artery-posterior inferior cerebellar artery aneurysm via the transcondylar fossa (supracondylar transjugular tubercle) approach or the transcondylar approach: surgical results and indications for using two different lateral skull base approaches. J Neurosurg 2001;95:268–74 [DOI] [PubMed] [Google Scholar]
- 11. Taguchi Y, Hoshikawa Y, Tanaka K, et al. Contralateral transcondylar approach for aneurysms of the posterior inferior cerebellar artery-vertebral artery complex. J Clin Neurosci 1996;3:156–61 [DOI] [PubMed] [Google Scholar]
- 12. Kawanishi M, Nagasawa S, Ohta T, et al. Simulation study on therapeutic vertebral artery occlusion for VA-PICA giant aneurysm. Neurol Res 1994;16:100–3 [DOI] [PubMed] [Google Scholar]