Abstract
BACKGROUND AND PURPOSE:
Partially thrombosed aneurysms as a distinct entity form a diverse collection of complex aneurysms characterized by organized intraluminal thrombus and solid mass. Endovascular treatment options are PVO or selective coil occlusion of the remaining lumen. We present long-term clinical and angiographic results of endovascular treatment of unruptured partially thrombosed aneurysms that presented with symptoms of mass effect.
MATERIALS AND METHODS:
Between 1994 and 2008, 30 partially thrombosed aneurysms were treated by selective coiling and 26 by PVO. Of 56 aneurysms, 53 (95%) were large or giant. Neurologic recovery during a mean clinical follow-up of 42.7 months was established. Evolution of aneurysm size during a mean follow-up of 26.6 months in 46 patients was assessed with MR imaging.
RESULTS:
Seventeen of 56 patients (30%) fully recovered, 22 patients (39%) partially recovered, 11 patients (20%) were unchanged, and 6 patients (11%) died. Complete recovery more often occurred after PVO than after coiling (12 of 26 versus 5 of 30, P = .02). Aneurysm size reduction occurred more often after PVO (17 of 18 versus 2 of 28, P < .001). Five aneurysms continued to grow after coiling, resulting in death in 3. During follow-up, 27 additional treatments were performed in 19 patients, all treated with coiling.
CONCLUSIONS:
In partially thrombosed aneurysms presenting with mass effect, the results of PVO are much better than those of selective coiling. After coiling, additional treatments are often needed, and some aneurysms keep growing. When PVO is not tolerated or not possible, surgical options should be considered before proceeding with coiling.
Partially thrombosed aneurysms are a diverse collection of complex aneurysms characterized by organized intraluminal thrombus and solid mass. Although size may vary from small to giant, most partially thrombosed aneurysms are in the large and giant size range. When located in the anterior circulation, these aneurysms frequently present with symptoms of mass effect on the cranial nerves. Neurologic symptoms by mass effect of the aneurysm depend on aneurysm location and size. Compression of the oculomotor nerve (CN III), trochlear nerve (CN IV), or abducens nerve (CN VI) results in ophthalmoparesis or ophthalmoplegia and is frequently associated with cavernous sinus and PcomA aneurysms. Compression of the optic nerve (CN II) results in decreased visual acuity and visual field deficits and is mostly associated with carotid ophthalmic and superior hypophyseal aneurysms. Giant aneurysms of the AcomA may cause a frontal syndrome and MCA aneurysms may cause dysphasia. Giant posterior circulation aneurysms may compress the brain stem. Large basilar tip and SCA aneurysms may cause CN III palsy, and PICA aneurysms can cause compression the trigeminal or the facial nerve.1–3
Partially thrombosed aneurysms are difficult to treat. The neurosurgical approach often requires techniques other than conventional clipping, such as thrombectomy with clip reconstruction, and is frequently associated with prolonged ischemia, failed reconstruction, and poor results.4–6 Sophisticated bypass surgery may have a good outcome in experienced hands but is not available in many centers. While surgery for these aneurysms is difficult, endovascular treatment generally is straightforward. Endovascular treatment consists of PVO with balloons or coils or selective coil occlusion with or without neck protection with a balloon or stent. In patients with ICA aneurysms who can tolerate therapeutic ICA occlusion, this therapy is effective in excluding the aneurysm from the circulation. Symptoms of mass effect are cured or improve in most patients. In addition, endovascular balloon occlusion of the ICA is simple to perform and safe and cheap.7–9 PVO is also possible in selected posterior circulation aneurysms. When PVO is not tolerated or is not possible because of aneurysm location, the aneurysm may be selectively occluded with coils. A well-known drawback of coiling of partially thrombosed aneurysms is the possibility of migration of coils into the thrombus during follow-up, resulting in reopening of the aneurysm lumen. In a recent study focusing on all unruptured aneurysms that presented with mass effect, the alleviation of symptoms of large and giant aneurysms was as good after coiling as after PVO.10 To our knowledge, the effect on mass symptoms after coiling and PVO in the subset of partially thrombosed aneurysms has not been studied yet. Results of endovascular treatment of partially thrombosed aneurysms are scarce and are often presented in case reports or small case series or are embedded in studies reporting the overall results of endovascular treatment of giant and large aneurysms.11–16
In this study, we present management and long-term clinical and angiographic results of 56 endovascularly treated partially thrombosed aneurysms that presented with symptoms of mass effect.
Materials and Methods
General Considerations for Treatment of Partially Thrombosed Aneurysms
We diagnosed partially thrombosed aneurysms with MR imaging or CT, followed by angiography in all patients. In our institution, all patients with unruptured partially thrombosed aneurysms presenting with mass effect are presented in a multidisciplinary meeting with neurologists, neuroradiologists, and neurosurgeons. The mode of proposed treatment is based on symptoms, aneurysm location, and geometry and is tailored to the individual patient. Patients with partially thrombosed unruptured ICA aneurysms are preferably treated with endovascular balloon occlusion of the ICA. The protocol for therapeutic ICA occlusion was described previously.9 Briefly, during balloon test occlusion in the awake patient, angiography of the contralateral ICA and/or vertebral artery is performed. Apart from clinical tolerance, synchronous opacification of the cerebral veins in the examined and occluded vascular territory indicates tolerance to permanent occlusion. After the patient passes the test, the parent vessel is permanently occluded with detachable balloons or coils.
In the posterior circulation, PVO is considered in distal vertebral artery aneurysms, including the PICA and vertebral junction.
In patients who cannot tolerate parent artery occlusion and in patients in whom PVO is not possible, other treatment options are considered, such as selective coiling with or without neck protection, direct neurosurgical clipping, thrombectomy with clip reconstruction, or bypass surgery.
Assessment of Neurologic Deficits
“Ophthalmoplegia” is defined as paresis of the oculomotor, trochlear, and abducens nerve or a combination with diplopia in single or multiple gazes with or without ptosis and pupillary dysfunction.
“Optic nerve dysfunction” is defined as decreased visual acuity with visual field deficits as assessed with perimetric or confrontation testing. The criteria for complete recovery of ophthalmoplegia were no diplopia in all direction of gazes, complete resolution of ptosis, and partial or complete recovery of pupillary reaction. Partial recovery of ophthalmoplegia was defined as residual diplopia in the upward, downward, lateral, or medial gaze with or without normal primary gaze, residual ptosis, and pupillary dysfunction. The criteria for complete recovery of optic nerve dysfunction were restoration of the normal visual acuity and the visual field. Residual visual field deficits indicated partial recovery.
In patients with neurologic presentations other than cranial nerve palsy with varying symptoms such as brain stem compression, frontal syndrome, hemiparesis, and dysphasia, recovery was assessed by the referring physician.
Clinical and Angiographic Follow-Up
Clinical status was assessed during every outpatient clinic visit. Clinical follow-up was extended by contacting the patient by phone, after the general practitioner was contacted to inquire if the patient was still alive. The medical records of patients who had died during the follow-up period were reviewed to retrieve the exact cause of death. Neurologic outcome was categorized as recovery, improvement, no change, or worsening, according to the treating physician and the patient's perception.
Imaging follow-up after selective coiling consisted of a follow-up angiogram at 6 months and extended angiographic and MR imaging follow-up at various intervals. Aneurysm occlusion was categorized as adequate (complete occlusion or neck remnant) or incomplete (residual aneurysm filling). Evolution of aneurysm occlusion at follow-up after coiling was categorized as worse, unchanged, or improved. When reopening of the aneurysm was apparent at follow-up, the necessity and mode of additional treatment were evaluated. For patients treated with PVO, follow-up imaging consisted of MR imaging or CT at 3 months and at various intervals thereafter. Evolution of aneurysm size after PVO was categorized as increased, unchanged, and decreased.
Data Analysis
Mode of treatment, procedural complications, and number and mode of additional treatments during follow-up were described. Evolution of aneurysm size was assessed as a proportion of each category. For aneurysms treated with selective coiling, occlusion status at imaging follow-up was described. Neurologic outcome of patients treated with selective coiling and PVO was separately analyzed. Patient sex, mean age, and mean clinical follow-up duration and aneurysm location and size were compared between patients treated by coiling and PVO by using the χ2 and independent t tests. The proportion of grown aneurysms and aneurysms with decreased size was compared for the 2 treatment modalities with χ2 tests. Procedural complications and clinical outcomes were compared between patients treated by coiling and PVO by using the χ2 and Fisher exact tests.
Results
Patient Characteristics and Clinical Presentation
We reviewed the patient data base of the St. Elisabeth Ziekenhuis in Tilburg, the Netherlands.
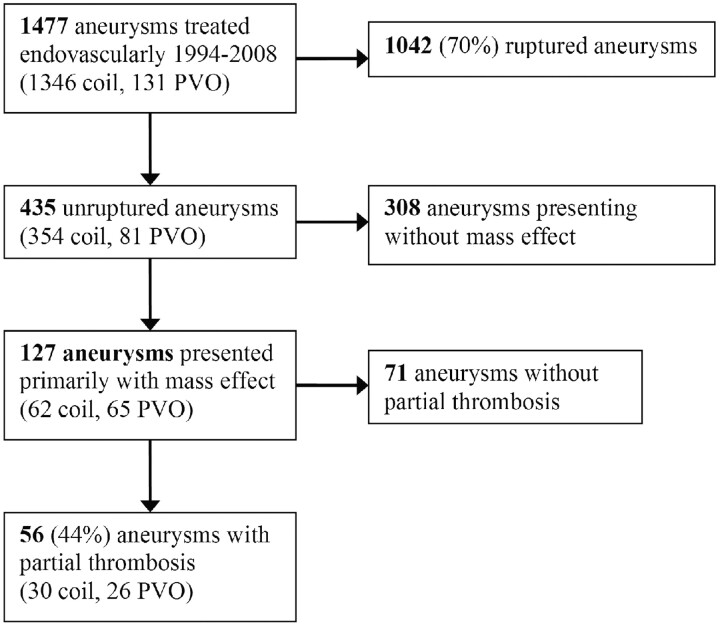
Patient selection is displayed in Fig 1. Between 1994 and 2008, 1192 patients with 1477 intracranial aneurysms were treated primarily endovascularly, of which 1346 aneurysms were treated by selective coil occlusion and 131, by PVO. Of 1477 aneurysms, 1042 in 1041 patients were ruptured aneurysms and 435 aneurysms in 384 patients were unruptured (30%). Of 127 unruptured aneurysms primarily presenting with mass effect, 56 (44%) were partially thrombosed, and these aneurysms form the subject of this study. Thirty of 56 (54%) were treated by selective coil occlusion and 26 (46%). by PVO.
Fig 1.
Flow chart showing patient selection.
There were 36 women (64%) and 20 men (36%) with a mean age of 56.7 years (median, 57.5 years; range, 10–81 years).
Aneurysm locations related to clinical presentation of patients is displayed in Table 1.
Table 1:
Aneurysm location and clinical presentation
| Aneurysm location | No. | Clinical Presentation (n) |
|---|---|---|
| Cavernous sinus | 20 | Ophthalmoplegia (14), CN III (3), CN VI (3) |
| Superior hypophyseal artery | 5 | CN II (4), ophthalmoplegia (1) |
| AcomA | 4 | Frontal syndrome (3), CN II (1) |
| MCA | 4 | Dysphasia (3), seizures (1) |
| Ophthalmic artery | 4 | CN II (2), CN III (2) |
| PcomA | 2 | CN III (2) |
| Carotid artery tip | 1 | Hemiparesis (1) |
| ACA | 1 | Frontal syndrome (1) |
| PICA | 6 | Stem compression (3), CN V (1), CN VI (1), CN VII (1) |
| SCA | 4 | CN V (2), CN III (1), CN IV (1) |
| Basilar tip | 3 | Brain stem compression (3) |
| Vertebral artery | 1 | Brain stem compression (1) |
| Vertebral artery junction | 1 | Brain stem compression (1) |
The clinical presentation of 56 the patients was ophthalmoplegia in 15, CN II palsy in 7, CN III palsy in 8, CN IV palsy in 1, CN V palsy in 3, CN VI palsy in 4, CN VII palsy in 1, brain stem compression in 8, frontal syndrome in 3, dysphasia in 4, seizures in 1, and hemiparesis in 1 patient.
Aneurysm Characteristics
Aneurysm location and size are displayed in Table 2. Aneurysms were located in the cavernous sinus (n = 20) and on the PICA (n = 6), superior hypophyseal artery (n = 5), AcomA (n = 4), SCA (n = 4), MCA (n = 4), ophthalmic artery (n = 4), basilar artery tip (n = 3), PcomA (n = 2), anterior cerebral artery (n = 1), carotid artery tip (n = 1), vertebral artery (n = 1), and vertebral artery junction (n = 1). Mean aneurysm size was 26 mm (median, 25 mm; range, 5–55 mm). One aneurysm (2%) was small (≤5 mm), 2 aneurysms (4%) were medium (6–10 mm), 23 aneurysms (41%) were large (11–24 mm), and 30 aneurysms (53%) were giant (≥25 mm).
Table 2:
Patient and aneurysm characteristics
| All Patients | Patients Treated with PVO | Patients Treated by Coiling | |
|---|---|---|---|
| No. patients | 56 | 26 | 30 |
| No. men | 20 (36%) | 7 (27%) | 13 (43%) |
| Mean age (yr) | 56.7 | 55.8 | 57.5 |
| Median age, range (yr) | 57.5, 10–81 | 58.6, 10–79 | 56.5, 38–81 |
| Mean follow-up (mo) | 42.7 | 32.0 | 51.9 |
| Median follow-up, range (mo) | 28.0, 0–179 | 25.5, 0–126 | 35.8, 0–179 |
| Mean aneurysm size (mm) | 26 | 26 | 26 |
| Median aneurysm size, range (mm) | 25, 5–55 | 25, 15–40 | 26, 5–55 |
| Anterior circulation aneurysms | 41 | 20 | 21 |
| Cavernous sinus | 20 | 18 | 2 |
| Superior hypophyseal artery | 5 | 1 | 4 |
| AcomA | 4 | 0 | 4 |
| MCA | 4 | 0 | 4 |
| Ophthalmic artery | 4 | 0 | 4 |
| PcomA | 2 | 0 | 2 |
| Carotid artery tip | 1 | 1 | 0 |
| ACA | 1 | 0 | 1 |
| Posterior circulation aneurysms | 15 | 6 | 9 |
| PICA | 6 | 3 | 3 |
| SCA | 4 | 1 | 3 |
| Basilar artery tip | 3 | 0 | 3 |
| Vertebral artery | 1 | 1 | 0 |
| Vertebral artery junction | 1 | 1 | 0 |
Aneurysm Management
Twenty-six aneurysms (46%) were treated by occlusion of the parent vessel. Twenty of these were treated by carotid artery occlusion (16 with balloons, 3 with coils, and 1 with a combination of balloon and coils). Three PICA aneurysms were treated by vertebral artery balloon occlusion, 1 vertebral artery aneurysm was treated by vertebral artery coil occlusion, and 1 distal SCA aneurysm was treated by selective coil occlusion of the SCA. One vertebral junction aneurysm was treated by bilateral vertebral artery occlusion by using both balloon and coils.
Thirty aneurysms (54%) were treated by selective coiling, of which 3 were coiled with balloon assistance, and 3, after stent placement in primary or additional coiling (Enterprise; Cordis Neurovascular, Miami Lakes, Florida). Coiling was performed with 50-cm-long straight coils (Detach-18; Cook, Copenhagen, Denmark) or GDC 18 helical coils (Boston Scientific, Fremont, California).
Procedural Complications
Procedural complications occurred in 7 of 56 patients (12.5%), in 6 patients after coiling and in 1 after PVO when 1 patient had a massive retroperitoneal hemorrhage needing surgical intervention. The massive hemorrhage led to hypoperfusion infarction and hemiplegia in this patient. After coiling, 2 patients had postprocedural infarctions, leading to hemipareses and aphasia in 1 and loss of function of 1 hand in the other patient. One patient died from procedural aneurysm perforation. One patient had persistent episodes of seizures after initial coiling. One patient had a massive retroperitoneal hemorrhage needing surgical intervention, which resulted in persistent leg pain and difficulty walking. One patient died after additional thrombectomy of a continuously growing AcomA aneurysm, 99 months after initial coiling.
Altogether, procedural mortality was 3.6% (2 of 56) and permanent procedural morbidity was 7.2% (4 of 56).
Angiographic Outcome, Size Evolution, and Additional Treatments
Angiographic and cross-sectional imaging follow-up was available in 46 patients (82%) for a mean 26.6 months (median, 9.9 months; range, 1 day to 143 months), totaling 102 follow-up patient-years (Table 3).
Table 3:
Evolution of aneurysm size after PVO or selective coiling in 46 patients with imaging follow-up
| Total (n = 46) | PVO (n = 18) | Coil (n = 28) | PVO vs Coil, P | |
|---|---|---|---|---|
| Continuous growth | 5 (11%) | 0 (0%) | 5 (18%) | – |
| Unchanged size | 22 (48%) | 1 (6%) | 21 (75%) | <.001 |
| Decreased size | 19 (41%) | 17 (94%) | 2 (7%) | <.001 |
Note:—indicates not calculated.
Aneurysm size evolution between pretreatment imaging and final follow-up indicated that 5 of 46 aneurysms had grown (11%), 22 aneurysms were unchanged (48%), and 19 had decreased in size (41%, Figs 2 and 3). All 5 aneurysms that had grown had been treated with selective coiling, and 17 of 19 aneurysms with decreased size had been treated with PVO.
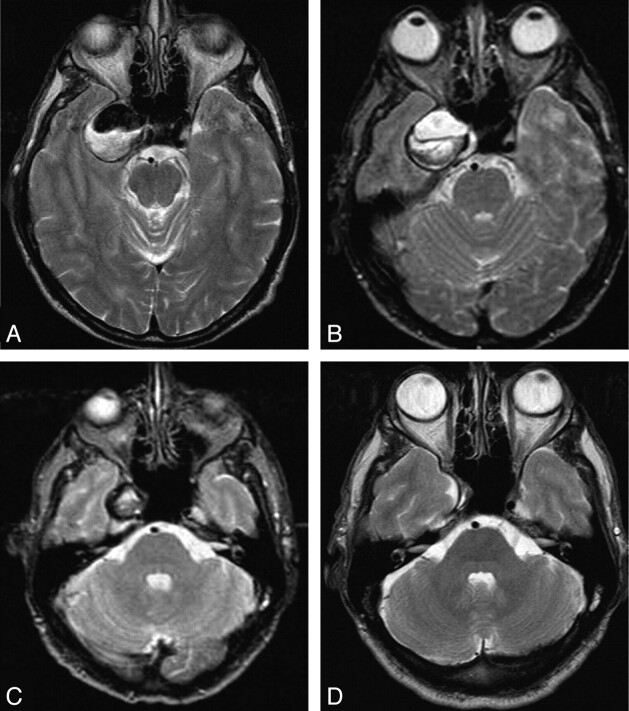
Fig 2.
A 64-year-old man with oculomotor nerve palsy from a partially thrombosed giant cavernous sinus aneurysm. A, Axial T2-weighted MR image at presentation shows a 30-mm right cavernous sinus aneurysm, partially thrombosed. B, Complete thrombosis of the aneurysm on an MR image 1 day after occlusion of the right ICA. C, Three months later, the aneurysm has decreased to half its initial size. D, Two and a half years after ICA occlusion, the aneurysm is completely obliterated. The patient is asymptomatic.
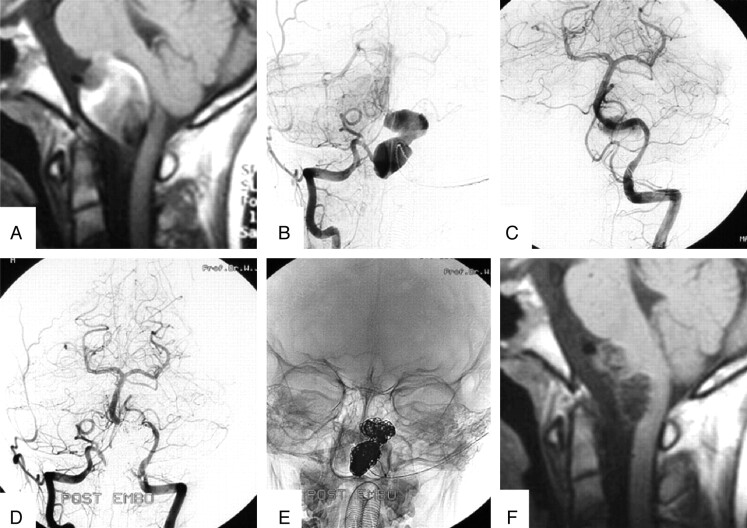
Fig 3.
A 10-year-old boy with symptoms of brain stem compression from a giant partially thrombosed distal vertebral aneurysm. A, Sagittal T1-weighted MR image shows a giant aneurysm with intraluminal thrombus located on the right vertebral artery compressing the brain stem. B, Right vertebral angiogram demonstrates the lumen of the aneurysm of the V4 segment distal to the PICA. C, Left vertebral angiogram shows supply of the posterior circulation with exception of the right PICA territory. D and E, Bilateral vertebral angiogram (D) and nonsubtracted image (E) after coil occlusion of the fusiform V4 aneurysm. Supply to all posterior circulation vessels is preserved. F, Sagittal T1-weighted MR image 6 months later shows reduction in aneurysm size and reduction of mass effect on the brain stem. The patient is asymptomatic.
Initial aneurysm occlusion after coiling was adequate in 27 of 30 coiled aneurysms (90%, Table 3). Of 30 coiled aneurysms, 28 had angiographic follow-up. Two patients died before follow-up. In 21 of 28 aneurysms (75%), first follow-up angiography showed aneurysm reopening, 6 of the aneurysms were unchanged (Fig 4), and 1 aneurysm had progressively thrombosed with improved occlusion. Eighteen of the 21 patients with aneurysm reopening were additionally treated, and in the other 3 patients, the reopening was treated conservatively and monitored with extended follow-up. Of 4 coiled aneurysms with unchanged size, 1 needed additional surgical removal of the aneurysm mass because of aggravating neurologic symptoms. In total, during the follow-up period, 27 additional treatments were performed in 19 patients: Aneurysms in 11 patients were coiled twice, those in 3 patients were coiled 3 times, the aneurysm in 1 patient was coiled once followed by surgical aneurysm thrombectomy, the aneurysm in 1 patient was coiled twice followed by PVO, the aneurysm in 1 patient was coiled twice followed by bypass surgery, and the aneurysm in 1 patient was coiled twice followed by clipping surgery and another surgery for mass removal. Final occlusion after all treatments was adequate in 21 of 28 patients with coiled aneurysms with follow-up and incomplete in 7 (25%). None of the patients treated with PVO needed additional treatment.
Fig 4.
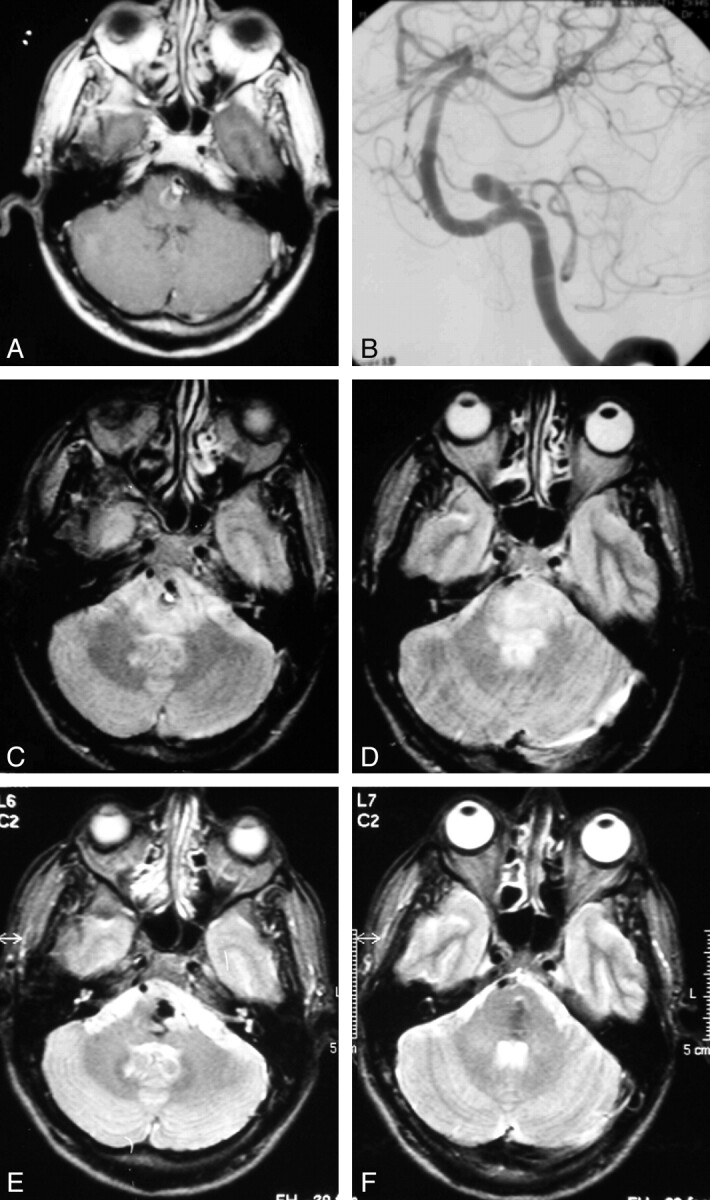
A 49-year-old man with abducens nerve palsy from a partially thrombosed PICA aneurysm projecting into the brain stem. A, Contrast-enhanced T1-weighted MR image at presentation shows a 10-mm aneurysm on the right PICA projecting into the brain stem with intraluminal thrombus. B, Left vertebral angiogram before coiling demonstrates the small aneurysm lumen. C and D, Axial T2-weighted MR image at presentation shows brain stem edema. E and F, One and a half years after selective coiling of the PICA aneurysm, the brain stem edema is resolved. The patient is asymptomatic.
Clinical Outcome
All patients were clinically followed for a mean of 42.7 months (median, 28.0 months; range, 0–179 months), totaling 199 follow-up patient-years (Table 4). Seventeen of 56 patients (30%) fully recovered from their presenting neurologic deficits, 22 patients (39%) partially recovered, 11 patients (20%) had unchanged symptoms, and 6 patients (11%) died as a result of aneurysmal disease. One patient with a 30-mm basilar tip aneurysm died from procedural perforation, and 1 patient with a coiled 50-mm AcomA aneurysm and persistent frontal syndrome died after 3 months in a nursing home. One patient died 5 months after occlusion of a vertebral artery for a 25-mm PICA aneurysm from persistent brain stem compression, and 1 patient died 14 months after coiling of a continuously growing 30-mm basilar artery tip aneurysm from increased brain stem compression. One patient died 22 months after coiling of a continuously growing 40-mm PcomA aneurysm from increased edema and subsequent brain stem compression, and 1 patient died during clipping surgery for a frontal syndrome aggravated by a continuously growing 30-mm AcomA aneurysm 99 months after initial treatment.
Table 4:
Clinical follow-up results of 56 patients treated with PVO or selective coiling
| Total (n = 56) | PVO (n = 26) | Coil (n = 30) | PVO vs Coil, P | |
|---|---|---|---|---|
| Procedural complications | 7 (13%) | 1 (4%) | 6 (20%) | .11 |
| Died from aneurysmal disease | 6 (11%) | 1 (4%) | 5 (17%) | .20 |
| Neurologically unchanged | 11 (20%) | 4 (15%) | 7 (23%) | .52 |
| Neurologically improved | 22 (39%) | 9 (35%) | 13 (43%) | .09a |
| Cured | 17 (30%) | 12 (46%) | 5 (17%) | .02 |
P value of combined outcomes improved and cured.
Two patients died from unrelated causes: One 84-year-old patient with unchanged symptoms of CN II palsy and one 66-year-old patient with a cured CN V palsy died from cardiovascular disease 50 and 72 months after coiling, respectively. In clinical outcome analyses, these 2 patients were considered neurologically unchanged and cured, respectively.
Effect of Treatment Technique on Outcome
Sex and age did not differ between patients treated by coiling or PVO (43% versus 27% men, P = .27). Mean clinical follow-up duration was longer for patients treated by selective coiling compared with patients treated with PVO (mean, 51.9 versus 32.0 months; P = .01) (Table 1).
Cavernous sinus aneurysms were significantly more often treated with occlusion of the ICA than with selective coiling (18 versus 2, P < .001). Anterior circulation aneurysms were equally frequently treated with selective coiling (51%) and PVO. Of 15 posterior circulation aneurysms, 9 (60%) were treated by selective coiling and 6 by PVO (Table 1).
Reduction of aneurysm size after PVO occurred significantly more often than after selective coiling (17 of 18 versus 2 of 28, P < .001). Continuous aneurysm growth after treatment occurred only after coiling and not after PVO. Five of 28 (18%) coiled aneurysms kept growing and eventually led to death in 3 patients.
One of 26 patients (4%) treated with PVO died during clinical follow-up versus 5 of 30 patients (17%) treated with selective coiling (P = .20). Complete neurologic recovery occurred significantly more often after PVO than after coiling (12 of 26 versus 5 of 30, P = .02). However, this difference was not statistically significant for both improved and cured outcome together (21 of 26 and 18 of 30, P = .09). Eighteen of 30 patients with giant aneurysms of ≥25 mm were cured or improved at follow-up versus 21 of 26 patients with aneurysms <25 mm (P = .09). Procedural complications occurred more frequently in selective coiling (7 of 30 versus 1 of 26, P = .06).
Discussion
We present a cohort of patients with partially thrombosed aneurysms presenting with symptoms of mass effect who were treated with endovascular techniques as a separate aneurysm subgroup. Fifty-six patients presented with neurologic symptoms from this diverse collection of complex aneurysms and were clinically followed for a mean of 3.5 years. Twenty-six patients were treated by PVO, and 30 patients by selective coiling. It is impossible to compare the 2 treatment modalities: Size and location of the partially thrombosed aneurysms presenting with mass effect in our patient cohort differed widely, and for some locations (basilar tip, AcomA), there was an imperative choice of selective coiling as an endovascular treatment. However, we compared outcomes between patients treated with either PVO or coiling to provide a better insight into prognosis once a treatment was selected.
In our patient cohort, it has become apparent that PVO to treat partially thrombosed aneurysms is safe. After PVO, almost all aneurysms decrease in size, with no aneurysms becoming larger. Symptoms of mass effect are cured or improve in most patients. PVO was a definitive treatment; no additional treatments during follow-up were needed. Selective coiling sparing the parent artery for partially thrombosed aneurysms was also safe. However, reopening after coiling during follow-up was frequent (75%), mostly as a result of migration of coils into the intraluminal thrombus, and retreatment was often needed (Fig 5). After coiling, the partially thrombosed aneurysm rarely decreased in size, and more striking, a substantial proportion of giant partially thrombosed coiled aneurysms showed continuous growth, especially basilar tip and AcomA aneurysms. This persistent aneurysm growth had a very poor prognosis and led to death in 3 of 5 patients in our study. From these data, one might conclude that in patients with partially thrombosed aneurysms presenting with mass effect, PVO is the treatment of choice. When occlusion of the parent vessel is not tolerated or PVO is not an option because of aneurysm location, surgical procedures should be considered. When surgery is not an option, selective coiling may be considered because it might improve the outcome of the patient.
Fig 5.
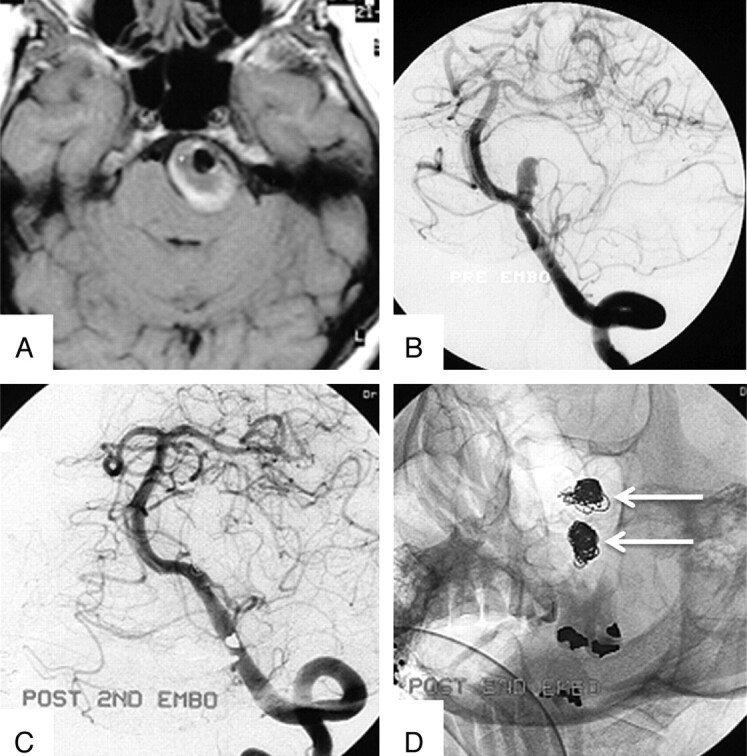
A 55-year-old woman with brain stem compression from a partially thrombosed PICA aneurysm. A, Axial T1-weighted MR image at presentation shows a 32-mm left PICA aneurysm with a large intraluminal thrombus. B, Left vertebral angiogram shows the small aneurysm lumen. C and D, Angiogram (C) and nonsubtracted image (D) after the second coiling show 2 separate coil meshes: The first coil mesh has migrated into the thrombus, and the second coil mesh occludes the lumen.
Our data show that selective coiling of partially thrombosed aneurysms does not always protect against continuous aneurysm growth with increasing symptoms of mass effect. These patients with growing aneurysms form an endovascular challenge when surgical thrombectomy is unfeasible: In an attempt to prevent dissections of the aneurysm wall that start in the remaining neck lumen, we now try to occlude the neck of the AcomA and basilar tip aneurysms as completely as possible, mostly after stent placement. On selected locations, placement of a flow diverter may completely seal the aneurysm neck and possibly prevent further growth, but this has not yet been confirmed.17,18
This study has several limitations. First, we have combined a diverse collection of complex aneurysms into 1 group of partially thrombosed aneurysms. We realize every aneurysm in this study is unique and has its own treatment options and challenges. In our study, we merely describe the outcomes of all these patients with the aim of collecting information that might contribute to better prediction of outcomes of future patients. Second, we were unable to compare different endovascular treatment modalities because in many aneurysms, 1 option was imperative. However, we think that the favorable outcome of PVO compared with coiling in our cohort is valuable new information for the individual prognosis and for counseling of patients.
In the literature, 21% of coiled aneurysms showed reopening at imaging follow-up and 10% of aneurysms were retreated.19 In our study, after coiling of partially thrombosed aneurysms, 75% showed reopening at angiographic follow-up, and 63% of aneurysms were retreated. It is likely that migration of the coil mass into the intraluminal thrombus caused the extremely higher rates of reopening and retreatment. Also, more than half of the partially thrombosed aneurysms treated in our center were giant aneurysms, and 94.6% were >10 mm. The angiographic and clinical prognosis of giant and large aneurysms is known to be worse than that of their smaller counterparts.20,21 In comparing ours to previous studies regarding clinical follow-up and neurologic outcome of patients presenting with neurologic symptoms from all types of endovascularly treated aneurysms, we found no apparent worse outcome of the endovascularly treated partially thrombosed aneurysms in our study.2,6,8,10,15, 22–24
Partially thrombosed aneurysms might have a different pathogenesis and, therefore, may form a different disease than aneurysms without intraluminal thrombus. Some propose that the vasa vasorum of the aneurysmal wall play a crucial role in the development and growth of aneurysms with intraluminal thrombus by proliferation, inflammation, and rupture.25–27 These authors suggest that these aneurysms should be treated by targeting the outer vessel wall and not the aneurysmal lumen. Our study does not confirm this theory. In our experience, supported by others, the growth of partially thrombosed aneurysms is incremental by dissection of the aneurysm wall. This sudden growth may result in acute symptoms of mass effect. After PVO, aneurysm growth was never seen, in our view because dissections originating in the lumen of the aneurysms can no longer occur. On the other hand, after selective coiling of the aneurysmal lumen, dissections may still originate in the remaining neck by continuous hemodynamic forces. This finding may explain the persistent growth of some aneurysms after selective coiling and justifies effort to completely occlude the neck of these aneurysms with the aid of stents or flow diverters.
In conclusion, patients who present with mass effect from a partially thrombosed aneurysm have mostly very large and giant aneurysms. These aneurysms preferably should be treated by occlusion of the parent vessel because this treatment has a low complication rate and good angiographic and clinical outcome. If PVO is not tolerated or is not possible because of aneurysm anatomy and vessel geometry, selective coiling is often the only alternative with a chance of a favorable clinical outcome, but the higher complication rate and the high retreatment rate should be kept in mind. In addition, if despite coiling, continuous growth of the aneurysm is detected on follow-up imaging, additional surgical or endovascular procedures should be considered because the prognosis of patients with growing aneurysms is poor. In selected aneurysms and patients, the use of modern flow-diverting stents to exclude the aneurysm from the circulation might be considered.17,18
Abbreviations
- ACA
anterior cerebral artery
- AcomA
anterior communicating artery
- CN
cranial nerve
- GDC
Guglielmi detachable coils
- ICA
internal carotid artery
- MCA
middle cerebral artery
- MRA
MR angiography
- PcomA
posterior communicating artery
- PICA
posterior inferior cerebellar artery
- PVO
parent vessel occlusion
- SCA
superior cerebellar artery
- TIA
transient ischemic attack
References
- 1. Mansour N, Kamel MH, Kelleher M, et al. Resolution of cranial nerve paresis after endovascular management of cerebral aneurysms. Surg Neurol 2007;68:500–04 [DOI] [PubMed] [Google Scholar]
- 2. Kazekawa K, Tsutsumi M, Aikawa H, et al. Internal carotid aneurysms presenting with mass effect symptoms of cranial nerve dysfunction: efficacy and imitations of endosaccular embolization with GDC. Radiat Med 2003;21:80–85 [PubMed] [Google Scholar]
- 3. Stiebel-Kalish H, Maimon S, Amsalem J, et al. Evolution of oculomotor nerve paresis after endovascular coiling of posterior communicating artery aneurysms: a neuro-ophthalmological perspective. Neurosurgery 2003;53:1268–74 [DOI] [PubMed] [Google Scholar]
- 4. Lawton MT, Quiñones-Hinojosa A, Chang EF, et al. Thrombotic intracranial aneurysms: classification scheme and management strategies in 68 patients. Neurosurgery 2005;56:441–54 [DOI] [PubMed] [Google Scholar]
- 5. Goldenberg-Cohen N, Curry C, Miller NR, et al. Long-term visual and neurological prognosis in patients with treated and untreated cavernous sinus aneurysms. J Neurol Neurosurg Psychiatry 2004;75:863–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Oliviera JG, Borba LA, Rassi-Neto A, et al. Intracranial aneurysms presenting with mass effect over the anterior optic pathways: neurosurgical management and outcomes. Neurosurg Focus 2009;26:E3. [DOI] [PubMed] [Google Scholar]
- 7. van der Schaaf IC, Brilstra EH, Buskens E, et al. Endovascular treatment of aneurysms in the cavernous sinus: a systematic review on balloon occlusion of the parent vessel and embolization with coils. Stroke 2002;33:313–18 [DOI] [PubMed] [Google Scholar]
- 8. Larson JJ, Tew JM, Jr, Tomsick TA, et al. Treatment of aneurysms of the internal carotid artery by intravascular balloon occlusion: long-term follow-up of 58 patients. Neurosurgery 1995;36:23–30 [PubMed] [Google Scholar]
- 9. van Rooij WJ, Sluzewski M, Metz NH, et al. Carotid balloon occlusion for large and giant aneurysms: evaluation of a new test occlusion protocol. Neurosurgery 2000;47:116–21 [DOI] [PubMed] [Google Scholar]
- 10. van Rooij WJ, Sluzewski M. Unruptured large and giant carotid artery aneurysms presenting with cranial nerve palsy: comparison of clinical recovery after selective aneurysm coiling and therapeutic carotid artery occlusion. AJNR Am J Neuroradiol 2008;29:997–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Möller V, Axmann C, Reith W. Clinical course of a partially thrombosed, symptomatic aneurysm of the basilar artery tip with partial recanalization subsequent to coiling [in German]. Radiologe 2006;46:417–20 [DOI] [PubMed] [Google Scholar]
- 12. Sluzewski M, Menovsky T, van Rooij WJ, et al. Coiling of very large or giant cerebral aneurysms: long-term clinical and serial angiographic results. AJNR Am J Neuroradiol 2003;24:257–62 [PMC free article] [PubMed] [Google Scholar]
- 13. Gruber A, Killer M, Bavinzski G, et al. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery 1999;45:793–803 [DOI] [PubMed] [Google Scholar]
- 14. Lubicz B, Gauvrit JY, Leclerc X, et al. Giant aneurysms of the internal carotid artery: endovascular treatment and long-term follow-up. Neuroradiology 2003;45:650–55 [DOI] [PubMed] [Google Scholar]
- 15. Lubicz B, Leclerc X, Gauvrit JY, et al. Giant vertebrobasilar aneurysms: endovascular treatment and long-term follow-up. Neurosurgery 2004;55:316–23 [DOI] [PubMed] [Google Scholar]
- 16. Cho YD, Park JC, Kwon BJ, et al. Endovascular treatment of largely thrombosed saccular aneurysms: follow-up results in ten patients. Neuroradiology 2010;52:751–58 [DOI] [PubMed] [Google Scholar]
- 17. Kessler IM, Mounayer C, Piotin M, et al. The use of balloon-expandable stents in the management of intracranial arterial diseases: a 5-year single-center experience. AJNR Am J Neuroradiol 2005;26:2342–48 [PMC free article] [PubMed] [Google Scholar]
- 18. Saatci I, Cekirge HS, Ozturk MH, et al. Treatment of internal carotid artery aneurysms with a covered stent: experience in 24 patients with mid-term follow-up results. AJNR Am J Neuroradiol 2004;25:1742–49 [PMC free article] [PubMed] [Google Scholar]
- 19. Ferns SP, Sprengers ME, van Rooij WJ, et al. Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke 2009;40:523–29 [DOI] [PubMed] [Google Scholar]
- 20. Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003;34:1398–403 [DOI] [PubMed] [Google Scholar]
- 21. Campi A, Ramzi N, Molyneux AJ, et al. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trial (ISAT). Stroke 2007;38:1538–44 [DOI] [PubMed] [Google Scholar]
- 22. Malish TW, Guglielmi G, Viñuela F, et al. Unruptured aneurysms presenting with mass effect symptoms: response to endosaccular treatment with Guglielmi detachable coils. Part I. Symptoms of cranial nerve dysfunction. J Neurosurg 1998;89:956–61 [DOI] [PubMed] [Google Scholar]
- 23. Hanse MC, Gerrits MC, van Rooij WJ, et al. Recovery of posterior communicating artery aneurysm-induced oculomotor palsy after coiling. AJNR Am J Neuroradiol 2008;29:988–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Gast AN, Sprengers ME, van Rooij WJ, et al. Midterm clinical and magnetic resonance imaging follow-up of large and giant carotid artery aneurysms after therapeutic carotid artery occlusion. Neurosurgery 2007;60:1025–29 [DOI] [PubMed] [Google Scholar]
- 25. Krings T, Piske RL, Lasjaunias PL. Intracranial arterial aneurysm vasculopathies: targeting the outer vessel wall. Neuroradiology 2005;47:931–37 [DOI] [PubMed] [Google Scholar]
- 26. Krings T, Alvarez H, Reinacher P, et al. Growth and rupture mechanism of partially thrombosed aneurysms. Intervent Neuroradiol 2007;13:117–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alvarez H. Etiology of giant aneurysms and their treatment. AJNR Am J Neuroradiol 2009;30:12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]