Abstract
BACKGROUND AND PURPOSE:
Occlusion of the AOP results in a characteristic pattern of ischemia: bilateral paramedian thalamus with or without midbrain involvement. Although the classic imaging findings are often recognized, only a few small case series and isolated cases of AOP infarction have been reported. The purpose of this study was to characterize the complete imaging spectrum of AOP infarction on the basis of a large series of cases obtained from multiple institutions.
MATERIALS AND METHODS:
Imaging and clinical data of 37 patients with AOP infarction from 2000 to 2009 were reviewed retrospectively. The primary imaging criterion for inclusion was an abnormal signal intensity on MR imaging and/or hypoattenuation on CT involving distinct arterial zones of the bilateral paramedian thalami with or without rostral midbrain involvement. Patients were excluded if there was a neoplastic, infectious, or inflammatory etiology.
RESULTS:
We identified 4 ischemic patterns of AOP infarction: 1) bilateral paramedian thalamic with midbrain (43%), 2) bilateral paramedian thalamic without midbrain (38%), 3) bilateral paramedian thalamic with anterior thalamus and midbrain (14%), and 4) bilateral paramedian thalamic with anterior thalamus without midbrain (5%). A previously unreported finding (the “V” sign) on FLAIR and DWI sequences was identified in 67% of cases of AOP infarction with midbrain involvement and supports the diagnosis when present.
CONCLUSIONS:
The 4 distinct patterns of ischemia identified in our large case series, along with the midbrain V sign, should improve recognition of AOP infarction and assist with the neurologic evaluation and management of patients with thalamic strokes.
The thalamus is predominantly supplied by multiple small vessels originating from the PcomA and P1 and P2 segments of the PCAs. Although there are significant variation and overlap, thalamic vascular supply is classically categorized into 4 territories: anterior, paramedian, inferolateral, and posterior (on-line Fig 1). The anterior territory is supplied by the polar (or thalamotuberal) arteries, which arise from the PcomA. The paramedian territory is supplied by the paramedian (or thalamoperforating) arteries, which arise from the P1 segment of the PCA. The inferolateral territory is supplied by the thalamogeniculate arteries, which arise from the P2 segment of the PCA. The posterior territory is supplied by the posterior choroidal arteries, which arise from the P2 segment of the PCA.1–8
The paramedian arteries have great variability with respect to number, size, and territorial contribution to the thalamus.1,2,6,9–13 Many authors have demonstrated that the paramedian arteries can supply both the paramedian and the anterior thalamic territories, especially when the polar artery is absent.1,4,6,9,10,14–19 The variable presence of the polar artery (absent in 30%–60% of the population)7,15,16 is not surprising because it arises from the PcomA, which itself is highly variable and can be absent or hypoplastic. One case series reported 7 of 8 bilateral paramedian thalamic infarcts extended into the anterior thalamic territory of the polar artery.10 Additionally, the arteries that supply the rostral midbrain—the superior mesencephalic (or rubral) arteries—can branch separately from P1 or share a common origin with the paramedian arteries. Thus, the paramedian arteries often supply the rostral midbrain.4,9,14,20–22
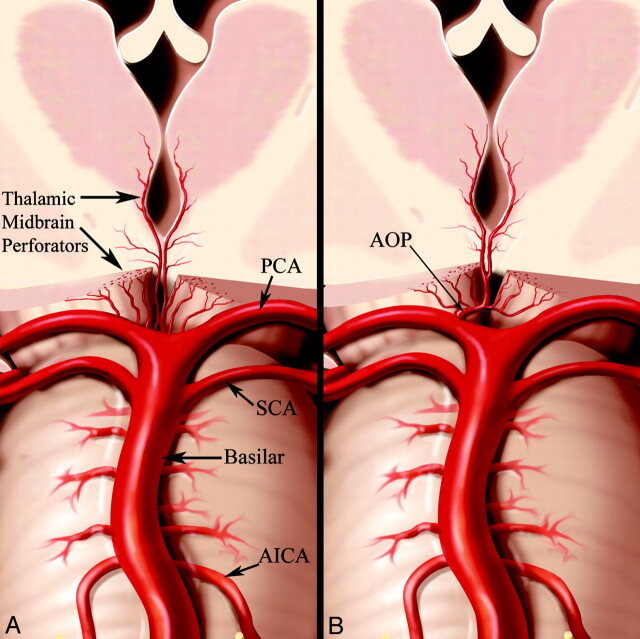
The focus of this article is the specific ischemic patterns that result from occlusion of an anatomic variant of the paramedian arteries called the AOP.4 The AOP is an uncommon anatomic variant, in which a single dominant thalamoperforating artery supplies the bilateral medial thalami with variable contribution to the rostral midbrain (Fig 1). Occlusion results in a characteristic pattern of ischemia: bilateral paramedian thalamic infarcts with or without midbrain involvement.1,17,20,21
Fig 1.
A, Conventional anatomy demonstrating paired thalamic and midbrain perforating arteries. B, AOP arising as a single unpaired trunk from P1 supplying the bilateral paramedian thalami and rostral midbrain. Reprinted with permission from Amirsys Inc.
Although the classic imaging findings of AOP infarction are often recognized, most investigations have reported only a few isolated cases, and to our knowledge, a comprehensive evaluation of its radiographic spectrum has not been documented. The imaging differential of bithalamic lesions is broad and includes arterial and venous occlusion, infiltrative neoplasm, and infectious and inflammatory lesions. Diagnosing an AOP infarction is critical to direct the appropriate time-sensitive management and to prevent additional unnecessary procedures. The purpose of this study, the largest case series to date, is to characterize the complete imaging spectrum of AOP infarction. We identify specific patterns consistent with variations in clinical presentation and thalamic vascular supply and describe a previously unreported finding (the “V” sign) on FLAIR and DWI sequences that supports the diagnosis when present.
Materials and Methods
We identified 37 patients with AOP infarctions (age range, 28–93 years; mean, 59 years) on the basis of radiographic and clinical data. Twenty-one patients from our institution were retrospectively identified by searching radiology reports from 2000 to 2009. Patients were excluded if bithalamic signal-intensity abnormality was shown to be due to pathology-proved neoplasm or an infectious or inflammatory etiology such as viral encephalitis. Sixteen patients with AOP infarction, from other institutions, were included. This study was approved by the institutional review board. The primary imaging criterion for inclusion was abnormal signal intensity on MR imaging and/or hypoattenuation on CT involving the bilateral paramedian thalami with or without rostral midbrain involvement. The abnormal signal intensity was defined as a hyperintense T2 or FLAIR signal intensity on MR imaging with or without restricted diffusion or postcontrast enhancement, within a specific bilateral paramedian thalamic distribution, corresponding to a distinct arterial territory. The CT hypoattenuation was defined as a region of decreased attenuation (compared with gray and white matter) representing edema in a specific bilateral paramedian thalamic distribution. A compatible clinical presentation (eg, rapid onset of altered mental status and ophthalmoplegia) was also used to support inclusion, when clinical data were available. Patients were excluded if there was pathology-proved direct involvement of the thalamus by neoplasm-mimicking ischemia on contrast-enhanced imaging.
In 2 patients, biopsy confirmed the presence of thalamic infarction. These patients presented with bilateral paramedian thalamic enhancing lesions and underwent stereotactic biopsy for suspected bithalamic glioma. They were subsequently discharged with follow-up imaging showing expected evolution of ischemic and postoperative changes.
MR imaging was performed in 32/37 patients; MRA, in 8/37; CT, in 20/37; CTA, in 8/37; CT perfusion, in 1/37; and conventional angiography, in 2/37 patients (On-line Table 1). Minimum MR imaging sequences included routine FLAIR and DWI with selected cases having T2, T2* gradient recalled-echo, T1, and T1 postcontrast sequences. A total of 13 patients had MRA, CTA, conventional angiography, or some combination of these 3 modalities. Specifically, 4 patients had MRA only, 3 patients had CTA only, 4 patients had MRA and CTA, 1 patient had CTA and conventional angiography, and 1 patient had conventional angiography only.
A clinical chart review was performed to identify age, sex, risk factors, and symptoms (on-line Table 2). Clinical data were available for 25/37 patients. The medical records were not available for 12. Each case was assigned to 1 of the following etiologies on the basis of clinical risk factors: cardiac embolism, large artery atherosclerosis, small artery occlusion, or undetermined.
Images from all 37 patients were reviewed by 3 senior staff neuroradiologists and a neuroradiology fellow, and conclusions were reached by consensus. The ischemic territories involved in each case were recorded under the following categories: bilateral paramedian thalamus (symmetric or asymmetric), anterior thalamus (right, left, or bilateral), and midbrain. Additional ischemic foci, both synchronous and metachronous, were also documented (on-line Table 3). These infarcts were considered synchronous if they demonstrated the same signal intensity on DWI images (restricted diffusion) and/or the same enhancement on postcontrast T1 sequences as the thalamic and midbrain infarcts. Ischemic foci were considered metachronous if there was no restricted diffusion or postcontrast enhancement.
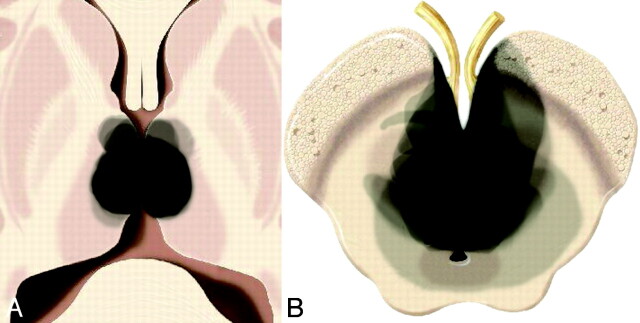
Using Adobe Photoshop (Adobe Systems, San Jose, California), 1 of the authors (N.A.L.) manually traced the ischemic territory involved in each stroke onto a graphic of the thalamus and midbrain at representative levels. Multiple territories were then superimposed to depict the total distribution of ischemic injury, with increased opacity representing more commonly affected regions (on-line Figs 2–5 and Fig 2). A graphic of the thalamus (on-line Fig 1) and an illustration of the AOP (Fig 1) depict the thalamic arterial supply.
Fig 2.
Collective extent of all 4 infarct patterns at the level of the thalamus (A) and midbrain (B) from all 37 cases superimposed.
Results
Bilateral paramedian thalamic infarctions were present in all patients (37/37). Thalamic involvement was asymmetric in 68% (25/37) and symmetric in 32% of patients (12/37). Bilateral paramedian thalamic with rostral midbrain infarction was identified in 57% of patients (21/37). Bilateral paramedian thalamic without midbrain infarction was demonstrated in 43% of patients (16/37). Bilateral paramedian thalamic with anterior thalamic involvement was present in 19% of patients (7/37). Of the 7 patients with paramedian and anterior thalamic territory involvement, 5 demonstrated midbrain involvement. Each case was found to fit into 1 of 4 patterns (on-line Figs 2–7 and Figs 3 and 4, on-line Table 1): bilateral paramedian thalamic with midbrain 43% (16/37), bilateral paramedian thalamic without midbrain 38% (14/37), bilateral paramedian thalamic with anterior thalamus and midbrain 14% (5/37), and bilateral paramedian thalamic with anterior thalamus without midbrain 5% (2/37). Figure 2 demonstrates the collective extent of infarction in all 37 patients. On the basis of clinical history, the most common presumed etiology was cardioembolic (on-line Table 2).
Fig 3.
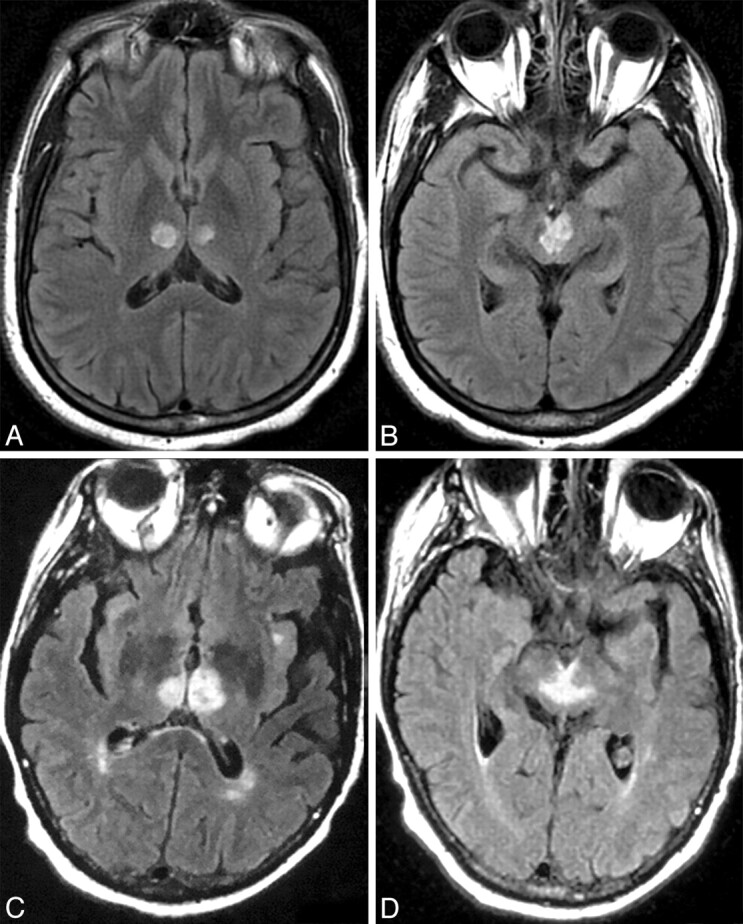
Case 2 (A and B) and case 16 (C and D). Axial FLAIR MR images at the level the thalamus (A and C) and midbrain (B and D) demonstrate bilateral paramedian thalamic and midbrain involvement (pattern 1). Notice the hyperintense signal intensity along the pial surface of the midbrain interpeduncular fossa representing the V sign (B and D).
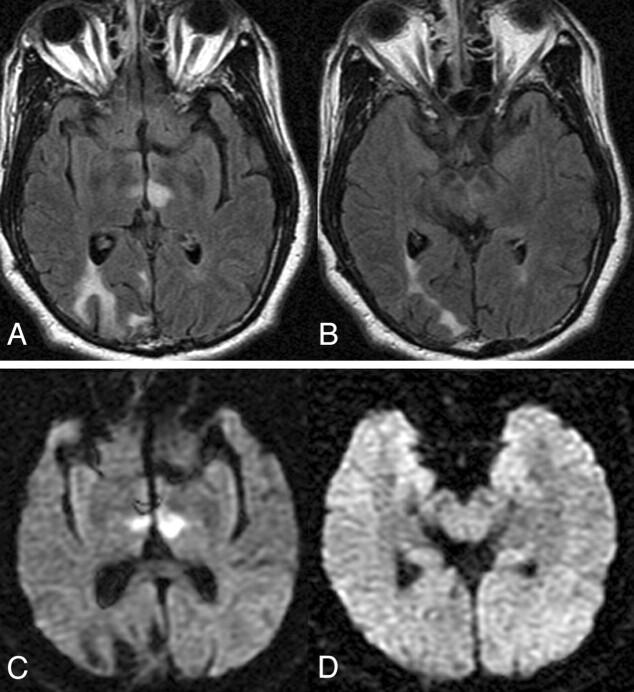
Fig 4.
Case 22. Axial FLAIR (A and B) and DWI (C and D) images at the level of the thalamus (A and C) and midbrain (B and D) demonstrate infarction of the bilateral paramedian thalami without midbrain involvement (pattern 2).
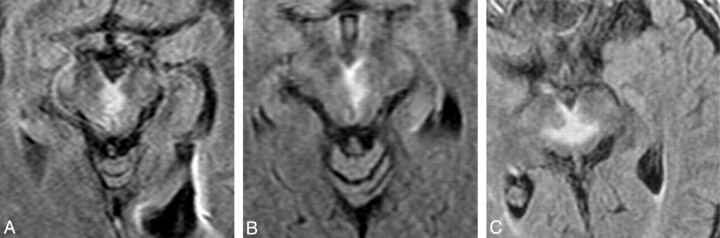
A distinct pattern of V-shaped hyperintensity on axial FLAIR and/or DWI (Fig 5) was present along the pial surface of the midbrain adjacent to the interpeduncular fossa in 38% of all patients (14/37) and 67% of patients with midbrain involvement (14/21). Synchronous or metachronous infarcts were identified in the cerebellum in 19% of patients (7/37), the occipital lobe in 11% of patients (4/37), and the MCA territory in 14% of patients (5/37) (on-line Table 3). The AOP was definitively visualized on conventional angiography in 1 patient (Fig 6). This was attributed to spontaneous resolution of the presumed embolus and probable luxury perfusion to the affected structures. No stenosis or occlusion of the basilar artery or PCA was observed in any of the 13 patients who underwent MRA, CTA, or conventional angiography.
Fig 5.
Axial FLAIR MR images through the midbrain from cases 31 (A), 5 (B), and 9 (C) show a V-shaped hyperintense signal intensity along the pial surface of the midbrain at the interpeduncular fossa (the V sign).
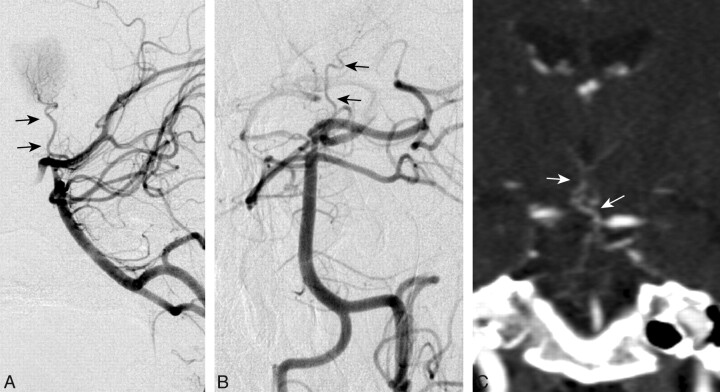
Fig 6.
DSA of the left vertebral injection, lateral (A) and anteroposterior (B) views, and a coronal CTA image (C) from case 23 demonstrate a large unpaired thalamic perforating artery (arrows) arising from the proximal P1 segment supplying the bilateral thalami (ie, an AOP).
Discussion
In our series, we identified 4 distinct patterns of AOP infarction (on-line Figs 6 and 7 and Figs 3 and 4): bilateral paramedian thalamic with rostral midbrain (43%), bilateral paramedian thalamic without midbrain (38%), bilateral paramedian and anterior thalamic with midbrain (14%), and bilateral paramedian and anterior thalamic without midbrain (5%). These 4 distinct patterns are consistent with known variations in the paramedian artery. Another distinctive imaging finding was a V-shaped hyperintense signal intensity on axial FLAIR and DWI images along the pial surface of the midbrain in the interpeduncular fossa (Fig 5). The sensitivity of this V sign is 67% in cases of AOP infarction with midbrain involvement.
The prevalence of the AOP is unknown. A small study of 15 cadaver brains demonstrated the AOP in 1 specimen.11 To date, the diagnosis of AOP infarction has been uncommon. Because of the variable presence and size of the P1 segment, which gives rise to the paramedian arteries, the AOP may be an underdiagnosed variant.23 At our institution, we identified 21 patients with AOP infarction during a 9-year period. In 2 large stroke series, the characteristic AOP infarct pattern was estimated to occur in 0.1% and 0.3% of all ischemic strokes.2,5 However, this is likely a conservative estimate due to limitations in their inclusion criteria. Other smaller studies have demonstrated the AOP ischemic pattern in 2% of all ischemic strokes24 and from 4% to 18% of all thalamic strokes.4,9,19,24,25 The AOP is rarely visualized with conventional angiography, and to our knowledge, only 3 other authors have successfully demonstrated this variant.18,26,27 We were able to capture a striking image of the AOP with conventional angiography in 1 of our patients (Fig 6).
Several clinical stroke patterns potentially involving the AOP have been described, including bilateral paramedian thalamic,5,10,15 paramedian and polar thalamic,16 and paramedian thalamic and mesencephalic.20,21,28 While these syndromes share some similarities in clinical presentation, there are notable differences that reflect the involvement of specific thalamic or mesencephalic structures.6
Bilateral paramedian thalamic strokes are typically characterized by a triad of altered mental status, vertical gaze palsy, and memory impairment.1,4–6,10,15–17,19,21,24,25,29–35 Altered mental status can present anywhere on the spectrum from drowsiness or confusion to hypersomnolence or coma. These disorders of vigilance generally occur with sudden onset and may persist until death, though cases of complete recovery have been documented.15,36,37 In our case series, 26% of the patients for whom the outcome at discharge is known (5/19) recovered completely. All of these patients showed lesions limited to the bilateral paramedian thalamus (pattern 2), and mental status that was only mildly altered on presentation. Vertical gaze palsies usually suggest mesencephalic involvement, but they have also been observed in patients without midbrain lesions, a finding that may be explained by disruption of cortical inputs that traverse the thalamus on their way to the rostral interstitial medial longitudinal fasciculus.38 Memory impairment, sometimes with confabulation, is frequently reported in patients with bilateral paramedian thalamic stroke but tends to resolve with time.10,15,16
When a bilateral paramedian thalamic stroke also involves the polar (anterior) territory, the most obvious clinical difference is more severe memory impairment.16 Several thalamic structures are commonly implicated in memory function: the mammillothalamic tract, anterior nucleus, and dorsomedial nucleus. The mammillothalamic tract and dorsomedial nucleus are found in the paramedian territory; the anterior nucleus belongs to the polar territory. Amnesia appears to be more profound and persistent when lesions affect all of these structures.16 Because the paramedian artery often takes over the territory of the polar artery, it seems likely that cases of bilateral paramedian stroke with polar involvement represent occlusions of an AOP, rather than synchronous occlusions of an AOP and the polar artery.
Bilateral paramedian thalamic lesions are often accompanied by rostral midbrain lesions, producing a “mesencephalothalamic” or “thalamopeduncular” syndrome.1,21 In addition to the triad of altered mental status, vertical gaze palsy, and amnesia, the syndrome is characterized by other oculomotor disturbances, hemiplegia, cerebellar ataxia, and movement disorders.1,21,28,29 The affected structures—interpeduncular nucleus, decussation of the superior cerebellar peduncle, medial part of the red nucleus, nucleus of cranial nerve III, and anterior part of the periaqueductal gray matter—compose the territory of the superior mesencephalic (or rubral) artery.1 This artery can branch separately from the proximal PCA or share a common origin with the paramedian thalamic artery,1,29 which may supply the thalamus bilaterally. Therefore, an infarction of the bilateral paramedian thalamus and the rostral midbrain may be explained by occlusion of a single AOP.1,21,28,29
Two limitations of our study should be mentioned. First, due to the retrospective nature of this imaging study, clinical data were incomplete and, in some patients, unavailable. Second, a single large embolus at the basilar tip could result in a similar infarct pattern, especially in combined paramedian thalamic and midbrain infarcts.1 However, this would typically manifest as the “top of the basilar” syndrome39 with additional characteristic posterior circulation infarcts, which are not present in our series. The cerebellar and occipital infarcts observed in some of our patients are more consistent with a cardioembolic setting. Additionally, none of the 13 patients who underwent MRA, CTA, or conventional angiography demonstrated an occluded basilar tip or embolus.
Although the imaging differential of bithalamic lesions includes venous infarction and infiltrative neoplasm, these entities should not be confused with AOP infarction. Venous infarction and infiltrative neoplasm do not respect the specific arterial territory of the paramedian or anterior thalamic arterial zones but rather involve multiple arterial regions.
Conclusions
The four distinct patterns of ischemia identified in our large case series, along with the midbrain V sign, should improve recognition of AOP infarction and assist with the neurologic evaluation and management of patients with thalamic strokes. The patterns are consistent with previously described clinical syndromes and variations in the thalamic vascular supply. AOP infarction may be more frequent than previously reported, perhaps due in part to the increased utility of imaging studies, especially MR imaging.
Supplementary Material
Abbreviations
- AF
atrial fibrillation
- AICA
anterior inferior cerebellar artery
- angio
angiography
- AOP
artery of Percheron
- CAD
coronary artery disease
- CardAbn
cardiac abnormalities (miscellaneous non-valvular)
- CE
cardiac embolism
- CHF
congestive heart failure
- CT perf
CT perfusion
- CTA
CT angiography
- CVA-P
previous cerebrovascular accident
- DM
diabetes mellitus
- DVI
deep venous thrombus
- DWI
diffusion-weighted imaging
- EtOH
heavy drinking
- FLAIR
fluid-attenuated inversion recovery
- FMD
fibromuscular dysplasia
- HHC
hyperhomocysteinemia
- HL
hyperlipidemia
- HTN
hypertension
- H/o
history of
- ICH
intracranial hemorrhage
- INO
internuclear ophthalmoplegia
- L
left
- LAA
large artery atherosclerosis (includes large artery thrombosis and artery-to-artery embolism)
- LV
left ventricular
- LVAD
left ventricular assist device
- MCA
middle cerebral artery
- MRA
MR angiography
- MRI or MR
MR imaging
- OCP
oral contraceptive pill
- ODC
stroke of other determined cause
- P1
first segment of the PCA
- P2
second segment of PCA
- PCA
posterior cerebral artery
- PcomA
posterior communicating artery
- PFO
patent foramen ovale
- PICA
posterior inferior cerebellar artery
- R
right
- SCA
superior cerebellar artery
- S/p
status post
- SVO
small vessel occlusion
- TIA-P
previous transient ischemic attack
- Tob
tobacco smoker
- UND
stroke of undetermined cause
- VA
vertebral artery
- ValvAbn
valvular abnormalities
- Y
yes
Footnotes
Indicates article with supplemental on-line tables.
Indicates article with supplemental on-line figures.
References
- 1. Castaigne P, Lhermitte F, Buge A, et al. Paramedian thalamic and midbrain infarct: clinical and neuropathological study. Ann Neurol 1981;10:127–48 [DOI] [PubMed] [Google Scholar]
- 2. Carrera E, Michel P, Bogousslavsky J. Anteromedian, central, and posterolateral infarcts of the thalamus: three variant types. Stroke 2004;35:2826–31 [DOI] [PubMed] [Google Scholar]
- 3. Takahashi S, Suzuki M, Matsumoto K, et al. Extent and location of cerebral infarcts on multiplanar MR images: correlation with distribution of perforating arteries on cerebral angiograms and on cadaveric microangiograms. AJR Am J Roentgenol 1994;163:1215–22 [DOI] [PubMed] [Google Scholar]
- 4. Bogousslavsky J, Regli F, Uske A. Thalamic infarcts: clinical syndromes, etiology, and prognosis. Neurology 1988;38:837–48 [DOI] [PubMed] [Google Scholar]
- 5. Kumral E, Evyapan D, Balkir K, et al. Bilateral thalamic infarction: clinical, etiological and MRI correlates. Acta Neurol Scand 2001;103:35–42 [DOI] [PubMed] [Google Scholar]
- 6. Schmahmann JD. Vascular syndromes of the thalamus. Stroke 2003;34:2264–78 [DOI] [PubMed] [Google Scholar]
- 7. Wang X, Fan YH, Lam WW, et al. Clinical features, topographic patterns on DWI and etiology of thalamic infarcts. J Neurol Sci 2008;267:147–53 [DOI] [PubMed] [Google Scholar]
- 8. Tatu L, Moulin T, Bogousslavsky J, et al. Arterial territories of the human brain: cerebral hemispheres. Neurology 1998;50:1699–708 [DOI] [PubMed] [Google Scholar]
- 9. Takahashi S, Goto K, Fukasawa H, et al. Computed tomography of cerebral infarction along the distribution of the basal perforating arteries. Part II. Thalamic arterial group. Radiology 1985;155:119–30 [DOI] [PubMed] [Google Scholar]
- 10. Gentilini M, De Renzi E, Crisi G. Bilateral paramedian thalamic artery infarcts: report of eight cases. J Neurol Neurosurg Psychiatry 1987;50:900–09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Uz A. Variations in the origin of the thalamoperforating arteries. J Clin Neurosci 2007;14:134–37 [DOI] [PubMed] [Google Scholar]
- 12. Kraft P, Waschbisch A, Wendel F, et al. Why it's important to know Percheron's artery: solitary carotid stenosis as a unique cause of anterior, posterior and bithalamic ischemia. J Neurol 2009;256:1558–60. Epub 2009 Apr 30 [DOI] [PubMed] [Google Scholar]
- 13. Marinkovic SV, Milisavljevic MM, Kovacevic MS. Anastomoses among the thalamoperforating branches of the posterior cerebral artery. Arch Neurol 1986;43:811–14 [DOI] [PubMed] [Google Scholar]
- 14. Percheron G. The anatomy of the arterial supply of the human thalamus and its use for the interpretation of the thalamic vascular pathology. Z Neurol 1973;205:1–13 [DOI] [PubMed] [Google Scholar]
- 15. Reilly M, Connolly S, Stack J, et al. Bilateral paramedian thalamic infarction: a distinct but poorly recognized stroke syndrome. Q J Med 1992;82:63–70 [PubMed] [Google Scholar]
- 16. Perren F, Clarke S, Bogousslavsky J. The syndrome of combined polar and paramedian thalamic infarction. Arch Neurol 2005;62:1212–16 [DOI] [PubMed] [Google Scholar]
- 17. Rangel-Castilla L, Gasco J, Thompson B, et al. Bilateral paramedian thalamic and mesencephalic infarcts after basilar tip aneurysm coiling: role of the artery of Percheron. Neurocirugia (Astur) 2009;20:288–93 [DOI] [PubMed] [Google Scholar]
- 18. Weidauer S, Nichtweiss M, Zanella FE, et al. Assessment of paramedian thalamic infarcts: MR imaging, clinical features and prognosis. Eur Radiol 2004;14:1615–26 [DOI] [PubMed] [Google Scholar]
- 19. Graff-Radford NR, Damasio H, Yamada T, et al. Nonhaemorrhagic thalamic infarction: clinical, neuropsychological and electrophysiological findings in four anatomical groups defined by computerized tomography. Brain 1985;108(pt 2):485–516 [DOI] [PubMed] [Google Scholar]
- 20. Matheus MG, Castillo M. Imaging of acute bilateral paramedian thalamic and mesencephalic infarcts. AJNR Am J Neuroradiol 2003;24:2005–08 [PMC free article] [PubMed] [Google Scholar]
- 21. Waterston JA, Stark RJ, Gilligan BS. Paramedian thalamic and midbrain infarction: the ‘mesencephalothalamic syndrome.’ Clin Exp Neurol 1987;24:45–53 [PubMed] [Google Scholar]
- 22. Tatu L, Moulin T, Bogousslavsky J, et al. Arterial territories of human brain: brainstem and cerebellum. Neurology 1996;47:1125–35 [DOI] [PubMed] [Google Scholar]
- 23. Krampla W, Schmidbauer B, Hruby W. Ischaemic stroke of the artery of Percheron (2007: 10b). Eur Radiol 2008;18:192–94. Epub 2008 Jan 4 [DOI] [PubMed] [Google Scholar]
- 24. Pezzini A, Del Zotto E, Archetti S, et al. Thalamic infarcts in young adults: relationship between clinical-topographic features and pathogenesis. Eur Neurol 2002;47:30–36 [DOI] [PubMed] [Google Scholar]
- 25. Saez de Ocariz MM, Nader JA, Santos JA, et al. Thalamic vascular lesions: risk factors and clinical course for infarcts and hemorrhages. Stroke 1996;27:1530–36 [DOI] [PubMed] [Google Scholar]
- 26. Roitberg BZ, Tuccar E, Alp MS. Bilateral paramedian thalamic infarct in the presence of an unpaired thalamic perforating artery. Acta Neurochir (Wien) 2002;144:301–04, discussion 304 [DOI] [PubMed] [Google Scholar]
- 27. Kostanian V, Cramer SC. Artery of Percheron thrombolysis. AJNR Am J Neuroradiol 2007;28:870–71 [PMC free article] [PubMed] [Google Scholar]
- 28. Lepore FE, Gulli V, Miller DC. Neuro-ophthalmological findings with neuropathological correlation in bilateral thalamic-mesencephalic infarction. J Clin Neuroophthalmol 1985;5:224–28 [PubMed] [Google Scholar]
- 29. Biller J, Sand JJ, Corbett JJ, et al. Syndrome of the paramedian thalamic arteries: clinical and neuroimaging correlation. J Clin Neuroophthalmol 1985;5:217–23 [PubMed] [Google Scholar]
- 30. Thurtell MJ, Halmagyi GM. Complete ophthalmoplegia: an unusual sign of bilateral paramedian midbrain-thalamic infarction. Stroke 2008;39:1355–57 [DOI] [PubMed] [Google Scholar]
- 31. Swanson RA, Schmidley JW. Amnestic syndrome and vertical gaze palsy: early detection of bilateral thalamic infarction by CT and NMR. Stroke 1985;16:823–27 [DOI] [PubMed] [Google Scholar]
- 32. Meissner I, Sapir S, Kokmen E, et al. The paramedian diencephalic syndrome: a dynamic phenomenon. Stroke 1987;18:380–85 [DOI] [PubMed] [Google Scholar]
- 33. Muller A, Baumgartner RW, Rohrenbach C, et al. Persistent Kluver-Bucy syndrome after bilateral thalamic infarction. Neuropsychiatry Neuropsychol Behav Neurol 1999;12:136–39 [PubMed] [Google Scholar]
- 34. Raphaeli G, Liberman A, Gomori JM, et al. Acute bilateral paramedian thalamic infarcts after occlusion of the artery of Percheron. Neurology 2006;66:E7. [DOI] [PubMed] [Google Scholar]
- 35. Wiest G, Mallek R, Baumgartner C. Selective loss of vergence control secondary to bilateral paramedian thalamic infarction. Neurology 2000;54:1997–99 [DOI] [PubMed] [Google Scholar]
- 36. Khoiny A, Goldberg M, Khoiny N. Atypical presentation with good outcome in a bilateral paramedian thalamic infarction. J Neurol Sci 2006;23:54–58 [Google Scholar]
- 37. Krolak-Salmon P, Croisile B, Houzard C, et al. Total recovery after bilateral paramedian thalamic infarct. Eur Neurol 2000;44:216–18 [DOI] [PubMed] [Google Scholar]
- 38. Clark JM, Albers GW. Vertical gaze palsies from medial thalamic infarctions without midbrain involvement. Stroke 1995;26:1467–70 [DOI] [PubMed] [Google Scholar]
- 39. Caplan LR. “Top of the basilar” syndrome. Neurology 1980;30:72–79 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.