Abstract
BACKGROUND AND PURPOSE:
Upper cervical spinal canal dimension may have a role in abnormal CSF dynamics in patients with Chiari I malformation. We attempted to measure spinal canal tapering from anteroposterior spinal canal dimensions in patients with Chiari I.
MATERIALS AND METHODS:
Twenty-one patients with Chiari I malformation, including 12 with syringomyelia and 7 patients with IS were identified from a local registry. Age- and sex-matched control subjects with cervical spine MR imaging findings reported as normal were selected from the PACS. The anteroposterior diameter of the spinal canal was measured at C1-C7 on T2-weighted sagittal MR images. The taper ratio of the spinal canal was calculated with the regression line. Goodness of fit was calculated as R2. Differences between patients with Chiari I and other patients were tested for significance with Kruskal-Wallis tests and multivariate analysis.
RESULTS:
Taper ratios averaged −0.6 ± 0.3 mm/level in the patients with Chiari and syrinx, −0.4 ± 0.2 mm/level (mean ± 1 SD) in the patients with Chiari without syrinx, and −0.3 ± 0.5 mm/level in the patients with IS; control groups had average taper ratios of −0.3 ± 0.2 mm/level. Mean R2 equaled 0.43. Taper ratios in patients with Chiari and syringomyelia differed significantly from those in the control group (P = .003). Taper ratios in the patients with Chiari without syrinx and in patients with IS did not differ significantly from their matched control groups (P = .60 and 0.76, respectively).
CONCLUSIONS:
Patients with Chiari I and a syrinx have steeper tapering of the upper cervical spinal canal than matched controls.
Patients with the Chiari malformation have abnormal CSF dynamics,1–3 which are thought to have a role in the pathogenesis of syringomyelia and perhaps some neurologic symptoms associated with the Chiari malformation. Tonsillar herniation and small posterior fossa dimensions have a role in the flow abnormalities, according to prevailing theories. The possibility that cervical spinal canal anatomy in the Chiari I malformation may influence CSF flow dynamics has not received sufficient study, to our knowledge. CSF velocities increase from the foramen magnum to C4 or C5 in patients with Chiari I,4–6 an observation explained by tapering of the spinal canal. The upper cervical spinal canal tapers in healthy subjects.7 Whether it tapers as much or more in patients with Chiari I has not been investigated, to our knowledge. Therefore we attempted to measure tapering of the cervical spinal canal in patients with Chiari I from the anteroposterior dimensions of the spinal canal. Diameters at each cervical spinal level, easily measured on sagittal MR images, correlated significantly with spinal canal areas.8 We chose to include patients with Chiari I with and without a syrinx. For comparison, we selected patients with IS, which is a syrinx without evidence of tumor, prior trauma, prior infection, or a Chiari I malformation. Like the Chiari I malformation, IS may have abnormal CSF velocities9 and possibly abnormal posterior fossa dimensions.10,11 For controls, we selected patients who had MR imaging of the cervical spine whose findings were reported to be normal. We tested the hypothesis that patients with Chiari I have steeper tapering of the spinal subarachnoid spaces than healthy subjects.
Materials and Methods
Our institutional review board approved this retrospective study and waived the requirement for informed consent. The study conformed to the Health Insurance Portability and Accountability Act standards.
Patients
Patients with a Chiari I malformation who had cervical spine MR imaging between January 4 and December 30, 2009, at our institution were identified. Inclusion criteria were: >5 mm of tonsillar herniation; exclusion criteria were the following: hydrocephalus, evidence of prior surgery, degenerative disk disease (except for mild disk bulging at C5-C6 or C6-C7), spinal cord disease (except syrinx), severe kyphosis or straightening of the cervical spine, evidence of spinal trauma, poor image quality of the T1 and T2 images, or multiple artifacts present in the images. The patients with Chiari were subdivided into those with and without a syrinx. Patients with IS were also identified from the registry. Exclusion criteria for patients with IS included the following: tonsils below the foramen magnum, history of neoplasm or injury, or infection or other cause of syrinx, plus the criteria used for exclusion for the patients with Chiari. The cases were anonymized and identified by a reference number.
The patient study log of the PACS was reviewed in reverse chronologic order starting with March 1, 2010, to find control patients with cervical MR imaging studies who matched the cases in age and sex. The inclusion criterion was a cervical spinal MR imaging study officially interpreted as showing no pathology, except minor disk bulging at either or both of the 2 lower cervical disk levels. The same exclusion criteria as those in patients were used for controls. The controls were anonymized and identified by a reference number.
Each patient and control during his or her clinical evaluation had cervical spine MR imaging studies on a 1.5T imager (Signa; GE Healthcare, Milwaukee, Wisconsin) that included sagittal T1-weighted (TR/TE, 520/10 ms; section thickness, 3 mm; 256 × 256 matrix; 16-cm FOV; NEX, 3) and sagittal T2-weighted (TR/TE, 3600/116 ms; section thickness, 3 mm; 512 × 512 matrix; FOV, 16 cm) images. Any other images acquired were reviewed as necessary to exclude pathology but were not otherwise used in this study.
An investigator measured tonsillar herniation with a standard technique, drawing a line from the posterior tip of the basion to the anterior tip of the opisthion and a second line perpendicular to the first line through the tip of the tonsils, along which the length of tonsillar herniation was measured with resident software. A neuroradiologist reviewed the cervical spine images to identify pathology or anomalies of the spine or spinal cord that would make the subject unsuitable for this study.
Measurements
An investigator, blinded to the patient name, demographics, and diagnosis, measured spinal canal diameters at each spinal level from C1 to T1. The investigator selected a midline T2-weighted MR image by finding the sagittal image that best displayed the spinous processes. If the T2-weighted image demonstrated significant artifacts secondary to motion or susceptibility, the T1-weighted image was chosen instead and the midline was evaluated in the same manner.
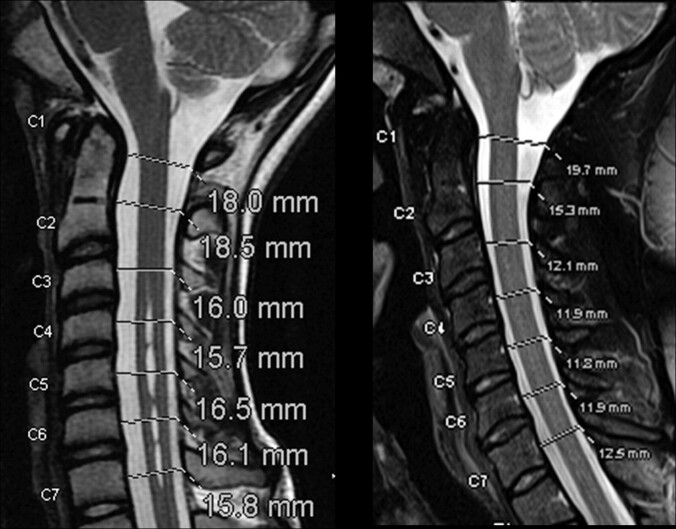
Sagittal images were zoomed, dragged, and windowed to facilitate visualization of the spinal canal and to optimize measurement with the software in PACS. A line was placed perpendicular to the spinal canal at the midpoint between the top and bottom of each vertebra (in the case of C1 through the midpoint of the anterior arch). On this line, the junctions between CSF and the epidural tissues anterior and posterior to the spinal cord were identified. The length of this line was measured as the anteroposterior diameter of the spinal canal to a tenth of a millimeter by using the annotation software in the PACS system at each level (Fig 1).
Fig 1.
Sagittal T2-weighted MR images (TR/TE, 3000/100 ms) in 2 patients illustrate the measurement of the spinal canal diameter at 7 cervical spinal canal levels.
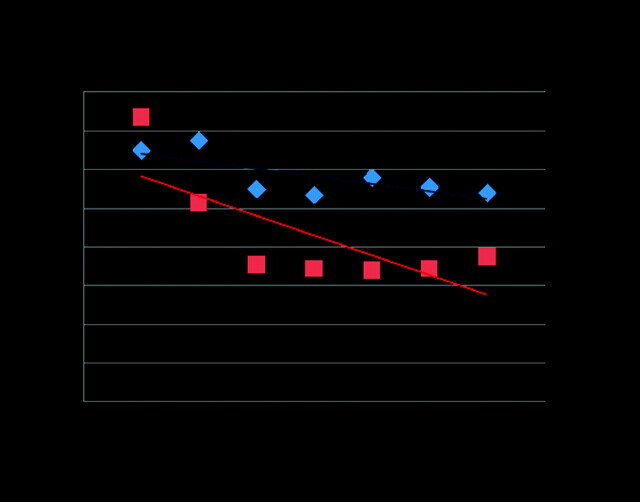
For each Chiari, IS, and control subject, the diameters of the spinal canal and syrinx were plotted against spine level. A trend line was fitted by linear regression to the diameters between C1 and T1, and the slope of this line was calculated (Fig 2). R2 was calculated for each subject to assess how well the linear approximation described the anteroposterior spinal canal diameters. The slope of the trend line was recorded as the taper ratio for that spine. For each group, the average diameters, average taper ratios, and corresponding SDs were tabulated.
Fig 2.
Plot of diameters for the 2 patients in Fig 1 and the fitting of a trend line. The trend line slopes were at the −0.4 and −1.0 m/levels, with the negative sign signifying that the canal tapers toward its caudal end.
Statistical Analysis
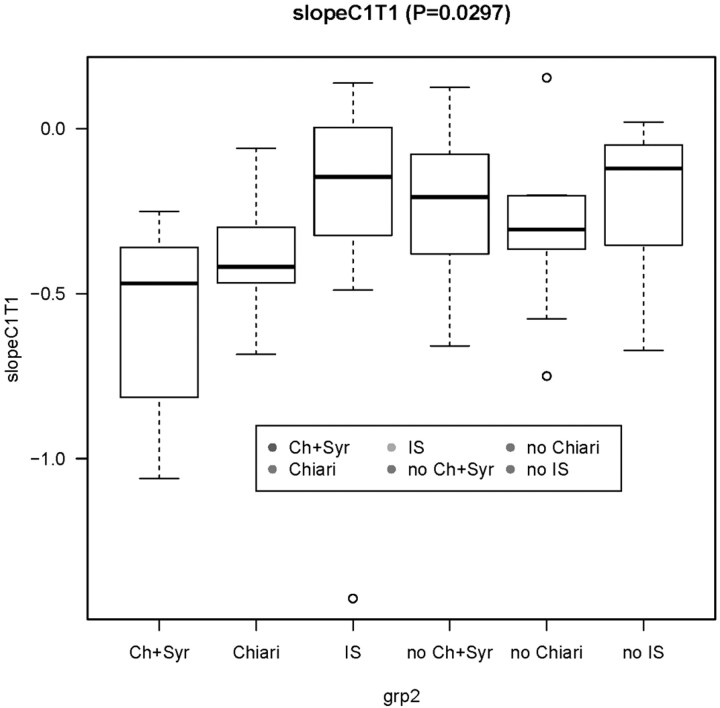
The median and IQR of the 56 R2 values were calculated. Taper ratios within a group were graphically summarized with box-and-whisker displays (Fig 3). Paired Welch t and Kruskal-Wallis tests were used to test for group differences in taper ratio due to group (Chiari, Chiari with syrinx, IS, control) and sex. Multiple linear regression was used to model the C1-T1 canal slope as a function of age, sex, and diagnosis group (Chiari without syrinx, Chiari with syrinx, IS, control). First, a baseline model with age was fitted. Diagnostic group (control, Chiari without syrinx, Chiari with syrinx, IS), interaction with age, sex by age interaction, and sex by diagnosis group interaction were added to the model sequentially, in the given order, strategically equivalent to a fitting analysis of covariance models. If the likelihood ratio F-test proved significant, the candidate variable was left in the model. Plots of the residuals were examined as diagnostics for possible violations in model assumptions. Parameter estimates were reported as point estimates followed by their SEs enclosed in parentheses. The criterion used for statistical significance was P < .05 (2-sided). No adjustment was made of P values for multiple testing. Statistical graphics and computations were obtained in R, version 2.10.0.12 Post hoc analyses included repeat multivariate analyses with exclusion of a possible wild value and calculation of the taper ratio for a segment of the cervical spine rather than for the whole of the cervical spine.
Fig 3.
Box-and-whisker display of taper ratios for the 3 patients and 3 control groups. The midline denotes the median, the box spans the middle 50% of the data (IQR), and the whiskers extend to the most extreme observation within 1.5 times the IQR; observations outside this range are displayed individually. The P value in the title is from a Kruskal-Wallis test.
Results
Patients
Twenty-one patients with a Chiari malformation including 12 with syringomyelia and 9 without syringomyelia, 7 patients with IS, and 28 age- and sex-matched control subjects were selected (Table 1). The average age of the 12 patients with Chiari with syringomyelia (5 males and 7 females) was 23 years (range, 4–58 years). Of the 9 patients with Chiari without syrinx, 5 males and 4 females were an average of 34 years of age (range, 7–60 years); of the 7 patients with IS, 4 males and 3 females were an average of 12 years of age (range, 5–39 years). The controls selected for the study were referred for evaluation of neck pain (8 cases); numbness, tingling, or radicular pain (7 cases); trauma (6 cases); possible metastases (6 cases); and possible CSF leak (1 case). None of the controls had tonsils extending into the cervical spinal canal.
Table 1:
Number of patients, average age, taper ratios and standard deviations for the 3 patient groups (Chiari I, Chiari I plus syrinx, IS) and the 3 matched control groupsa
| No. | Age (yr) | Taper Ratio | SD | |
|---|---|---|---|---|
| Chiari | 9 | 34 | −0.4 | 0.2 |
| Control | 9 | 34 | −0.3 | 0.3 |
| Chiari plus syrinx | 12 | 23 | −0.6 | 0.3 |
| Control | 12 | 23 | −0.2 | 0.2 |
| IS | 7 | 12 | −0.3 | 0.5 |
| Control | 7 | 12 | −0.2 | 0.3 |
Two-tailed t test of the means were significant (P = .033) for the Chiari I plus syrinx versus controls; borderline (P = .054) for the Chiari versus controls; and not significant for the IS versus controls.
Patients with Chiari I with or without a syrinx had downward tonsil herniation ranging from 5.1 to 28.7 mm (average, 12.02 ± 6.9 mm). The patients with Chiari I with and without syrinx had similar tonsillar ectopia. No anomalies or pathology was identified in the patients or controls except for the tonsillar herniation and syringomyelia. Twelve patients with Chiari had syrinxes located from C1 to T7 and the 7 patients with IS had syrinxes located from C4 to T8. By inspection, taper ratios differed subtly among patients (Fig 1).
Measurements
Taper ratios averaged −0.6 ± 0.3 mm/level in the patients with Chiari with syrinx, −0.4 ± 0.2 mm/level in the patients with Chiari without syrinx, and −0.3 ± 0.5 mm/level in the patients with IS. The control groups had average taper ratios of −0.2 to −0.3 ± 0.2 mm/level (Table 1, Fig 3). Taper ratios in patients with Chiari and syringomyelia differed significantly from those in the control group (P = .003). Taper ratios in the patients with Chiari without syrinx and in the patients with IS did not differ significantly from their matched control group (P = .054 and 0.076, respectively). Diameter measurements were made on T2-weighted images in 55 instances and on a T1-weighted image in 1 patient. The taper ratios calculated for each patient and control fit the actual plotted diameters (Fig 2) variably. The median R2 for the taper ratios was 0.41 (IQR, 0.17–0.57).
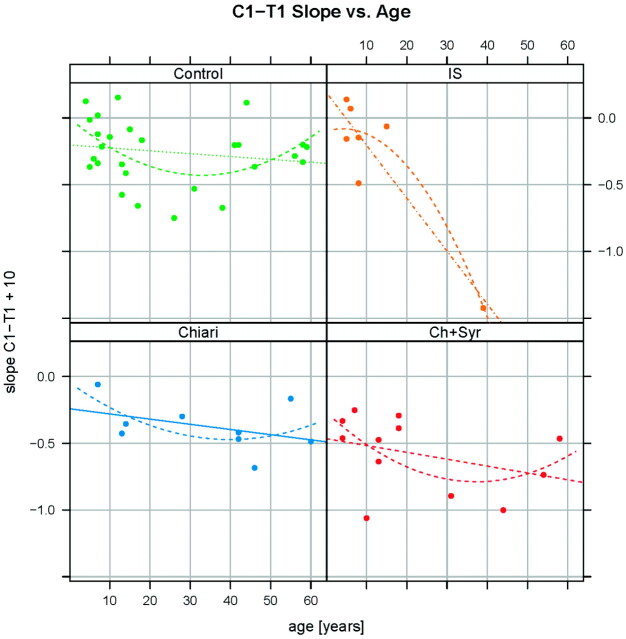
In plots of taper ratio versus age for the IS, Chiari, and control groups, trend lines had similar slopes if 1 aberrant value in the IS group and its matched control were disregarded (Fig 4). If the aberrant value was retained, the slope of the taper ratio–versus-age plot for IS was steeper. Tapering appeared nonlinear with more tapering apparent in the upper cervical spine. The taper ratios for C1-C4 (averaging between −0.8 and −1.0) were steeper than those for C1 to T1 in each group. The median R2 for these taper ratios was 0.84 (IQR, 0.70–0.89).
Fig 4.
C1-T1 slope versus age, by diagnostic group. Dots represent patient ages and their C1-T1 taper ratios. Continuous lines and dashed curves are least-square straight line and quadratic fits, respectively. Analysis repeated with omission of the lowest taper ratio in the IS group revealed a linear fit with a slope more consistent with the slopes in the other groups.
Multivariate Model and Sensitivity Analyses
In the final parameter estimates (Table 2), effects were parameterized as offsets with respect to the Chiari group. Compared with the taper ratio of 0.01 mm/level for the patients with Chiari, the ratios for the controls, patients with Chiari with syrinx, and the patients with IS were not significantly different (P = .8, .14, and .21, respectively). The Chiari and syrinx group did not differ significantly from the Chiari-alone subjects in either intercept or slope. The effect of age on slope in the Chiari group was significant (P = .009), with slope decreasing by 0.03 mm/level per year of age (SE = 0.010 mm). Age had no significant effect in the Chiari-plus-syrinx group or in the control group. The effect of the square of age was also significant. Age had a significant effect on the taper ratio in patients with IS (P = .003). Overall, these results show a significant effect of age and Chiari malformation on spinal canal dimensions, with both variables interacting. The coefficient of determination R2 = 0.54 indicates that slightly more than half of the variability in the taper ratios is explained by the model.
Table 2:
Coefficients of taper ratios as a function of age for Chiari, IS, and control patients from final multivariate modela
| Parameter | Slope Estimate in Model | Standard Error | P Value |
|---|---|---|---|
| Taper ratio | |||
| Chiari | 0.01 mm/level | 0.188 | 1.0 |
| Controls | −0.03 mm/level | 0.178 | .8 |
| Chiari + syrinx | −0.3 mm/level | 0.194 | .14 |
| IS | 0.3 mm/level | 0.214 | .21 |
| Effect of age on taper ratio | |||
| Chiari | −0.03 mm/level/year of age | 0.010 | .009 |
| Effect of age2 on taper ratio | |||
| Chiari | 0.0003 mm/level/year of age2 | 0.0001 | .01 |
| Effect of age on taper ratio | |||
| Controls | 0.003 mm/level/year of age | 0.005 | .5 |
| Chiari + syrinx | 0.0003 mm/level/year of age | 0.006 | 1.0 |
| IS | −0.03 mm/level/year of age | 0.010 | .003 |
| Effect of age2 on taper ratio | |||
| IS | 0.0003/level/year of age2 | 0.0001 | .01 |
Age and the presence of a Chiari malformation affect spinal canal taper ratios, with both variables interacting. The multivariate analysis estimates the taper ratio (millimeter/level) for Chiari patients as equal to −0.445–0.009 (age of patient in years), for the controls as −0.114–0.074 (age of the patient in years), and for the IS group as 0.191–0.039 (age of the patient in years).
When the influential wild value in the IS group and its matched control were removed, C1-T1 taper ratios were found to depend on age (P = .007), its square (P = .016), and diagnosis group (P < .001). The lack of statistical significance of the age-diagnosis interaction (P = .86) suggests that parallel lines with different diagnosis-specific intercepts suffice in the statistical model. The R2 (0.43) demonstrates that less than half the variation in taper ratios is accounted for in the model. If taper ratios are computed over C1-C4 instead of C1-T1, evidence of differences in taper ratios due to diagnosis group persists (P = .005); also, age still interacts with the diagnosis group (P = .02), but the quadratic age term is no longer needed (P = .28). Explanatory power remains low, with R2 = 0.43.
Discussion
To our knowledge, taper ratios in patients with Chiari I have not been reported previously. This study demonstrates that the cervical spinal canal tapers more steeply in patients with Chiari I with a syrinx than in control subjects. Age of the patient or control subject has a significant effect on tapering. The study suggests that besides the anatomy of the cerebellar tonsils and posterior fossa, spinal anatomy may be a significant factor in the Chiari malformation with syringomyelia.
Previous studies document that the cervical spinal canal tapers mildly in healthy subjects.7 Reported taper ratios in our controls do not agree perfectly with those in previous reports, probably because of differences in ages, measuring techniques, and the method for calculating the taper ratios. For example, diameters were measured for the boney margins of the spinal canal, for C2 through C7, and for adults only.7
This study has many limitations and warrants additional data collection. Spinal canal diameters were measured, but due to the lack of suitable cross-sectional images at all levels, areas were not measured. Cervical spinal canal diameters, however, correlate significantly with canal areas.8 To minimize inter-reader variability in this study, a single investigator measured all diameters. A potential confounding effect in the differences we observed is the reported association of Chiari I with HDCT.13 Lack of clinical data prevented screening for possible HDCT in our subjects. Although a straight line characterizes spinal canal taper adequately for most subjects, a quadratic or other nonlinear equation may have been better in other cases. The cervical spinal canal does not taper uniformly along its length. Tapering is steeper from C1 to C4 than from C4 to C7. The proportion of variability explained by the statistical model was relatively low (R2 = 0.54), implying 46% of the variation in the response is still unaccounted for.
The steeper tapering of the cervical spinal canal in the patients with Chiari has a number of implications. Because of tapering, CSF velocities are faster and pressures are relatively lower at the caudal than at the cranial end of the spinal canal. In patients with Chiari, with steeper tapering, the differences in velocity and pressure between cranial and caudal ends of the cervical spinal canal are greater. Pressure gradients along the spinal canal are larger in patients with Chiari than in healthy individuals, who have less steep tapering of the spinal canal. The steepness of the taper and the length of the taper, which vary from 1 patient to another, may explain where or if a syrinx forms. The combination of obstruction at the foramen magnum plus tapering may create very abnormal CSF dynamics. The age at which syrinx develops may relate to the age at which spinal canal tapering produces critical changes in CSF dynamics. Additional studies of spinal canal tapering in patients with Chiari are needed. Specifically, measuring the change in the subarachnoid space cross-sectional area compared with the cervical spinal level will better characterize tapering of the cervical spinal canal. Comparison of tapering in patients with syrinx and neurologic signs or symptoms with the tapering in patients with an incidental asymptomatic tonsillar ectopia may help identify patients with critical abnormalities in CSF flow.
These measurements of spinal canal tapering may lead to another conclusion. Chiari I malformations include an abnormally small posterior fossa, an abnormal location of the cerebellar tonsils, and, based on this study, an abnormal development of the cervical spine. These data support the theory that the Chiari I malformation is related to abnormal mesodermal development.14–16
Conclusions
The study shows steeper-than-normal tapering of cervical spinal canals in patients with Chiari I with a syrinx.
ABBREVIATIONS:
- HDCT
hereditary disorders of connective tissue
- IQR
interquartile range
- IS
idiopathic syringomyelia
- SE
standard error
Footnotes
Disclosures: Victor Haughton—UNRELATED: Expert Testimony: I have testified on causes of back pain and on the risk of neck pain in cases of Chiari I; I have no cases presently on the subject of CSF flow or Chiari malformation, Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: travel support for European Society of Neuroradiology to present on the subject of the dehydrated disk.
References
- 1. Quigley MF, Iskandar B, Quigley ME, et al. Cerebrospinal fluid flow in foramen magnum: temporal and spatial patterns at MR imaging in volunteers and in patients with Chiari I malformation. Radiology 2004;232:229–36 [DOI] [PubMed] [Google Scholar]
- 2. Heiss JD, Patronas N, DeVroom HL, et al. Elucidating the pathophysiology of syringomyelia. J Neurosurg 1999;91:553–62 [DOI] [PubMed] [Google Scholar]
- 3. Hofman E, Warmuth-Metz M, Bendzus M, et al. Phase-contrast MR imaging of the cervical CSF and spinal cord: volumetric motion analysis in patients with Chiari I malformation. AJNR Am J Neuroradiol 2000;21:151–58 [PMC free article] [PubMed] [Google Scholar]
- 4. Shah S, Haughton V, del Río AM.. CSF flow through the upper cervical spinal canal in Chiari I malformation. AJNR Am J Neuroradiol 2011;32:1149–53. Epub 2011 Apr 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Linge SO, Haughton V, Løvgren AE, et al. CSF flow dynamics at the craniovertebral junction studied with an idealized model of the subarachnoid space and computational flow analysis. AJNR Am J Neuroradiol 2010;31:185–92. Epub 2009 Sep 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roldan A, Wieben O, Haughton V, et al. Characterization of CSF hydrodynamics in the presence and absence of tonsillar ectopia by means of computational flow analysis. AJNR Am J Neuroradiol 2009;30:941–46. Epub 2009 Mar 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tatarek NE.. Variation in the human cervical neural canal. Spine 2005;5:623–31 [DOI] [PubMed] [Google Scholar]
- 8. Gepstein R, Folman Y, Sagiv P, et al. Does the anteroposterior diameter of the bony spinal canal reflect its size? An anatomical study. Surg Radiol Anat 1991;13:289–91 [DOI] [PubMed] [Google Scholar]
- 9. Struck AF, Haughton VM.. Idiopathic syringomyelia: phase-contrast MR of cerebrospinal fluid flow dynamics at level of foramen magnum. Radiology 2009;253:184–90. Epub 2009 Jun 30 [DOI] [PubMed] [Google Scholar]
- 10. Bogdanov EI, Heiss JD, Mendelevich EG, et al. Clinical and neuroimaging features of “idiopathic” syringomyelia. Neurology 2004;62:791–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sekula RF, Jr, Jannetta PJ, Casey KF, et al. Dimensions of the posterior fossa in patients symptomatic for Chiari I malformation but without cerebellar tonsillar descent. Cerebrospinal Fluid Res 2005;18:2–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The R Project for Statistical Computing. http://www.r-project.org. Accessed October 2011 [Google Scholar]
- 13. Milhorat TH, Bolognese PA, Nishikawa M, et al. Syndrome of occipitoatlantoaxial hypermobility, cranial settling, and Chiari malformation type I in patients with hereditary disorders of connective tissue. J Neurosurg Spine 2007;7:601–09 [DOI] [PubMed] [Google Scholar]
- 14. Stovner LJ, Bergan U, Nilsen G, et al. Posterior cranial fossa dimensions in the Chiari I malformation: relation to pathogenesis and clinical presentation. Neuroradiology 1993;35:113–18 [DOI] [PubMed] [Google Scholar]
- 15. Nishikawa M, Sakamoto H, Hakuba A, et al. Pathogenesis of Chiari malformation: a morphometric study of the posterior cranial fossa. J Neurosurg 1997;86:40–47 [DOI] [PubMed] [Google Scholar]
- 16. Nyland H, Krogness KG.. Size of posterior fossa in Chiari type 1 malformation in adults. Acta Neurochir (Wien) 1978;40:233–42 [DOI] [PubMed] [Google Scholar]