SUMMARY:
RCVS is a clinical condition of recurrent severe headaches that may be associated with ischemic or hemorrhagic stroke and that is defined by the presence of segmental vasoconstriction in multiple cerebral arteries. The angiographic appearance resembles vasculitis, except that the abnormalities resolve during the course of several months. Because the treatment of RCVS differs from that for vasculitis, radiologists must understand the clinical and radiologic features so as to better guide imaging algorithms and facilitate diagnosis. We present a series of 6 cases of RCVS that highlight the imaging features across multiple modalities.
RCVS is an under-recognized clinical-radiologic entity characterized by a history of sudden severe headaches, sometimes associated with ischemic or hemorrhagic stroke, focal neurologic deficits, or seizures. First described by Call et al in 19881, RCVS encompasses a wide variety of entities previously described by other names, including postpartum angiopathy, migrainous vasospasm, migrainous stroke, drug-induced angiopathy, and benign angiopathy of the CNS.2,3 The condition is defined by reversible segmental cerebral vasoconstriction on angiography.1–3 The angiographic findings are similar to those of other vasculopathies, including PACNS.4 Unlike PACNS, the vascular abnormalities of RCVS resolve within several months.1,2,5,6
RCVS typically occurs following exposure to a trigger, commonly sympathomimetic or vasoactive agents, including amphetamines, phenylpropanolamine, pseudoephedrine, serotonergic antidepressants, nicotine, caffeine, cannabis, and triptan- or ergot-containing medications.2,7–9 Other triggers include the peripartum period,10 eclampsia, strenuous physical activity, bathing or showering, sexual activity, and binge alcohol drinking.2 Most patients with RCVS are young and middle-aged women.3
Patients with RCVS exhibit a range of parenchymal abnormalities, including convexal SAH, IPH, ischemic infarcts, and PRES.2,9,11–13 Alternatively, imaging findings may be normal.2,9 Findings of CSF analysis are usually normal or near-normal.3 Because of the overlap with other causes of headache and stroke in adults and because the vascular abnormalities early in the course of the disease may be subtle or absent, the diagnosis of RCVS is easily missed. Early recognition of RCVS allows appropriate clinical management aimed at reducing the frequency, duration, and severity of vascular complications, primarily through identification and removal of triggers. Because management and outcome of RCVS differ from those of other vasculopathies, it is critical that radiologists recognize its typical imaging appearance, time course, and clinical features.
Case Series
Case 1
A 24-year-old woman with a history of diabetes and migraine headaches was 7 months postpartum when she developed severe episodic occipital headaches several days after minor head trauma. On physical examination, her blood pressure was elevated at 238/127 mm Hg. CSF showed mildly elevated protein but no markers of inflammation. Initial NCCT findings were normal, but on day 3, showed a right occipital IPH. Head CTA findings were normal. She was discharged home but returned to an outside hospital on day 15 with persistent headaches, new vision changes, and confusion. A diagnosis of vasculitis was made, but despite treatment with high-dose intravenous methylprednisolone, her symptoms worsened and she was transferred to our facility.
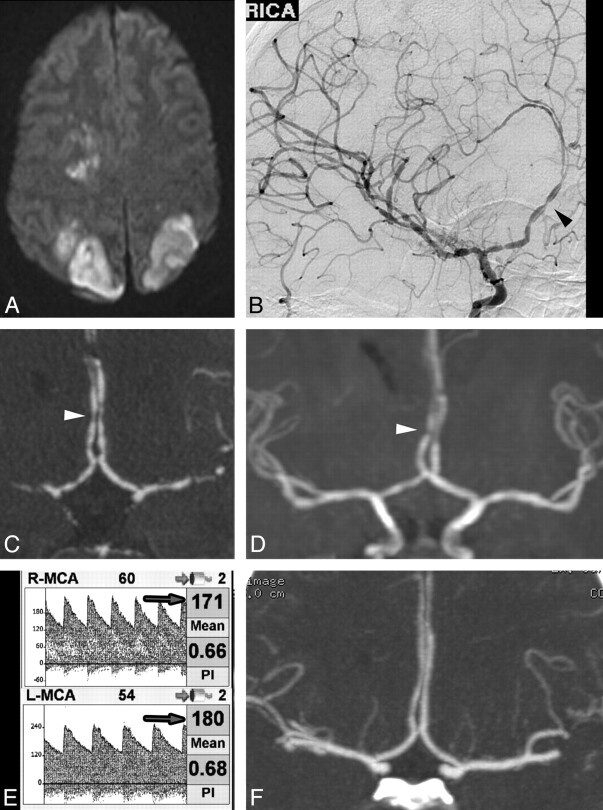
On initial examination, she was emotionally labile and oriented to year only. She could perceive light but could not count fingers. She had a left-sided facial droop and hemiplegia. Laboratory testing for autoimmune and infectious causes of vasculopathy was negative. MR imaging showed acute ischemic infarctions in multiple vascular territories bilaterally (Fig 1A) and a subacute IPH. DSA showed widespread segmental vasoconstriction and vasodilation, with a characteristic “sausages-on-a-string” appearance in multiple vessels (Fig 1B). Similar findings were evident on CTA (Fig 1C) and MRA (Fig 1D). TCD 1 week later showed flow-velocity characteristics consistent with moderate vasospasm (Fig 1E).
Fig 1.
A, MR imaging DWI sequence demonstrates acute infarctions in several vascular territories. Angiography (DSA, CTA, and MRA) performed at the same time reveals widespread areas of severe vascular narrowing alternating with vasodilation (arrowheads). B, DSA, right ICA transorbital oblique projection. C, CTA, coronal MIP reformat. D, MRA, coronal MIP reformat. E, TCD demonstrates elevated MCA flow velocities bilaterally (arrows). F, Coronal MIP reformat of CTA performed 11 months later demonstrates normalization of vessel caliber and contour.
She was treated with verapamil, a calcium channel blocker, and gradually her headaches resolved and her neurologic function improved. She had a persistent left inferior quadrantanopia and left hemiparesis, but she was able to ambulate with a cane and return to independent living. Follow-up CTA at 11 months (Fig 1F) demonstrated near-complete resolution of the vascular abnormalities.
This case illustrates severe vascular abnormalities of RCVS visible on MRA and CTA in addition to DSA. Complications of RCVS may be devastating and include IPH and stroke. Note that the initial anatomic imaging findings were normal, and the triggers of hypertension and minor head trauma were nonspecific. Thus, a high index of suspicion was needed to make the diagnosis.
Case 2
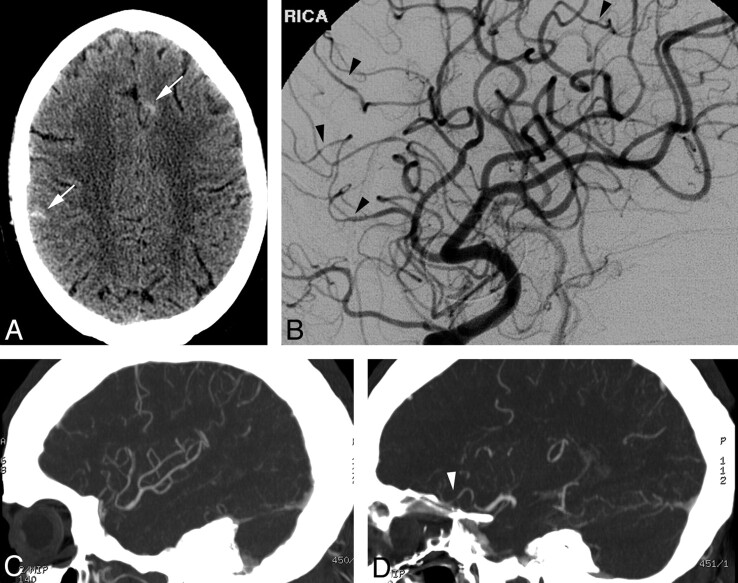
A 27-year-old man presented with headache, ataxia, and confusion after taking a “pick-me-up” pill. He smoked 1.5 packs of cigarettes per day and occasional marijuana. On examination, he was tachycardic and normotensive. Neurologic examination findings were nonfocal. Urine toxicology was positive for amphetamines and methamphetamines. NCCT (Fig 2A) and subsequent MR imaging (not shown) demonstrated scattered convexal SAH. CTA findings were normal. DSA performed that day (Fig 2B) showed multiple subtle irregularities of the small-to-medium-caliber vessels within the anterior and posterior circulations. Even on re-analysis of the CTA with dedicated MIP reformats of the abnormal vessels, CTA did not convincingly demonstrate the abnormalities found on DSA (Fig 2C, -D). Laboratory testing for systemic and infectious vasculopathies was negative. CSF analysis was consistent with presumed chemical meningitis related to SAH. On clinical follow-up, his symptoms were improved.
Fig 2.
A, NCCT demonstrates scattered sulcal SAH (arrows). B, DSA, right ICA oblique lateral projection, demonstrates subtle areas of vessel narrowing and dilation in several small-caliber arteries. C and D, MIP reformats from CTA performed the same day demonstrate normal large and medium right MCA branches (C) but do not convincingly demonstrate the abnormal small-caliber arteries (arrowhead, D), illustrating the limitations of CTA.
This case illustrates the presentation of RCVS as isolated convexal SAH. In this case, the trigger was likely amphetamines or cannabis. It also illustrates that subtle vascular abnormalities in RCVS may require DSA for diagnosis.
Case 3
A 21-year-old woman presented 1 day after the sudden onset of a severe bifrontal headache, accompanied by nausea, vomiting, and word-finding difficulties. She had drunk ≥5 energy drinks daily for the preceding several days, each containing 160 mg of caffeine. She smoked 1 pack of cigarettes per day. On examination, she was agitated and anxious. NCCT showed a moderate-sized left temporal IPH and tentorial SDH (Fig 3A). CTA findings were initially reported as normal, but in retrospect, there was subtle vascular irregularity in medium-sized MCA branches, highlighted on MIP reformat views (Fig 3B). DSA demonstrated marked segmental vasoconstriction in multiple small- and medium-caliber vessels (Fig 3C). TCD obtained a few days later demonstrated slightly elevated MCA flow velocities, consistent with hyperdynamic flow (data not shown). Laboratory testing for systemic and infectious vasculopathies was negative. She was treated with nimodipine for 3 weeks, with subsequent improvement in headache severity and resolution of word-finding difficulties. Follow-up DSA after 2 months showed complete resolution of the vascular abnormalities (Fig 3D).
Fig 3.
A, NCCT demonstrates left temporal IPH (asterisk) and tentorial SDH (arrow). B, CTA MIP reformat, demonstrates subtle irregularity of the distal right MCA branches (not prospectively recognized). C, DSA, right common carotid artery, Haughton projection, shows multiple areas of vascular narrowing of medium- and small-caliber vessels (arrowheads). D, Findings resolved on follow-up DSA, right ICA Haughton projection, 2 months later.
This case illustrates a third presentation of RCVS: IPH and SDH. The trigger was likely caffeine or other energy drink components. While in retrospect the vascular changes were evident on CTA, the findings were extremely subtle and the diagnosis would be difficult to make prospectively, illustrating the utility of DSA for the diagnosis of vasculopathy. This case also illustrates the hallmark of this disease: reversal of the vascular abnormalities after removal of triggers, possibly hastened by short-term use of calcium channel blockers.
Case 4
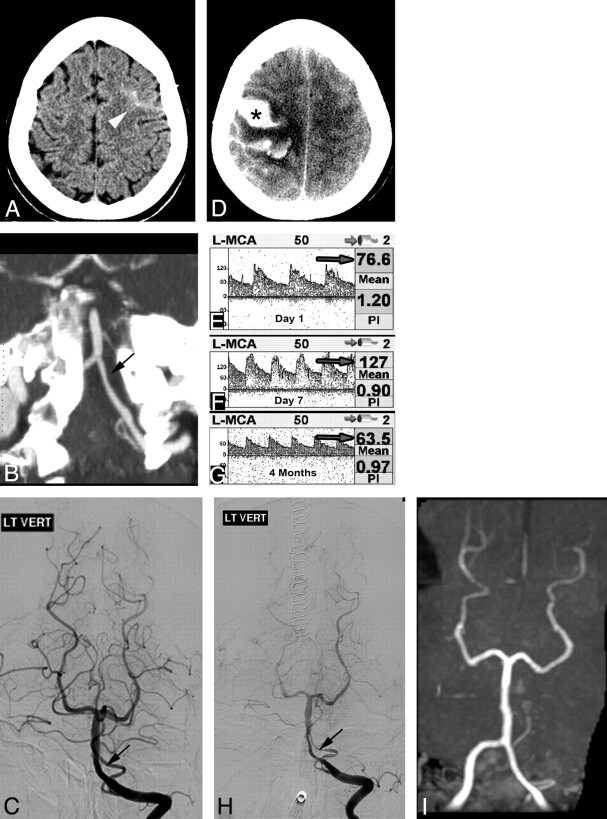
A 36-year-old woman developed a severe occipital headache while diving into cold water. Her headache recurred episodically during the next week. On initial examination, her blood pressure was mildly elevated, but neurologic examination, NCCT, and CSF evaluation findings were all normal. She was treated with antiemetics, NSAIDs, dexamethasone, sumatriptan, and dihydroergotamine. On day 4, repeat NCCT showed a left frontal IPH, which was surgically evacuated. On day 7, NCCT showed a new right IPH, new SDH, and an evolving subacute left IPH (Fig 4A). MRA showed irregular narrowing of multiple MCA branches bilaterally (Fig 4B), worse on the left. DSA confirmed multiple areas of segmental vasoconstriction (Fig 4C). The extent of vascular abnormality appeared overestimated on MRA, likely due to decreased flow-related signal intensity resulting from focal vessel narrowing of the M2 branches after the bifurcation. Extensive laboratory investigations were negative except for elevated C-reactive protein at 40 mg/L and weakly positive antinuclear antibody results at 1:40. Verapamil treatment was instituted, and her headaches ceased. Follow-up DSA 3 weeks later showed significant improvement in vascular abnormalities, which resolved completely on subsequent DSA 3 months later (Fig 4D).
Fig 4.
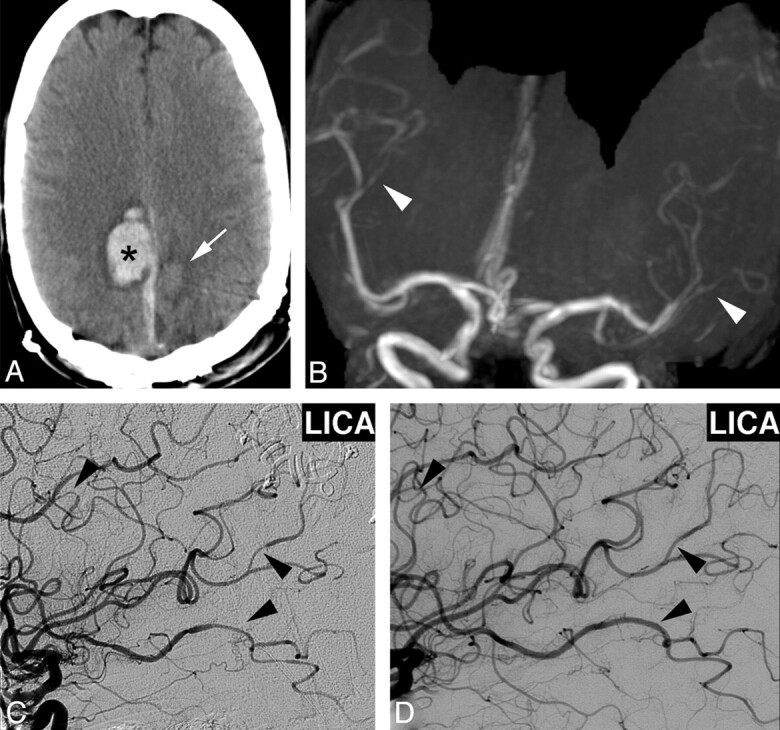
A, NCCT 1 week after initial presentation demonstrates right posterior frontal IPH (asterisk), right parafalcine SDH, and subacute left posterior frontal IPH (arrow). B and C, MRA coronal MIP reformat shows irregular narrowing of distal MCA branches (white arrowheads, B), which was confirmed on DSA, left ICA injection (arrowheads, C). D, Repeat DSA 3 months later demonstrates resolution of vascular findings.
This case illustrates the potentially delayed nature of the complications of RCVS, including IPH and SDH. The initial trigger was likely cold water diving. If the diagnosis had been suspected earlier, this patient might not have received sumatriptan and dihydroergotamine, which are vasoactive medications that likely exacerbated her vasoconstrictive response.
Case 5
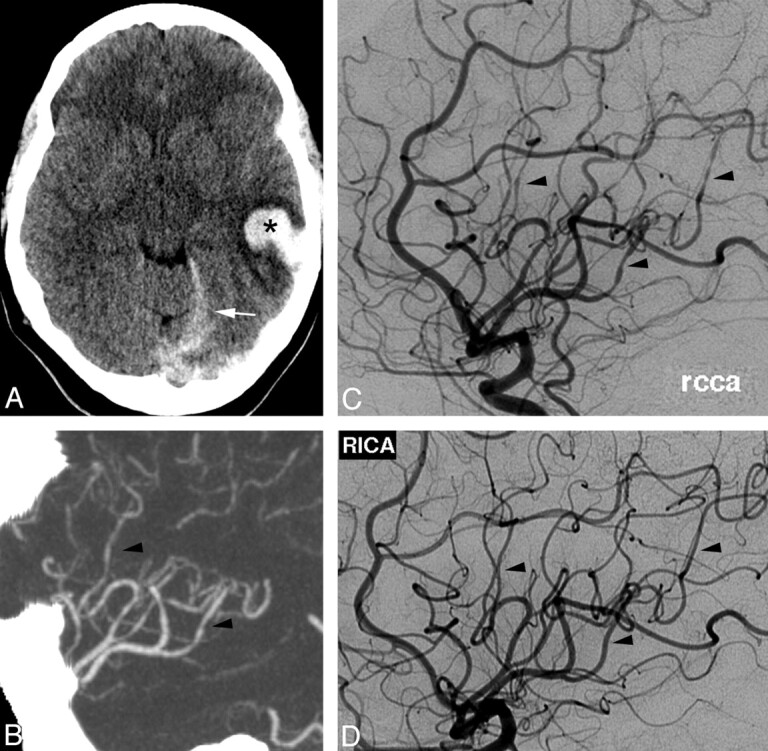
A 56-year-old woman with depression and migraine headaches presented several days after a thunderclap headache accompanied by nausea and vomiting. She sought medical attention after her headache recurred while swimming in hot springs. Her medications included a serotonergic antidepressant. Physical and neurologic examination and laboratory investigation findings were normal. NCCT showed convexal SAH (Fig 5A). CTA (not shown) and DSA (Fig 5B) findings were normal, as was TCD performed 2 days later (Fig 5C). Repeat TCD on day 4 showed increasing flow velocities (Fig 5D). Repeat DSA on day 5 showed segmental vasoconstriction and vasodilation in multiple vessels including the extracranial circulation (Fig 5E) and bilateral posterior cerebral arteries (Fig 5F). She was treated with nimodipine, and her headaches resolved. She has had no further severe headaches on follow-up 9 months after discharge.
Fig 5.
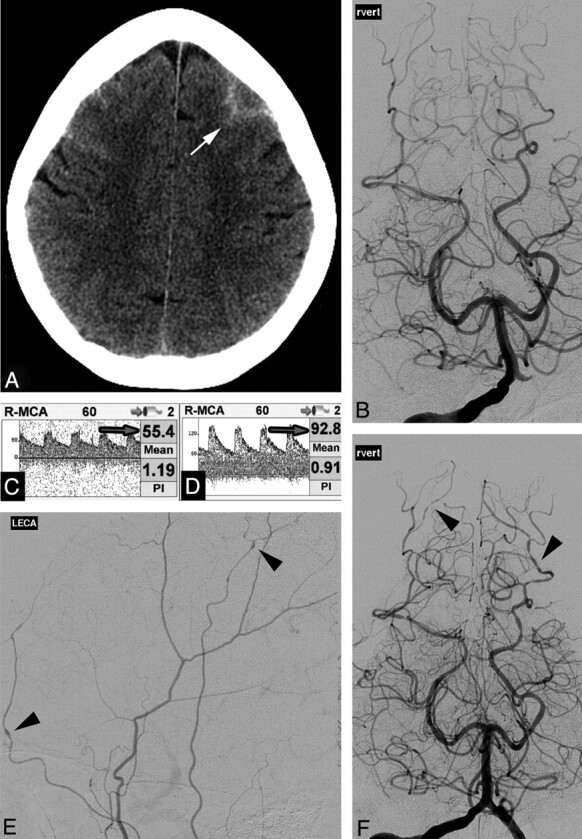
A, NCCT demonstrates frontal convexal SAH (arrow). B, DSA findings, right vertebral injection, are normal on presentation. C and D, Right MCA TCD demonstrates normal flow velocities (arrow, C), which increased on day 4 (arrow, D). E and F, Repeat DSA 5 days after normal findings on the initial study demonstrates multifocal areas of constriction and dilation in the extracranial circulation (left external carotid artery injection, arrowheads, E) and intracranial circulation (vertebral injection, arrowheads, F).
This case illustrates a key challenge in diagnosing RCVS: the delayed onset of radiographically evident vascular abnormalities. TCD in this case was helpful in detecting the earliest vascular abnormalities. Inciting factors in this case included migraine headaches, a serotonergic antidepressant, and hot water. This case also demonstrates that vascular abnormalities may occur in the external circulation, as previously reported.9
Case 6
A 62-year-old woman with frequent migraine headaches and depression presented with the sudden onset of a severe headache accompanied by visual disturbance. Her symptoms were unlike her usual migraines. Her medications included a serotonergic antidepressant. She smoked marijuana daily. NCCT findings were initially normal, but CSF revealed 57 red blood cells. On examination, blood pressure was mildly elevated at 140/70 mm Hg. Neurologic examination findings were nonfocal. Laboratory investigation findings were normal except for an elevated erythrocyte sedimentation rate at 30 mm/hr. Repeat NCCT showed a small left frontal SAH (Fig 6A). CTA findings were normal except for a subtle irregularity of the left intradural vertebral artery (Fig 6B). DSA the following day was interpreted as having normal findings, but in retrospect, there was a mild irregularity of the left vertebral artery (Fig 6C). MR imaging (not shown) redemonstrated SAH, but findings were otherwise normal. TCD on day 2 demonstrated mildly elevated flow velocities (Fig 6E).
Fig 6.
A, NCCT on admission shows convexal SAH (arrowhead). B and C, CTA, oblique coronal MIP reformat (B), suggests subtle left vertebral irregularity, but DSA findings are normal except for questionable irregularity in the left vertebral artery (arrow) not appreciated prospectively (C). E, TCD on admission demonstrates mildly elevated left MCA flow velocity (arrow). D, Repeat NCCT at day 5 demonstrates new IPH (asterisk) with worsened mass effect. F, TCD on day 7 demonstrates increased flow velocity consistent with vasospasm (arrow). H, Repeat DSA shows marked segmental vasoconstriction and poor contrast opacification of vessels. Follow-up TCD at 4 months (G) demonstrates normalization of flow velocities, and MRA shows resolution of vascular abnormalities (I).
Despite treatment with nimodipine, antiemetics, naproxen, and opioids, her headaches persisted and she developed left-sided weakness and somnolence on day 5. Repeat NCCT (Fig 6D) showed new right frontoparietal and occipital IPHs, with associated midline shift that required decompressive hemicraniectomy. Repeat TCD on day 7 demonstrated increasing flow velocities bilaterally (Fig 6F). Repeat DSA showed marked diffuse narrowing of all cerebral vessels, with segmental vasoconstriction involving multiple vessels (Fig 6H). Repeat TCD findings at 4 months were normal (Fig 6G). Follow-up MRA showed significant improvement in vascular abnormalities (Fig 6I). She improved clinically but did have persistent left spastic hemiparesis.
This case illustrates the potentially devastating sequelae of RCVS despite accurate diagnosis and early treatment. Inciting factors in this case included migraine headaches, cannabis, and a serotonergic antidepressant. As in the previous case, the angiographic findings were delayed, and TCDs were helpful in documenting initial vasospasm.
Discussion
RCVS is gaining recognition clinically but may not be familiar to radiologists. This case series illustrates the typical clinical and imaging presentations of RCVS, including the time course of development and resolution of vascular abnormalities. Patients with RCVS may present with hemorrhage, infarctions, or PRES.2,5,12,14 If the initial head CT findings are normal, hemorrhage or infarctions may develop during the course of the illness. RCVS-induced intracranial hemorrhage is more common in women and people with migraine headaches.11 In patients younger than 60 years of age, RCVS is reportedly a common cause of nontraumatic convexal SAH.12 Vascular imaging findings may be evident on DSA, CTA,15 MRA,5 or TCD16 and include multifocal segmental constrictions alternating with focal dilations or normal-caliber vessels3 in multiple vascular distributions. The vascular abnormalities typically resolve by 3 months.2,3,5,6 Initial vascular imaging findings may be normal, with abnormalities developing during days to weeks.2 Thus, despite normal angiography findings, the diagnosis of RCVS must be considered in the appropriate clinical setting.
Resolution of angiographic abnormalities within 3 months of the initial episode is the hallmark of RCVS. The clinical course, however, varies depending on the extent of brain parenchymal injury. A recent study suggests that patients with RCVS with either IPH or SAH have a more severe clinical course.11
The imaging findings of RCVS may be similar to those found in PRES. While the classic imaging findings in PRES include vasogenic edema in the parietal, occipital, and posterior frontal lobes, restricted diffusion may also be present. PRES may also present with intracranial hemorrhage, including isolated SAH.17 Vasculopathy can occur in PRES, with vasoconstriction, focal vasodilation, or a string-of-beads appearance in the second- and third-order arteries visible on DSA and MRA.18 There may be an overlap between these 2 entities because Chen et al5 reported a correlation between involvement of the P2 segment of the posterior cerebral artery in RCVS and the presence of concomitant PRES and hypothesized a similar pathogenesis underlying these 2 entities.
The evaluation, treatment, and outcome of RCVS differ markedly from those of PACNS,19 which often requires brain biopsy for diagnosis.20 Unlike RCVS, PACNS typically is associated with marked CSF pleocytosis. Treatment for PACNS is more aggressive, often including steroids and other immunosuppressant agents with substantial adverse effects. Untreated, PACNS has a clinically progressive course with severe neurologic impairment, often resulting in coma or death within 1 year. In contrast, the diagnosis of RCVS does not require brain biopsy, the condition is usually not progressive, and identification and elimination of the causative vasoactive agent or stimulus can effectively treat the syndrome.7 Short-term treatment with calcium channel antagonists is often used as supplemental therapy,3,21–23 though a recent retrospective analysis did not find calcium channel blockers to have a significant effect on outcome.9 Others have reported intra-arterial therapy with milrinone,24 verapamil,25 or balloon angioplasty.25 Overall, RCVS has a better prognosis than PACNS, particularly when recognized early.
Standard imaging algorithms for RCVS have not been established.3,19,23 While DSA is probably the criterion standard for diagnosis, CTA and MRA may be useful alternatives for diagnosis or follow-up. The potential limitations of CTA or MRA in the diagnosis of RCVS include decreased sensitivity for subtle cases or those predominantly affecting small-caliber vessels. TCD findings are abnormal in 69%–81% of patients with RCVS2,16 and may be another useful adjunct test. Because confident diagnosis and follow-up of RCVS require serial vascular evaluations, it is reasonable to develop imaging algorithms that minimize the risk of radiation and contrast.
While the sensitivity and specificity of various imaging modalities for RCVS have not been established, CTA and MRA have been evaluated for other intracranial vascular diseases. In a study of intracranial atherosclerosis, both CTA and MRA were found to be sensitive for detecting vessel stenosis in the circle of Willis and proximal cerebral arteries (first- and second-order vessels), though CTA was more sensitive than MRA (98% versus 70%, P < .001).26 RCVS can involve large- and medium-caliber vessels but may be limited to small-caliber distal vessels, in which case CTA and MRA may be less sensitive.
For the evaluation of small-caliber vessels, Villablanca et al27 found that CTA was capable of detecting most (≥90%) of the targeted small intracranial vessels depicted on DSA, with the smallest vessel size detected by multidetector row CT being 0.7 mm, compared with 0.4 mm for DSA. Despite finding CTA capable of detecting small-caliber vessels, the study authors did not advocate CTA in the initial diagnosis of small-vessel disease such as vasculitis due to the better visualization of the distal portions of these vessels on DSA. Similarly, Agid et al28 found that while small-vessel irregularity diagnosed on DSA can be retrospectively detected on CTA, prospective diagnosis can be difficult. Both groups advocated DSA for the initial diagnosis of vasculitis.
Conclusions
RCVS is an increasingly recognized cause of intracranial hemorrhage, infarction, and PRES in younger adult patients who responds well to early removal of triggers and possibly with treatment with calcium channel blockers. The diagnosis requires a high index of suspicion, including recognition of the typical presentation, the patient population at risk, and the usual inciting stimuli. Timely diagnosis and treatment can potentially minimize the complications of the disease. With appropriate treatment, there is the potential to prevent debilitating neurologic deficits.
ABBREVIATIONS:
- IPH
intraparenchymal hemorrhage
- MIP
maximum intensity projection
- NSAID
nonsteroidal anti-inflammatory drug
- PACNS
primary angiitis of the CNS
- PRES
posterior reversible encephalopathy syndrome
- RCVS
reversible cerebral vasoconstriction syndrome
- SDH
subdural hematoma
- TCD
transcranial Doppler
References
- 1. Call GK, Fleming MC, Sealfon S, et al. Reversible cerebral segmental vasoconstriction. Stroke 1988;19:1159–70 [DOI] [PubMed] [Google Scholar]
- 2. Ducros A, Boukobza M, Porcher R, et al. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome: a prospective series of 67 patients. Brain 2007;130:3091–101 [DOI] [PubMed] [Google Scholar]
- 3. Calabrese LH, Dodick DW, Schwedt TJ, et al. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med 2007;146:34–44 [DOI] [PubMed] [Google Scholar]
- 4. Gerretsen P, Kern RZ. Reversible cerebral vasoconstriction syndrome or primary angiitis of the central nervous system? Can J Neurol Sci 2007;34:467–77 [PubMed] [Google Scholar]
- 5. Chen SP, Fuh JL, Wang SJ, et al. Magnetic resonance angiography in reversible cerebral vasoconstriction syndromes. Ann Neurol 2010;67:648–56 [DOI] [PubMed] [Google Scholar]
- 6. Hajj-Ali RA, Furlan A, Abou-Chebel A, et al. Benign angiopathy of the central nervous system: cohort of 16 patients with clinical course and long-term followup. Arthritis Rheum 2002;47:662–69 [DOI] [PubMed] [Google Scholar]
- 7. Singhal AB, Caviness VS, Begleiter AF, et al. Cerebral vasoconstriction and stroke after use of serotonergic drugs. Neurology 2002;58:130–33 [DOI] [PubMed] [Google Scholar]
- 8. Koopman K, Teune LK, ter Laan M, et al. An often unrecognized cause of thunderclap headache: reversible cerebral vasoconstriction syndrome. J Headache Pain 2008;9:389–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singhal AB, Hajj-Ali RA, Topcuoglu MA, et al. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol 2011;68:1005–12 [DOI] [PubMed] [Google Scholar]
- 10. Singhal AB, Benstein RA. Postpartum angiopathy and other cerebral vasoconstriction syndromes. Neurocrit Care 2005;3:91–97 [DOI] [PubMed] [Google Scholar]
- 11. Ducros A, Fiedler U, Porcher R, et al. Hemorrhagic manifestations of reversible cerebral vasoconstriction syndrome: frequency, features, and risk factors. Stroke 2010;41:2505–11 [DOI] [PubMed] [Google Scholar]
- 12. Kumar S, Goddeau RP, Jr, Selim MH, et al. Atraumatic convexal subarachnoid hemorrhage: clinical presentation, imaging patterns, and etiologies. Neurology 2010;74:893–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Edlow BL, Kasner SE, Hurst RW, et al. Reversible cerebral vasoconstriction syndrome associated with subarachnoid hemorrhage. Neurocrit Care 2007;7:203–10 [DOI] [PubMed] [Google Scholar]
- 14. Moustafa RR, Allen CM, Baon JC. Call-Fleming syndrome associated with subarachnoid haemorrhage: three new cases. J Neurol Neurosurg Psychiatry 2008;79:602–05 [DOI] [PubMed] [Google Scholar]
- 15. Rosenbloom MH, Sighal AB. CT angiography and diffusion-perfusion MR imaging in a patient with ipsilateral reversible cerebral vasoconstriction after carotid endarterectomy. AJNR Am J Neuroradiol 2007;28:920–22 [PMC free article] [PubMed] [Google Scholar]
- 16. Chen SP, Fuh JL, Chang FC, et al. Transcranial color Doppler study for reversible cerebral vasoconstriction syndromes. Ann Neurol 2008;63:751–57 [DOI] [PubMed] [Google Scholar]
- 17. Batynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol 2008;29:1036–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bartynski WS, Bordman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol 2008;29:447–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benstein RA. Reversible cerebral vasoconstriction syndromes. Curr Treat Options Cardiovasc Med 2006;8:229–34 [DOI] [PubMed] [Google Scholar]
- 20. Kadkhodayan Y, Alreshaid A, Moran CJ, et al. Primary angiitis of the central nervous system at conventional angiography. Radiology 2004;233:878–82 [DOI] [PubMed] [Google Scholar]
- 21. Nowak DA, Rodiek SO, Henneken S, et al. Reversible segmental cerebral vasoconstriction (Call-Fleming syndrome): are calcium channel inhibitors a potential treatment option? Cephalalgia 2003;23:218–22 [DOI] [PubMed] [Google Scholar]
- 22. Chen SP, Fuh JL, Lirng JF, et al. Recurrent primary thunderclap headache and benign CNS angiopathy: spectra of the same disorder? Neurology 2006;67:2164–69 [DOI] [PubMed] [Google Scholar]
- 23. Sattar A, Manousakis G, Jensen MB. Systematic review of reversible cerebral vasoconstriction syndrome. Expert Rev Cardiovasc Ther 2010;8:1417–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bouchard M, Verreault S, Gariepy JL, et al. Intra-arterial milrinone for reversible cerebral vasoconstriction syndrome. Headache 2009;49:142–45 Epub 2008 Jul 21 [DOI] [PubMed] [Google Scholar]
- 25. Farid H, Tatum JK, Wong C, et al. Reversible cerebral vasoconstriction syndrome: treatment with combined intra-arterial verapamil infusion and intracranial angioplasty. AJNR Am J Neuroradiol 2011;32:E184–87 Epub 2011 Jan 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bash S, Villablanca JP, Jahan R, et al. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am J Neuroradiol 2005;26:1012–21 [PMC free article] [PubMed] [Google Scholar]
- 27. Villablanca JP, Rodriguez FJ, Stockman T, et al. MDCT angiography for detection and quantification of small intracranial arteries: comparison with conventional catheter angiography. AJR Am J Roentgenol 2007;188:593–602 [DOI] [PubMed] [Google Scholar]
- 28. Agid R, Andersson T, Almqvist H, et al. Negative CT angiography findings in patients with spontaneous subarachnoid hemorrhage: when is digital subtraction angiography still needed? AJNR Am J Neuroradiol 2010;31:696–705 [DOI] [PMC free article] [PubMed] [Google Scholar]