Abstract
BACKGROUND AND PURPOSE:
Little is known about how commonly the internal jugular vein is compressed by extrinsic structures in the upper neck. The purpose of this paper was to identify the frequency and cause of external compression of the superior segment of the internal jugular vein.
MATERIALS AND METHODS:
Retrospective review of CT angiograms of the neck was performed in 108 consecutive patients. Axial source images were evaluated for moderate (>50%) or severe (>80%) stenosis of the internal jugular vein on the basis of external compression. The cause of extrinsic compression was also recorded. In cases with stenosis, the presence of ipsilateral isoattenuated collateral veins was recorded and considered representative of collateral flow.
RESULTS:
Moderate stenosis was seen in 33.3% of right and 25.9% of left internal jugular veins. Severe stenosis was seen in 24.1% of right and 18.5% of left internal jugular veins. The most common causes of extrinsic compression included the styloid process and the posterior belly of the digastric muscle. In patients with severe internal jugular vein stenosis, 53.8% of right sides and 55% of left sides had associated condylar collaterals.
CONCLUSIONS:
Extrinsic compression of the superior segment of the internal jugular vein is a common finding in unselected patients, often caused by the styloid process or the posterior belly of the digastric muscle. Presence of severe stenosis is not universally associated with collateral formation.
Little is known about how commonly the internal jugular veins are subject to extrinsic compression in the upper neck. Recent literature has suggested that restriction of extracranial venous drainage may be a risk factor in the development of MS.1–3 Published series ofg treatment with angioplasty and/or stent placement have begun to emerge based on this yet unproved hypothesis.4,5 Jugular venous pathology has also been linked with transient global amnesia6,7 and migraines,8–11 though this is controversial11 and no clear etiologic link to venous pathology has been established for either disease.
To better understand the significance of extracranial IJV stenosis, we need to have a better understanding of normal jugular venous anatomy and associated variants. We are familiar with the concept of jugular asymmetry, where 1 IJV may be larger than the other due to preferential intracranial venous drainage through 1 sigmoid sinus versus the other. However, normal variants and their incidence in the extracranial IJV circulation are not well documented. The purpose of this article is to identify the frequency and cause of extrinsic compression of the upper jugular vein in consecutive unselected patients presenting for CT angiography of the neck.
Materials and Methods
Institutional review board approval was obtained for this retrospective analysis. The PACS at a large teaching hospital was queried for all consecutive patients who underwent an isolated CTA of the neck for evaluation of traumatic injury, atherosclerotic disease, or possible dissection between July 1, 2009 and June 30, 2010. Normal controls were not included. Pediatric patients were excluded, as were those in whom the CTA of the neck was combined with other contrast-enhanced imaging (such as brain or chest CTA). The provided indication for the examination was reviewed, and none of the studies were completed for suspected venous pathology. A total of 108 studies were then reviewed independently by 1 of 5 Certificate of Added Qualification–certified neuroradiologists (M.V.J., J.L.B., L.M.D., R.A.H., J.M.R.), with 5–25 years of postfellowship experience.
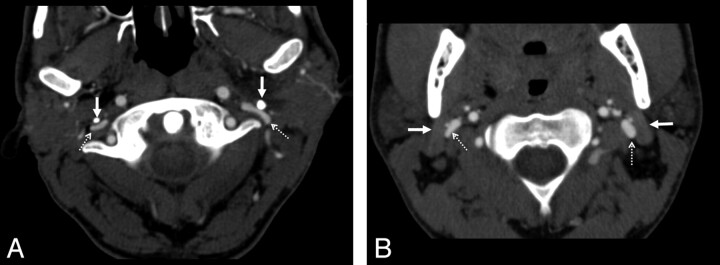
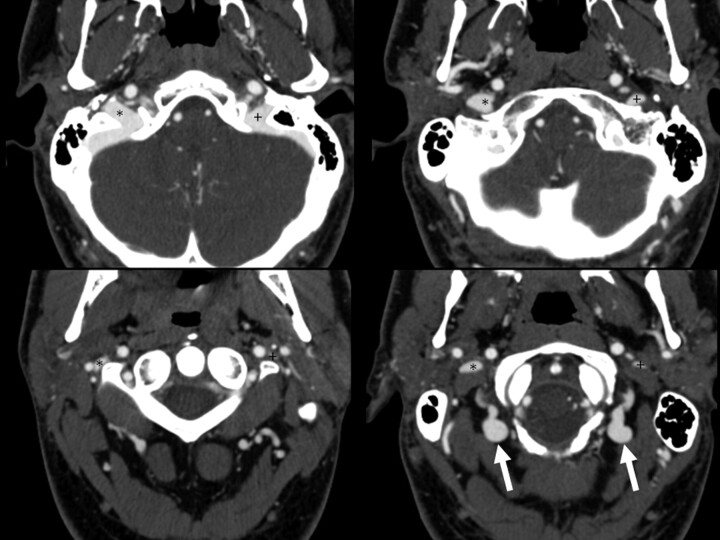
All patients were scanned in neutral supine position. For each study, 4 parameters were scored. The first parameter was jugular dominance, as determined by which jugular bulb was larger in diameter. The second was the presence of either moderate (>50%) or severe (>80%) stenosis caused by extrinsic compression, based on diameter reduction on axial source images compared with diameter of normal vein proximal to the stenosis (Fig 1). The largest diameter of the vein proximal to the stenosis, but distal to the jugular bulb, was used as the reference diameter. The segment of vein analyzed was from the superior jugular bulb down to the bottom of the C3 vertebral body. We chose this segment because we felt that the IJV below that level is unlikely to be subjected to extrinsic compression by osseous or muscular causes. The third parameter was the cause of the extrinsic compression (styloid process, posterior belly of the digastric muscle, C1 transverse process). Examples are given in Fig 1. In all cases, the compression was caused by 2 factors. The posteromedial aspect of the compression was caused by the adjacent cervical vertebra, which provides a noncompressible structure against which the vein is pressed. It is the anterolateral cause that varies and was recorded. The fourth parameter scored was the presence of significant collaterals (Fig 2). In order for collaterals to be considered significant, the attenuation of contrast in the collateral veins would need to be similar to that in the adjacent ipsilateral sigmoid sinus or jugular bulb, implying that venous drainage from the brain was going through these veins (Fig 3). This primarily refers to the lateral and posterior condylar veins and their recipient upper deep cervical veins. San Millán Ruíz et al provide an elegant description of the relevant anatomy of this region in their review.12
Fig 1.
Examples of extrinsic compression by the styloid process and the posterior belly of the digastric muscle. A, In this axial CT image, there is bilateral compression of the internal jugular veins (dashed arrows) by the styloid processes (solid arrows). The vein takes a pancake-like configuration at this level. B, Similar extrinsic compression, caused by the posterior belly of the digastric muscle (solid arrows) of both internal jugular veins (dashed arrows), though to a lesser degree. In both cases, the vein is compressed anterolaterally by the described structure (styloid process or digastric muscle). Along the medial and posteromedial aspect of the vein, the adjacent vertebra is the most rigid structure and will provide the other side of the extrinsic compressive “pincer.”
Fig 2.
Example of bilateral collateral vein filling. Four axial images from a CTA of the neck demonstrate bilateral posterior condylar and upper deep cervical vein filling (arrows). Note how contrast in these veins is isoattenuated with the jugular bulb. The left (+) and right (*) internal jugular veins are marked.
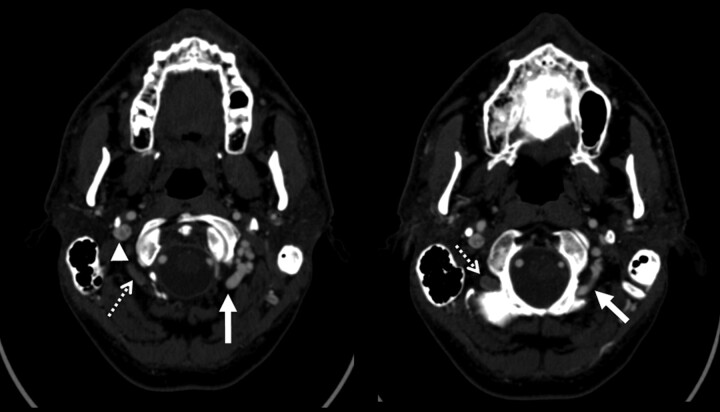
Fig 3.
Examples of condylar veins with and without collateral flow. Two axial images from a CTA of the neck demonstrate differential contrast attenuation in the condylar veins. The left condylar veins (solid arrows) are isoattenuated with the right internal jugular vein (arrowhead). The left internal jugular vein is markedly attenuated. Note that the right-sided condylar veins (dashed arrows) have similar caliber to the left but are not filled with contrast of the same attenuation. This would suggest that the venous drainage of the brain in this patient does use the left condylar veins but not the right.
Once the data were tabulated, the percentage of patients with moderate and severe stenoses of each or both jugular veins was tabulated, as was the percentage of patients with collaterals for each group. The scores were calculated independently for right and left jugular veins. In addition, we also scored the percentage of patients with moderate or severe stenosis of the dominant jugular vein (or bilateral stenosis, if codominant).
To determine whether other literature has described the incidence of these findings in the past, we performed a National Library of Medicine (PubMed) search with the terms “extrinsic compression internal jugular vein” and, as of August 16, 2011, only 5 search items were returned. None of these specifically addressed the issue of extrinsic compression of the internal jugular vein in the upper neck.
Results
Of the 108 consecutive patients reviewed, 53 (49.1%) were right jugular dominant, 21 (19.4%) were left jugular dominant, and 34 (31.5%) were codominant. The distribution of patients with moderate and severe stenosis by jugular vein are detailed in Table 1.
Table 1:
Relationship between the presence or absence of collaterals based on the degree of cross-sectional narrowing of the 108 jugular veins analyzed on each side
| Collaterals Present | No Collaterals Present | |
|---|---|---|
| Right internal jugular vein, degree of stenosis | ||
| None (n = 46; 42.6%) | 9/46 (19.6%) | 37/46 (80.4%) |
| Moderate (n = 36; 33.3%) | 14/36 (38.9%) | 22/36 (61.1%) |
| Severe (n = 26; 24.1%) | 14/26 (53.8%) | 12/26 (46.2%) |
| Left internal jugular vein, degree of stenosis | ||
| None (n = 60; 55.6%) | 13/60 (21.7%) | 47/60 (78.3%) |
| Moderate (n = 28; 25.9%) | 10/28 (35.7%) | 18/28 (64.3%) |
| Severe (n = 20; 18.5%) | 11/20 (55%) | 9/20 (45%) |
Interestingly, only 35 patients (32.4%) had 2 widely patent upper jugular veins. When evaluating the relationship between the presence of stenosis with condylar collateral formation, we noted that collateral filling was more commonly seen in conjunction with stenosis, but 19.6% of normal right and 21.7% of normal left jugular veins had ipsilateral isoattenuated condylar vein filling on CTA, and nearly half of the patients with severe stenoses had no collateral formation (Table 1).
Because substantial asymmetry in jugular drainage can exist, we sought to examine the relationship between the presence of any moderate or severe compressive stenosis of the dominant jugular vein (or, bilaterally, of codominant veins) and collateral formation. These results are summarized in Table 2. Indeed, only 10/18 (55.6%) patients with stenosis of the dominant jugular vein had ipsilateral collateral filling. The cause of the extrinsic narrowing for right and left sides separately is listed in Table 3.
Table 2:
Per patient analysis of flow pattern, and the presence or absence of collaterals on the side of the dominant jugular vein (or bilateral, if codominant)
| Overall (n = 108) | Collaterals Present | No Collaterals Present | |
|---|---|---|---|
| No stenosis bilaterally | 35 (32.4%) | ||
| Mild or moderate stenosis of nondominant jugular vein | 24 (22.2%) | ||
| Moderate stenosis of dominant jugular vein | 31 (28.7%) | 11/31 (35.5%) | 20/31 (64.5%) |
| Severe stenosis of dominant jugular vein | 18 (16.7%) | 10/18 (55.6%) | 8/18 (44.4%) |
Table 3:
Causes of moderate or severe narrowing by side
| Cause | Right Side (n = 108) | Left Side (n = 108) |
|---|---|---|
| Styloid process | 26/108 (24.1%) | 33/108 (30.6%) |
| Digastric muscle | 26/108 (24.1%) | 12/108 (11.1%) |
| Artery (ECA, ICA, or branches thereof) | 3/108 (2.8%) | 2/108 (1.8%) |
| Styloid process and digastric muscle | 5/108 (4.6%) | 0 |
| Other | 2/108 (1.9%) | 1/108 (0.9%) |
| None | 46/108 (42.6%) | 60/108 (55.6%) |
Note:—See Figure 1 for examples of narrowing by the styloid process and posterior belly of the digastric muscle. ECA indicates external carotid artery; ICA, internal carotid artery.
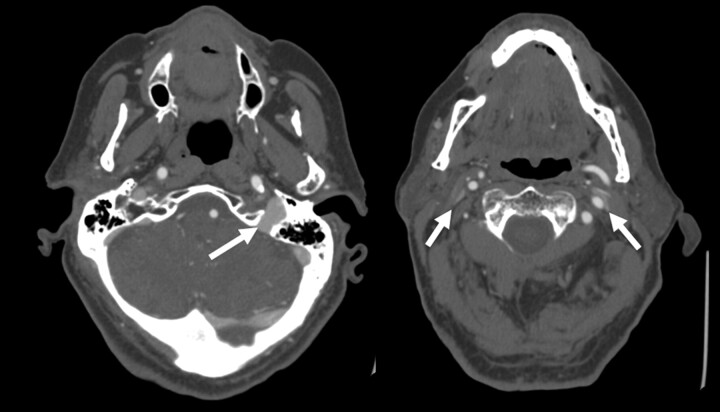
Severe bilateral extrinsic compression was seen in 10/108 patients (9.3%) and, of these patients, 8/10 (80%) had collateral filling. We find it interesting that even with severe, bilateral stenosis, collateral filing was not universally present (Fig 4). The cause(s) of moderate or severe narrowing by side are summarized in Table 3.
Fig 4.
Example of severe bilateral jugular venous stenosis without collateral formation. Two axial images from a CTA of the neck demonstrate a dominant left internal jugular vein (arrow, left image). The more caudal image (right) shows severe pancake-like flattening of the internal jugular veins bilaterally (arrows) but with no condylar collateral filling seen.
Discussion
Little has been published about the incidence of extrinsic compression of the internal jugular veins in the superior segment (between the jugular foramen and the level of C3). Our goal was to describe the frequency and causes of extrinsic compression of the internal jugular vein in unselected patients. We believe this has potential implications with respect to purported relationships between extracranial venous stenosis and demyelinating disease.3,4
We found that extrinsic compression of the internal jugular vein is common in unselected patients. The most common causes of this are the styloid process or posterior belly of the digastric muscle, often adjacent to the lateral mass of C1. One could postulate that the positional variability in venous drainage may play a role in collateral formation. When humans are upright, the predominant venous drainage of the brain is via the vertebral venous plexus, rather than the internal jugular vein.12–15 In contrast, the jugular veins are the dominant drainage for the supine patient.12,15 One described major connecting pathway is the anterior condylar confluence, which has been shown to have connections between the jugular bulb and the anterior, lateral, and posterior condlylar veins, and may have a significant role in the postural redirection of flow.12
Most interestingly, the development of collaterals was not consistently seen, even with severe stenosis. In fact, only 10 of 18 patients (55.6%) with severe stenosis of the dominant jugular (or bilateral, if codominant) had condylar collaterals. Given the variability in the presence of these collaterals in unselected patients, we feel that it is unlikely that these stenoses are of physiologic significance. In many other regions of the body, venous stenoses are considered significant when collateral vessels are found.
Our study has limitations. First, this is a retrospective series of patients with a neurologic reason for undergoing the CTA, which could be different from a control population of normal subjects. However, given the large number of patients in our study, we feel that the incidence of jugular stenosis is likely representative of a larger cohort of patients. If future studies of control patients are performed, MRV may be preferable to CTV to eliminate radiation exposure. However, in our study, the CT had the advantage (compared with MR) of allowing us to better visualize the cause of the extrinsic compression. Finally, we did all imaging with the patient in a supine position and the head in a neutral position. It is possible that turning the head may change the degree of compression.
Conclusions
Extrinsic compression of the superior segment of the internal jugular vein (between the jugular foramen and C3) is a common finding in unselected patients, caused by either the styloid process or the posterior belly of the digastric muscle and the transverse process of an adjacent cervical vertebra. Collateral formation was not uniformly seen in patients with moderate or severe stenosis. These findings suggest that extrinsic compression of the IJV at this level is unlikely to be pathologic in nature.
ABBREVIATION:
- IJV
internal jugular vein
Footnotes
Disclosures: Mahesh V. Jayaraman—UNRELATED: Consultancy: DePuy Institute, Comment: One-time consulting fee from DePuy Institute to provide feedback on physician education programs. Jeffrey M. Rogg—UNRELATED: Other: Bayer Pharmaceuticals* Comments: Participating as a researcher in Phase III trial for Gadavist detection of supra-aortic stenosis. (*Money paid to institution.)
References
- 1. Zamboni P. The big idea: iron-dependent inflammation in venous disease and proposed parallels in multiple sclerosis. J R Soc Med 2006;99:589–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zamboni P, Menegatti E, Weinstock-Guttman B, et al. The severity of chronic cerebrospinal venous insufficiency in patients with multiple sclerosis is related to altered cerebrospinal fluid dynamics. Funct Neurol 2009;24:133–38 [PubMed] [Google Scholar]
- 3. Zamboni P, Galeotti R, Menegatti E, et al. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 2009;80:392–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zamboni P, Galeotti R, Menegatti E, et al. A prospective open-label study of endovascular treatment of chronic cerebrospinal venous insufficiency. J Vasc Surg 2009;50:1348–58; e1–3 [DOI] [PubMed] [Google Scholar]
- 5. Ludyga T, Kazibudzki M, Simka M, et al. Endovascular treatment for chronic cerebrospinal venous insufficiency: is the procedure safe? Phlebology 2010;25:286–95 [DOI] [PubMed] [Google Scholar]
- 6. Agosti C, Borroni B, Akkawi NM, et al. Cerebrovascular risk factors and triggers in transient global amnesia patients with and without jugular valve incompetence: results from a sample of 243 patients. Eur Neurol 2010;63:291–94 [DOI] [PubMed] [Google Scholar]
- 7. Schreiber SJ, Doepp F, Klingebiel R, et al. Internal jugular vein valve incompetence and intracranial venous anatomy in transient global amnesia. J Neurol Neurosurg Psychiatry 2005;76:509–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung CP, Chao AC, Hsu HY, et al. Decreased jugular venous distensibility in migraine. Ultrasound Med Biol 2010;36:11–16 [DOI] [PubMed] [Google Scholar]
- 9. Chou CH, Chao AC, Lu SR, et al. Cephalic venous congestion aggravates only migraine-type headaches. Cephalalgia 2004;24:973–79 [DOI] [PubMed] [Google Scholar]
- 10. Doepp F, Schreiber SJ, Dreier JP, et al. Migraine aggravation caused by cephalic venous congestion. Headache 2003;43:96–98 [DOI] [PubMed] [Google Scholar]
- 11. Daugaard D, Thomsen LL, Olesen J. No relation between cephalic venous dilatation and pain in migraine. J Neurol Neurosurg Psychiatry 1998;65:260–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. San Millán Ruíz D, Gailloud P, Rufenacht DA, et al. The craniocervical venous system in relation to cerebral venous drainage. AJNR Am J Neuroradiol 2002;23:1500–08 [PMC free article] [PubMed] [Google Scholar]
- 13. Doepp F, Schreiber SJ, von Munster T, et al. How does the blood leave the brain? A systematic ultrasound analysis of cerebral venous drainage patterns. Neuroradiology 2004;46:565–70 [DOI] [PubMed] [Google Scholar]
- 14. Schreiber SJ, Lurtzing F, Gotze R, et al. Extrajugular pathways of human cerebral venous blood drainage assessed by duplex ultrasound. J Appl Physiol 2003;94:1802–05 [DOI] [PubMed] [Google Scholar]
- 15. Valdueza JM, von Munster T, Hoffman O, et al. Postural dependency of the cerebral venous outflow. Lancet 2000;355:200–01 [DOI] [PubMed] [Google Scholar]