Abstract
BACKGROUND AND PURPOSE:
A new curative embolization technique with Onyx for selected small and medium-sized superficially located brain AVMs was developed, which consists of obliteration of the nidus, including incremental occlusion of the draining veins. We report our first clinical results.
MATERIALS AND METHODS:
Between June 2008 and July 2011, 24 patients (7 women, 17 men; mean age, 41 years; range, 6–74 years) with AVMs were selected for curative embolization with Onyx. Presentation was hemorrhage in 14 and seizures in 10 patients. AVM location was frontal in 11, occipital in 6, parietal in 4, and temporal in 3. AVM size was a mean of 2.2 cm (median, 2; range, 1–3 cm).
RESULTS:
Complete angiographic obliteration of the AVM with Onyx in a single session was achieved in all 24 patients. There were no hemorrhagic or ischemic complications (0%; 95% CI, 0%–16.3%), and no new deficits induced by the treatment. Of 14 patients with ruptured AVMs, 1 patient who presented with a large frontal hematoma died shortly after surgical evacuation of the hematoma following complete embolization of a micro-AVM. Follow-up angiography at 3 months in 23 patients demonstrated a small AVM remnant in 1 that was treated with gamma knife radiosurgery. The other 22 AVMs remained completely occluded.
CONCLUSIONS:
In selected patients with small and medium-sized superficial brain AVMs, as defined in our study, injection of Onyx by using a curative embolization technique in a single session seems to provide a safe and effective alternative to radiosurgery or surgery.
Treatment of brain AVMs includes different modalities (embolization, surgery, radiosurgery) either alone or in combination. Substantial progress has been made in the embolization of AVMs in the past decade. The introduction of the nonadhesive EVOH Onyx (ev3, Irvine California) has largely replaced the use of acrylic glue for the obliteration of the nidus in many centers. With growing experience, advanced biplane imaging with rapid subtraction fluoroscopy, and refinements of technique in the use of Onyx, a considerable proportion of small and intermediate-sized brain AVMs can be completely obliterated at a low complication rate, on the condition that the nidus is accessible by the microcatheter with feeders suitable for the injection with Onyx, allowing of 2–3 cm of reflux.1–8 In AVMs presenting with intracranial hemorrhage, better recognition of certain angioarchitectural features of the AVM as likely weak spots prone to recurrent hemorrhage, such as intranidal- and flow-related aneurysms and venous stenoses, has prompted many operators to perform a partial targeted embolization treatment when complete AVM obliteration is unlikely or impossible.9–14
The growing experience with the use of Onyx in our department together with advancement in imaging possibilities and the adjustment of the technique of curative embolization have gradually resulted in the adaptation of the treatment paradigm of patients with AVMs in our hospital. Embolization is no longer basically restricted to partial nidus obliteration of large AVMs to facilitate subsequent surgery or radiosurgery. Curative embolization is now offered as an alternative to radiosurgery or surgery in selected patients with small or intermediate-sized unruptured or ruptured AVMs.
In this study, we report our experience with curative embolization of selected brain AVMs with Onyx.
Materials and Methods
Patient Selection for Curative Embolization
Our hospital has the largest neurosurgical service in the country and is a tertiary referral center for brain AVMs. A gamma knife (Perfexion; Leksell, Stockholm, Sweden) is situated next to the radiology department.
Patients were evaluated by an interdisciplinary conference consisting of neuroradiologists, neurologists, and neurosurgeons for assessment of the therapeutic options based on MR imaging, CT, and angiography. Curative embolization was considered in patients with small-to-medium-sized superficially located AVMs with feeding arteries accessible for microcatheters and allowing for reflux of Onyx for 2–3 cm, with a well-delineated nidus and recognizable proximal parts of the draining veins emerging from the nidus (Figs 1–3). Curative embolization was generally not considered in large AVMs with feeders from different territories; in AVMs located in basal ganglia, thalamus, or brain stem with perforating arteries as feeding vessels; in AVMs with a diffuse nidus; and “en passage”, leptomeningeal, or tiny and elongated feeders.
Fig 1.
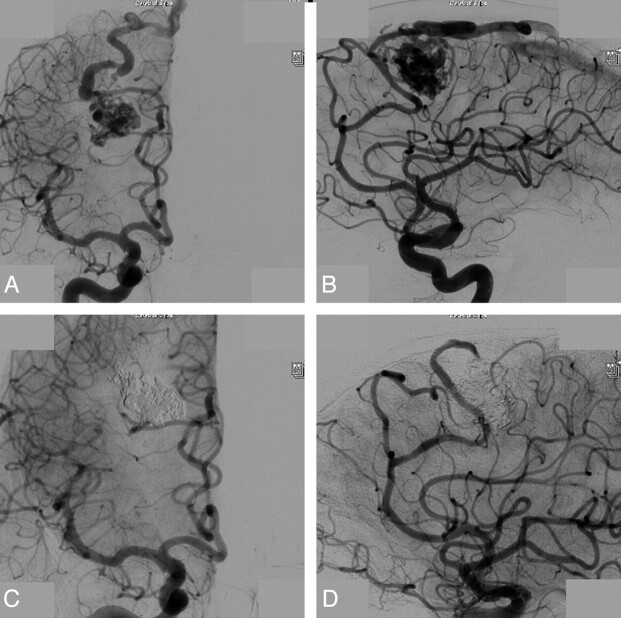
A 23-year-old man with a ruptured small left occipital brain AVM. A, Frontal view of a simultaneous right vertebral and left internal carotid angiogram shows a small occipital AVM with a single dominant feeder from the middle cerebral artery and a single dominant superficial draining vein (arrow). B, Complete obliteration after embolization with Onyx in a single injection. C–F, Different stages of a 47-minute Onyx injection show progressive filling of the nidus, including the dominant draining vein (arrow in F).
Fig 3.
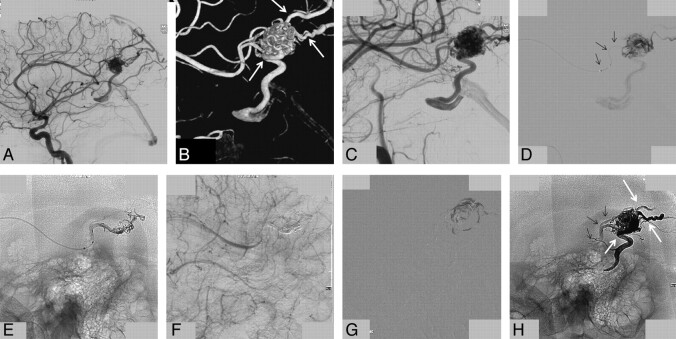
Embolization technique with Onyx of a small frontal AVM (same patient as in Fig 2). A, Late phase of the lateral view of an internal carotid artery angiogram shows the dominant draining vein. B, Superselective angiogram shows the lower part of the nidus and the origin of the draining vein. C–F, Different stages of a 64-minute Onyx injection show progressive filling of the nidus, including the dominant draining vein (arrows in F).
Fig 2.
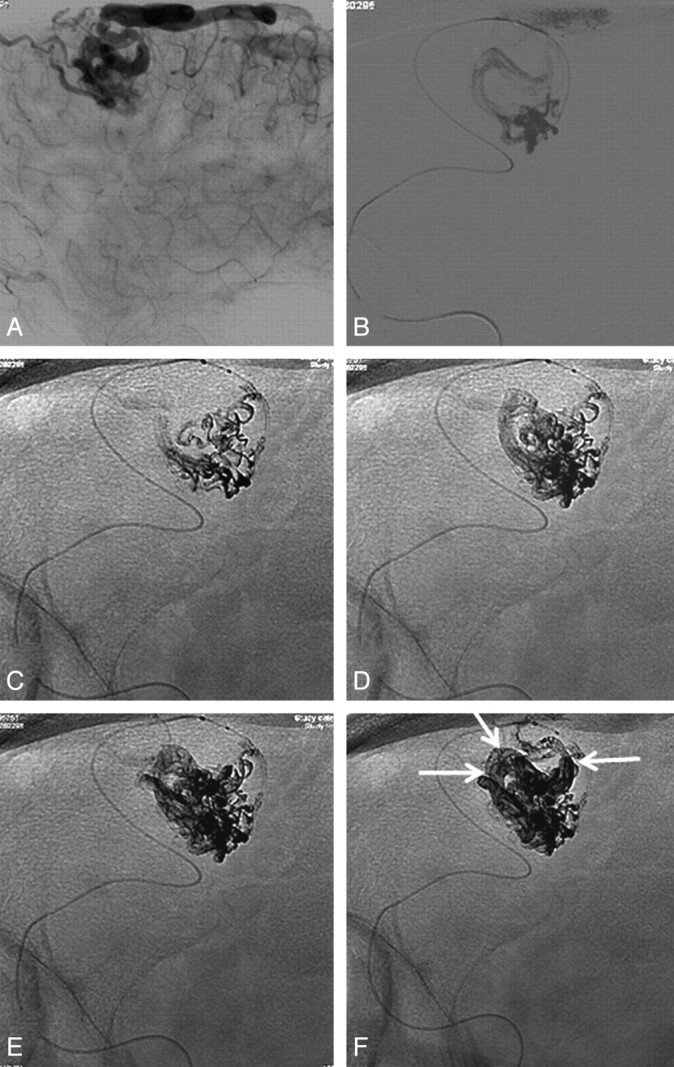
A 53-year-old man with seizures from a small frontal AVM. Frontal (A) and lateral (B) views from a right internal carotid angiogram demonstrate a small frontal AVM with 2 feeders from the anterior cerebral artery, a well-delineated nidus, and a single draining vein. C and D, Complete obliteration after embolization with Onyx.
In patients with unruptured small AVMs considered suitable for >1 treatment technique (curative embolization, radiosurgery, surgery, and conservative management), the pros and cons of the different treatments were discussed in the outpatient clinic. In patients with small AVMs presenting with hemorrhage, either curative embolization in the acute stage or surgery was offered as first-line treatment. In patients with large ruptured AVMs not amenable for curative embolization and with a flow-related or intranidal aneurysm as the source of hemorrhage, partially targeted embolization was offered to eliminate the aneurysm from the circulation and to reduce the size of the AVM to facilitate surgery or radiosurgery.
Patients
Between June 2008 and July 2011, 24 patients (7 women, 17 men) with AVMs were selected for curative embolization with Onyx. In the same timeframe, another 42 patients with AVMs were embolized to reduce the AVM size preceding surgery or radiosurgery or to primarily exclude intranidal or flow-related aneurysms as the source of AVM-related hemorrhage. In addition, during the study period, another 6 patients with AVMs underwent surgery primarily, and another 72 patients with AVMs were treated with gamma knife radiosurgery for the first time, either as a sole therapy or after embolization in other institutions.
The 24 patients with intended curative embolization were a mean of 41 years of age (median, 40; range, 6–74 years). Presentation was hemorrhage in 14 and seizures in 10 patients. AVM location was frontal in 11, occipital in 6, parietal in 4, and temporal in 3. AVM size was a mean of 2.2 cm (median, 2; range, 1–3 cm). Grading according to Spetzler and Martin15 was grade I in 6, grade II in 14, and grade III in 4.
Curative Embolization Technique with Onyx
Onyx is a liquid embolic agent consisting of 48-mol/L ethylene and 52-mol/L EVOH, dissolved in DMSO and mixed with micronized tantalum powder (35% weight per volume) for radiopaque visualization. Onyx is available in ready-to-use vials of 1.5 mL in 3 different viscosities, Onyx 18, 20, and 34 (concentrations of EVOH in centipoise: 6%, 6.5%, and 8% respectively). The vials must be placed on a mixer and shaken for at least 20 minutes to obtain a homogeneous solution consisting of the embolic component and the tantalum powder. Embolizations were performed with the patients under general anesthesia on a biplane angiographic system (Allura Neuro; Philips Healthcare, Best, the Netherlands) equipped with high-speed filming, 3D rotational angiography, cerebral tomographic scanning, 3D road-mapping, and rapid subtracted fluoroscopy. A 6F guiding catheter was positioned in the internal carotid or vertebral artery that supplied the AVM. The guiding catheter was continuously flushed with slightly heparinized saline (2000 IU of heparin per liter). Systemic heparin was not administered.
A microcatheter (Marathon, ev3; or Sonic, Balt, Montmorency, France) was placed in the dominant feeder as close as possible to the AVM nidus with its tip in the most distal part of the arterial feeder just before the transition to the venous part of the nidus. An angiographic series through the microcatheter with a 3-mL syringe was performed to evaluate the AVM architecture, adjusting the microcatheter tip in a safe position with allowance for 2- to 3-cm reflux of Onyx. Microangiography was also useful in evaluating the local AVM flow and illustrating the anatomy of the proximal part of the draining veins emerging from the nidus (Fig 3). In normal flow conditions, Onyx 18 was used; Onyx 34 was reserved for cases with high-flow intranidal fistulas, often in combination with a microballoon (Magic MDBE, Balt) to block the flow proximally to prevent premature migration of Onyx into the draining veins.8 No coils or acrylic glue were used for the occlusion of high-flow fistulas. The microcatheter was flushed with saline, its dead space was filled with DMSO, an equal volume of Onyx was injected into the microcatheter, and the injection of Onyx was started under subtracted fluoroscopy. In case of reflux around the catheter tip, the injection of Onyx was discontinued to form a cast around the tip of the microcatheter. Another slow injection was started with a new subtraction road map, after waiting for 10–30 seconds. If there was forward extension, the injection was continued at the same pace with no acceleration; and if reflux occurred, the injection was stopped again.
This cycle was patiently repeated until a compact plug formed to provide continuous forward penetration of the Onyx. When Onyx entered the proximal draining veins in a laminated fashion, the injection was stopped for 10–20 seconds to redirect Onyx advancement into another nidal compartment. Venous filling was identified directly as a vascular space with a diameter much larger than the arterial feeder or indirectly when Onyx was flowing rapidly or laminating along the venous wall. Utmost care was taken to preserve venous outflow until the entire nidus was filled with Onyx.
Nidus obliteration was regularly monitored by biplane angiographic runs. Embolization from a specific pedicle was terminated when no more forward advancement of Onyx was achieved and reflux was progressive. If necessary, a new microcatheter was positioned into another pedicle and embolization was continued in the same way. The most crucial part of the procedure was the approximation of complete obliteration. Toward the end of the embolization, Onyx was progressively allowed to enter the draining veins but injection was stopped frequently to redirect Onyx to the remaining parts of the nidus and even retrogradely into different arterial feeders. Even when repeated angiography indicated apparent complete obliteration of the AVM, Onyx was further injected to completely occlude the draining veins until repeated reflux in the feeder precluded further Onyx advancement (Fig 4).
Fig 4.
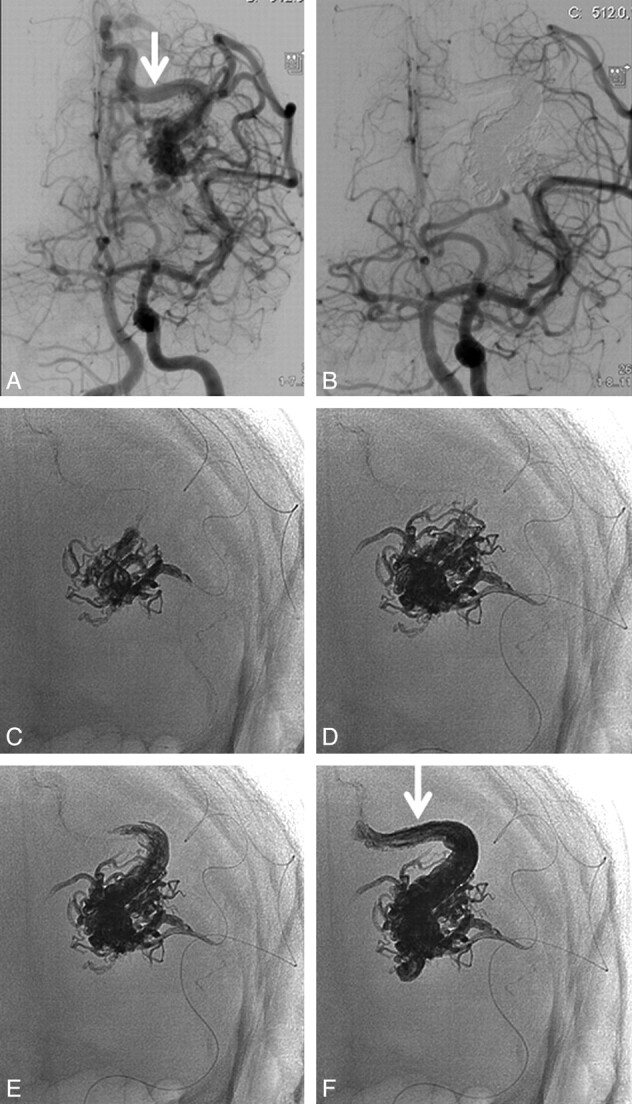
A 33-year-old man with a ruptured small parietal AVM. A and B, Lateral view of a right internal carotid angiogram and off-lateral 3D rotational image demonstrate a small parietal AVM supplied by the pericallosal artery with 1 deep and 2 superficial draining veins (arrows in B). C, Off-lateral magnification angiogram, identical to the 3D projection in B, best shows the proximal draining veins emerging from the nidus. D, Superselective angiogram through the microcatheter demonstrates a 3-cm distal end allowing reflux of Onyx (arrows). Early phase of Onyx injection shows a 2-cm reflux with little penetration into the nidus (E), but the angiogram suggests complete occlusion (F). G, Subtracted fluoroscopic image demonstrates progressive nidus filling with Onyx after apparent complete angiographic obliteration. H, Final Onyx cast with a 2-cm reflux in the feeder (small arrows) and occlusion of all 3 draining veins (arrows, compare with B).
The microcatheter was removed by slowly increasing traction. Mostly, the catheter was easily removed. If not, traction was incrementally increased every 20–30 seconds until the catheter came out. A cerebral tomography was performed to exclude hemorrhagic complications before the patient was awakened and detubated. No antiepileptic or steroid drugs were given routinely. We recorded the following parameters: the use of a balloon microcatheter to block the flow in high-flow fistulas, the number of Onyx injections, the duration of Onyx injections, and the total volume of Onyx injected.
After complete angiographic obliteration, a control angiography was performed after 3 months. If a remnant of the AVM persisted despite intended curative embolization, gamma knife radiosurgery was scheduled within 2 months.
Results
Results of Embolization
Complete angiographic obliteration of the AVM with Onyx in a single session was achieved in all 24 patients. There were no hemorrhagic or ischemic complications of the embolizations (0%; 95% CI, 0%–16.3%). In 19 patients, the AVM was obliterated with a single Onyx injection, and in 5 patients, with 2 injections. Onyx injections from a single pedicle ranged from 22 to 104 minutes (mean, 37 minutes), and the total amount of Onyx injected ranged from 1.5 to 17 mL. In 3 patients with high-flow fistulas, a microballoon was used to temporarily block the flow during Onyx injection.
Clinical and Angiographic Follow-Up at 3 Months
All 10 patients with unruptured AVMs were neurologically intact at 3-month follow-up, and angiography confirmed lasting complete obliteration of their AVMs. Of 14 patients with ruptured AVMs, 1 patient who presented with a large frontal hematoma died of an uncontrollable rise of intracranial pressure shortly after surgical evacuation of the hematoma following complete embolization of a micro-AVM. Of the surviving 13 patients with ruptured AVMs, 4 had neurologic deficits at 3 months directly related to the presenting hematoma (hemiparesis in 2 and hemianopsia in 2). Follow-up angiography at 3 months demonstrated a small remnant of the AVM in 1 patient that was subsequently treated with gamma knife radiosurgery. The remaining 12 AVMs were completely occluded.
Discussion
In this study of 24 patients with small-to-medium-sized AVMs selected for intended curative embolization with Onyx, curative embolization proved to be feasible in 23 (96%). In 1 patient, a small remnant was apparent on 3-month follow-up angiography that was subsequently treated with radiosurgery. There were no complications of the endovascular treatments.
Since the introduction of Onyx in our practice in 2000, this nonadhesive liquid embolic agent rapidly replaced the use of acrylic glue for the embolization of brain AVMs. We now use acrylic glue only for some micro-AVMs. During the years with growing experience and encouraged by the results of others,1–3,5–7 the technique of Onyx injection has evolved and so has the indication for embolization. We now offer intended curative embolization to selected patients with small AVMs with angioarchitecture suitable for Onyx injection, either as an alternative to radiosurgery in patients with unruptured AVMs or as a definitive treatment to prevent recurrent hemorrhage in patients with AVMs who present with hemorrhage.16 A few points need special attention for success in curative embolization with Onyx. First, the selection of the AVM is important. For curative embolization, the AVM should be small or medium-sized (1–3 cm) with feeders from a single vascular territory, not located in brain stem or deep structures, and with arterial feeders readily accessible by a microcatheter with the possibility of reflux of 2–3 cm. Second, the location of the proximal parts of the draining veins should be clear for the operator to recognize venous filling with Onyx in time.
We found it helpful to choose 2 projections on our biplane system, mostly from 3D rotational angiography, to use throughout the embolization to monitor reflux along the microcatheter as well as reflux in remote arterial feeders and to recognize early venous filling with Onyx. With these 2 projections as reference images on slave monitors, we used subtracted fluoroscopy with frequent new subtractions to monitor the course of the injected Onyx. At every moment that Onyx entered the veins, the injection was stopped to redirect the flow of Onyx to the remaining parts of the nidus. Only when complete obliteration of the nidus seemed apparent, were the draining veins completely filled with Onyx. At this point, reflux of Onyx via the nidus into remote arterial feeders regularly occurred. The procedure was stopped when Onyx progressively entered the veins after complete nidus filling or when repeated reflux along the microcatheter occurred. In most cases, this was beyond the point of angiographic obliteration of the AVM, especially in AVMs with a single dominant feeding pedicle (Fig 4).
Although in our small patient group no complications occurred, possible complications of brain AVM embolization with Onyx are technical, hemorrhagic, and ischemic. Technical complications include perforations with the guidewire or microcatheter or a retained microcatheter glued in the nidus. Hemorrhagic complications may occur by vessel perforation during catheterization or withdrawal or by altering the hemodynamics, especially by occlusion of the draining veins before complete obliteration of the nidus. Hemorrhagic complications may go undetected during embolization; therefore, cerebral tomographic scanning is recommended to detect such complications in a timely manner before waking the patient. Ischemic complications may occur with inadvertent occlusion of normal arteries, either by extended reflux along the microcatheter or by unrecognized reflux in remote arterial feeders.
Our results are in line with several recently published studies. In the recent large series by Saatci et al,2 using a comparable technique, complete obliteration with Onyx was achieved in 179 of 350 AVMs with a cure rate of small AVMs (Spetzler and Martin grades I and II) of 98%. Also other operators reported cure rates ≤94% of embolization of selected AVMs.1,3–7 In all studies, the same angioarchitectural features of the AVMs predispose them to complete obliteration.
Conclusions
The encouraging results of curative embolization of selected small AVMs with Onyx have changed the multimodality treatment paradigm for AVMs in several large centers, including ours. Although a gamma knife is situated next to the radiology department, we now offer curative embolization as a valid alternative to radiosurgery in selected patients. Radiosurgery is now mostly reserved for small AVMs with angioarchitecture not suitable for curative embolization, AVMs located in deep structures or in the brain stem, or as adjuvant treatment after partial embolization.
ABBREVIATIONS:
- CI
confidence interval
- DMSO
dimethyl-sulfoxide
- EVOH
ethylene-vinyl alcohol copolymer
References
- 1. Katsaridis V, Papagiannaki C, Aimar E.. Curative embolization of cerebral arteriovenous malformations (AVMs) with Onyx in 101 patients. Neuroradiology 2008;50:589–97 [DOI] [PubMed] [Google Scholar]
- 2. Saatci I, Geyik S, Yavuz K, et al. Endovascular treatment of brain arteriovenous malformations with prolonged intranidal Onyx injection technique: long-term results in 350 consecutive patients with completed endovascular treatment course. J Neurosurg 2011;115:78–88 [DOI] [PubMed] [Google Scholar]
- 3. Andreou A, Ioannidis I, Lalloo S, et al. Endovascular treatment of intracranial microarteriovenous malformations. J Neurosurg 2008;109:1091–97 [DOI] [PubMed] [Google Scholar]
- 4. van Rooij WJ, Sluzewski M, Beute GN.. Brain AVM embolization with Onyx. AJNR Am J Neuroradiol 2007;28:172–77 [PMC free article] [PubMed] [Google Scholar]
- 5. Abud DG, Riva R, Nakiri GS, et al. Treatment of brain arteriovenous malformations by double arterial catheterization with simultaneous injection of Onyx: retrospective series of 17 patients. AJNR Am J Neuroradiol 2011;32:152–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Panagiotopoulos V, Gizewski E, Asgari S, et al. Embolization of intracranial arteriovenous malformations with ethylene-vinyl alcohol copolymer (Onyx). AJNR Am J Neuroradiol 2009;30:99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weber W, Kis B, Siekmann R, et al. Endovascular treatment of intracranial arteriovenous malformations with Onyx: technical aspects. AJNR Am J Neuroradiol 2007;28:371–77 [PMC free article] [PubMed] [Google Scholar]
- 8. van Rooij WJ, Sluzewski M.. Endovascular occlusion of high-flow intracranial arteriovenous shunts: technical note. Neuroradiology 2007;49:1029–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alexander MJ, Tolbert ME.. Targeting cerebral arteriovenous malformations for minimally invasive therapy. Neurosurgery 2006;59(5 suppl 3):178–83 [DOI] [PubMed] [Google Scholar]
- 10. Hademenos GJ, Massoud TF.. Risk of intracranial arteriovenous malformation rupture due to venous drainage impairment: a theoretical analysis. Stroke 1996;27:1072–83 [DOI] [PubMed] [Google Scholar]
- 11. Mansmann U, Meisel J, Brock M, et al. Factors associated with intracranial hemorrhage in cases of cerebral arteriovenous malformation. Neurosurgery 2000;46:272–79 [DOI] [PubMed] [Google Scholar]
- 12. Stefani MA, Porter PJ, terBrugge KG, et al. Angioarchitectural factors present in brain arteriovenous malformations associated with hemorrhagic presentation. Stroke 2002;33:920–24 [DOI] [PubMed] [Google Scholar]
- 13. Krings T, Hans FJ, Geibprasert S, et al. Partial “targeted” embolisation of brain arteriovenous malformations. Eur Radiol 2010;20:2723–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Graf CJ, Perret GE, Torner JC.. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg 1983;58:331–37 [DOI] [PubMed] [Google Scholar]
- 15. Spetzler RF, Martin NA.. A proposed grading system for arteriovenous malformations. J Neurosurg 1986;65:476–83 [DOI] [PubMed] [Google Scholar]
- 16. Mast H, Young WL, Koennecke HC, et al. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet 1997;350:1065–68 [DOI] [PubMed] [Google Scholar]